Obesity and Microbes: The Role of Bariatric Surgery
Received: 02-Feb-2023 / Manuscript No. JOWT-23-89611 / Editor assigned: 04-Feb-2023 / PreQC No. JOWT-23-89611 (PQ) / Reviewed: 18-Feb-2023 / QC No. JOWT-23-89611 / Revised: 21-Feb-2023 / Manuscript No. JOWT-23-89611 (R) / Published Date: 28-Feb-2023 DOI: 10.4172/2165-7904.1000541
Abstract
Obesity is a worldwide ongoing pandemic and its consequences have a dramatic effect on society. Bariatric surgery is a key method to solve obesity, with 800,000 ca cases worldwide yearly and high outcome variability, which includes gut microbiota change. The relationship between weight and gut microbiota should be studied as it can affect the efficacy of weight-losing techniques.
Keywords
Obesity is defined as the accumulation of excess fat in the body that can compromise health: this corresponds to a BMI equal to or greater than 30 kg/m2, with three different likely levels that are class I (BMI = 30.0 – 39.9 kg/m2), class II (BMI = 40.0 – 49.9 kg/m2) and class III (BMI > 50 kg/m2) [1].
Obesity is considered a risk factor for various chronic illnesses such as cardiovascular diseases and hypertension, type 2 diabetes and thyreopathies, non-alcoholic fatty liver disease, and cancer. Over the past thirty years, the prevalence of obesity is rapidly getting higher not only in developed countries but also in developing countries. Even though physical inactivity and excessive food intake are usually thought of as the cause of obesity, its etiology is quite complex. There are many factors to be taken into account, such as environment, genetics, and lifestyle [2]. Besides, the microbiota has been reported as one of the key factors in obesity etiology [3-6].
Obesity is one of the easiest diseases to diagnose but one of the hardest to treat and it should be treated effectively to avoid its consequences on health. Obesity management must be planned in a personalized way [7]. At the moment, treatment methods for obesity are behavior modification therapy, diet therapy, medical treatment, and surgical treatment [8]. Surgery is not always the first choice and it should be applied only if the appropriate indications are present; after its application, body weight loss occurs with changes in the metabolism of bile acids, gastric pH, the metabolism of hormones, and in microbiota [9].
Bariatric Surgery
Bariatric surgery is one of the most effective therapeutic treatments for obesity and complications [10]. Thanks to it, long-term permanent body weight loss is achieved, metabolic effects of obesity are reduced, many diseases are prevented and quality of life is markedly increased [11]. Body weight loss with bariatric surgery is fulfilled through the change of food preferences, reduction of nutrient digestion, acceleration of gastric void, regulation of hormonal fluctuation (e.g. glucagon-like peptide 1, GLP-1, and peptide tyrosine tyrosine, PYY), and alterations in the metabolism of bile acids. In spite of the fact that bariatric surgery is suitable for obesity treatment, some complications can rarely occur and they should be taken into account in evaluating surgical risk. These are gastroesophageal reflux, nutritional deficiencies, gastric outlet obstruction, mesh erosion and marginal ulcerations, slippage, and internal herniation [12]. Indications for bariatric surgery were established by the United States National Institute of Health in 1991 (Table 1) [13].
| Indication for surgical operation [13] |
|---|
| BMI=40 kg/m2 (or) BMI > 35 kg/m2 + type 2 diabetes, hypertension, sleep apnea, or hyperlipidemia |
| Acceptance of surgical risk |
| Failure of nonsurgical treatments |
| Psychiatric stability, no alcohol and drug dependence |
| Well-established motivation, knowledge of the operation and its sequelae |
| No medical problems that will harm the surgeon |
| No uncontrolled psychotic and depressive disorder |
| Complete family and social support |
Table 1: Indication for surgical operation in obesity according to the United States National Institute of Health 1991.
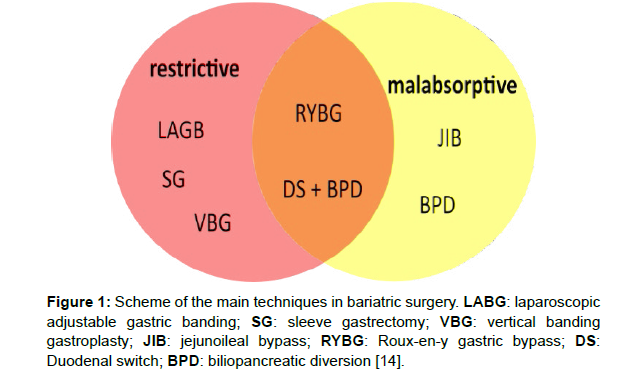
There are various bariatric surgical methods according to their effect mechanisms (Figure 1) [14].
Surgical techniques
The most common surgical techniques in bariatric surgery are described as follows.
RYBG: Roux-en-Y gastric bypass is the gold standard in the surgical treatment of obesity so it is the most commonly practiced bariatric surgery in the world [15]. This method consists of two steps. First of all, the stomach capacity is left to be about 30 cm3; Roux sputum can then be pulled up from the stomach, the front of the colon and back of the stomach, or behind the colon and stomach for gastro-jejunostomy [16]. The input of food and energy goes down due to the reduction in stomach volume. In certain cases, fat malabsorption can happen [17].
LSG: Laparoscopic sleeve gastrectomy consists of the removal of 80% ca of the lateral aspect of the stomach in a vertical fashion, leaving a long gastric tube [18]. LSG is preferred for patients who have super obesity and a BMI < 50 kg/m2 [19]. Due to the reduction of gastric volume, nutrient, and energy intake are restricted; however, there is a reduction in plasma levels of ghrelin [20].
LAGB: Laparoscopic adjustable gastric banding involves the placement of an adjustable silicone band around the upper part of the stomach, thus forming a small gastric space over the gastric band. The size of the gap between the upper stomach space and the posterior part of the stomach can be tailored by filling it with sterile 0.9% saline solution injected through the abdominal wall. Adjustment of the band can be done piecemeal during postoperative follow-up [21]. This method provides body weight loss by reducing nutrient uptake with a completely restrictive effect [14].
BPD: Biliopancreatic diversion (BPD) is composed of three main components: a gastric tube with preserved pylorus, distal ileoanal anastomosis, and anastomosis of the proximal duodenal bile duct. Body weight loss is provided by the reduction of gastric volume and decrease of ghrelin hormone, increasing PYY, as it happens in LSG. In this technique, hormonal changes with anatomical changes are thought to be the route to body weight loss [22].
Surgery induces important changes in both intrinsic and extrinsic factors, as well as in the anatomy of the gastrointestinal system (Table 2) [23, 24].
Changes |
RYBG | LSG | LAGB |
|---|---|---|---|
| Food intake | ↓ | ↓ | ↓ |
| Food transit time | ↑ | = | ↓ |
| Food choices | ↓preference for food rich in fats and sugars | ↓ | ↑preference for food poor in fibers |
| Chewing time | ↑ | ↑ | ↑ |
| Acid production | disrupted | = | = |
| Ghrelin | = | ↓ | = |
| GLP-1 and PYY | ↑ | = | = |
| Insulin | ↓ | ↓ | ↓ |
| Leptin | ↓ | ↓ | ↓ |
| Adiponectin | ↑ | ↑ | ↑ |
Table 2: Behavioural and biochemical changes in RYBG, LSG and LAGB. RYBG: Roux-en-y gastric bypass, LSG: laparoscopic sleeve gastrectomy, LAGB: laparoscopic adjustable gastric banding. GLP-1: glucagon-like peptide 1, PYY: peptide tyrosine tyrosine.
The gut microbiome
The human body is colonized by a huge variety of microbes, commonly referred to as the human microbiota. It comprises commensal, symbiotic, and pathogenic microbes. Microbiome, instead, is the genetic pool of microbiota living in a specific place and their relation with the environment [25]. It is estimated that there are about 1,014 microorganisms in the human body, more than 70% of which are in the colon, and more than 35,000 bacterial strains in the gastrointestinal tract [26]. Microbiota is determined by various factors such as delivery type, breastfeeding time, transition time to complementary feeding, diet, and use of antibiotics from birth to death but also host weight and sugar consumption [27].
The gut microbiota is a complex ecosystem, which provides major functions to the host such as metabolism regulation, modulation of the immune system, and thus protection against pathogens [28-31]. Gut microbiota can be classified into six bacterial clusters in healthy individuals and these include Firmicutes (including gram+ve strains of Clostridium, Eubacterium, Ruminococcus, Butyrivibrio, Anaerostipes, Roseburia, Faecalibacterium, etc.), Bacteroidetes (including gramnegative strains of Bacteroides, Porphyromonas, Prevotella, etc.), Proteobacteria (including gram- strains such as Enterobacteriaceae), Actinobacteria (including the gram+ve Bifidobacterium genus), Fusobacteria and Verrucomicrobia (including Akkermansia, etc.) [32]. Bacteroidetes and Firmicutes form more than 90% of the total intestinal microbiota. The most important components of the human intestinal microbiota are obligate anaerobes of the genus Bacteroides, Eubacterium, Clostridium, Ruminococcus, Peptococcus, Peptostreptococcus, Bifidobacterium, and Fusobacterium and facultative anaerobes such as Escherichia, Enterobacter, Enterococcus, Klebsiella, Lactobacillus, and Proteus. Methanogenic archaea have also been pointed out and the most important in the human gut is Methanobrevibacter smithii [33]. Changes in microbiota content shape human health at a significant level. It is outlined that many non-communicable diseases such as obesity, type 2 diabetes, asthma and allergies, inflammatory bowel disease, metabolic syndrome, and atherosclerosis are intimately linked with gut microbiota [34].
Gut disbiosis in obesity
It is reported that genetic and environmental factors affect obesity etiology. Researchers have also noted that intestinal microbiota contributes to the regulation of energy and fat metabolism and that it affects obesity and its complications [35]. It has been outlined that obese patients have less variability in the intestinal microbiota than normal-weight individuals [36]. The fundamental function that splits up microbial strains from obese and thin individuals is the inability to obtain fermentation; another difference is that short-chain fatty acids cannot be produced from indigestible food [37].
Intestinal microbiota studies in both human and animal models have helped in understanding the role of microbial activity in the etiology of obesity. It has been outlined that patients with obesity have fewer Bacteroidetes and more Firmicutes in their microbiota than normal-weight people. It is well known that diets rich in saturated fatty acids lead to obesity and hepatic steatosis, increasing the Firmicutes/ Bacteroidetes ratio in the gut microbiota [38]. On the other hand, fat and carbohydrate-restricted diets and body weight loss cause the amount of Bacteroidetes to increase and thus the Firmicutes/ Bacteriodetes ratio to decrease [39]. These shreds of evidence are controversial so that some studies show that there is no relationship between BMI and Firmicutes/Bacteroidetes [40,41] although other studies display an increase in Firmicutes/Bacteroidetes ratio in obesity and insulin resistance. Reduction of carbohydrate intake in obese patients decreases the butyrate levels in feces which corresponds to a decrease in the level of Roseburia spp. and Eubacterium rectale [42]. The microbiota is affected by body weight loss caused by diet and exercise. It has been reported that the quantities of Bacteroides and Lactobacillus increase as a result of energy restriction and exercise in patients with obesity. Nonetheless, no changes were seen in overweight adolescents who lost less than 2 kg in body weight [43].
Obesity can affect the human immune system in a significant way and gut microbiota is likely to express a notable function. This is a controversial statement because there is much disagreeing evidence in scientific literature. For example, it has been outlined that a higher BMI compromises immunization following COVID-19 vaccination [44] but other studies, instead, have reported that is no relationship between BMI and COVID-19 severity, even in the most critical cases [45]. Moreover, it has been stated that obesity could have a protective role against infectious diseases: that is the case of pouchitis by Clostridioides difficile. [46]
What is true is far from easy to be defined. This is mostly due to the fact that obesity is complex to the extent that it cannot be evaluated as a single variable. In any case, intestinal microbiota modification may be a therapeutic treatment for the prevention or even reversal of obesity.
Bariatric surgery and microbiota
It is reported that significant changes in gut microbiota occur after bariatric surgery; the most likely mechanisms include changes in food choices and preferences, reduction of food intake, and nutrient malabsorption [47].
First of all, short-term dietary changes may cause fast changes in the composition of intestinal microbiota. As an example, it has been outlined that Prevotella enterotypes are associated with complex carbohydrate-rich and simple carbohydrate-rich diets, whereas Bacteroidetes enterotype is correlated with a typical “Western diet”, full of animal protein and saturated fatty acids [48]. In detail, some diets can affect the quantity of specific strains of gut microbiota, such as diets low in fats and high in carbohydrates but also diets high in carbohydrates with a low glycemic index [49].
A second factor regulating the change in gut microbiota after bariatric surgery has been stated to be bile acids [8]. Bile acids can rule their synthesis and their intestinal reabsorption through modulation of the nuclear-located farnesoid X receptor (FXR). Another pathway of auto regulation is the G-linked protein TGR5, but this pathway is yet to be 100% understood [50]. Recently, the physiological role of bile acids has been associated with pancreatic beta cell function and thus glucose homeostasis but also energy consumption. Even these roles of bile acids are correlated with FXR and TGR5 pathways [8]. Bile and pancreatic secretions are separated from nutrients in RYGB and they come together only in the more distal part of the intestine; as a result, the distal jejunum and proximal ileum are excessively exposed to the nutrients. Dietary lipids are surrounded by the bile acids, while bile acids cycling in the upper intestine become blunted: this leads to an increase of serum bile acids level and of serum FGF15/19 levels that normalize the postprandial bile acids answer after surgery [51]. The pathway underlying the beneficial effects of bariatric surgery has been outlined to be changes in bile acids metabolism [43]. The change in bile acids flow has a definite effect on the alterations in gut microbiota after bariatric surgery, too. In the proximal jejunum, the absence of nutrient transit and the decrease in mobility alter the number of bacteria [24]. The changes in bile acids flow also change the 7α-dehydroxylation capacity of the intestinal microbiota, which is implied in the synthesis of the secondary (intermediate) bile acids. In these terms, administration of a diet supplemented with the primary bile acid colic acid to rats increases the presence of Firmicutes, which contains the enzyme 7α-hydroxylase such as Clostridium spp [52].
Even hormones, such as leptin and ghrelin, may change after bariatric surgery. Hormonal changes are linked to both energy metabolism and microbiota [53]. Despite the relationship between gut microbiota and ghrelin is not clearly comprehended, prebiotics are reported to modulate gut microbiota and decrease serum ghrelin levels [54]. On the other hand, leptin has a controversial role. Serum leptin levels have been outlined to have a positive correlation with Mucispirillum, Lactococcus. Another study stated that leptin has a positive correlation with Bifidobacterium and Lactobacillus whilst a negative correlation with Bacteroides, Clostridium, and Prevotella [55]. Researchers have emphasized that further studies are necessary, even though hormones have been reported to influence the intestinal microbiota [38, 56-58].
Another important factor affecting microbiota is changes in pH. After surgery, pH increases as the volume of the stomach decreases. The changing pH influences every part of the gastrointestinal system after the stomach. Increased pH can affect microbiota at an important level. It has been reported that Bacteroidetes decrease due to pH fluctuations after surgery, while Firmicutes and Actinobacteria increase [59].
After bariatric surgery, microbiota diversity changes due to the reasons mentioned above. Table 3 briefs how microorganisms are affected by bariatric surgery [8].
↑ bacteria |
↓ bacteria |
|---|---|
| Proteobacteria Gammaproteobacteria Escherichia coli Klebsiella pneumoniae Shigella boydii Salmonella enterica Enterobacter cancerogenus Enterobacter hormaechei Citrobacter spp Pseudomonas spp Enterococcus faecalis Fusobacterium nucleatum |
Gelicobacter spp Treponema pallidum Bachyspira hyodysenteriae Archae spp |
Table 3: Bacterial diversity of the intestinal microbiota after surgery.
Conclusion
Bariatric surgery is one of the main treatments of obesity. It is considerably effective in achieving and protecting weight loss. The effectiveness of obesity treatments after bariatric surgery is not only related to food consumption but also to microbiota alteration. Malabsorption status after bariatric surgery, changes in the metabolism of bile acids, gastric pH, and the metabolism of hormones give rise to gut microbiota alteration. Changes in microbiota also influence energy homeostasis. Because of these reasons, microbiota should be highlighted as a key factor in body weight loss after bariatric surgery.
References
- http://www.who.int/topics/obesity/en/.
- Panuganti KK, Lenehan CP (2017) Obesity. StatPearls. StatPearls Publishing, Florida.
- Duranti S, Ferrario C, van Sinderen D, Ventura M, Turroni F (2017) Obesity and microbiota: an example of an intricate relationship. Genes Nutr 12: 18.
- Seganfredo FB, Blume CA, Moehlecke M, Giongo A, Casagrande DS, et al. (2017) Weight-loss interventions and gut microbiota changes in overweight and obese patients: a systematic review. Obes Rev 18: 832-851.
- Boulangé CL, Neves AL, Chilloux J, Nicholson JK, Dumas ME (2016) Impact of the gut microbiota on inflammation, obesity, and metabolic disease. Genome Med 8: 42.
- Wang B, Yao M, Ling Z, Li L (2017) The human microbiota in health and disease. Engineering 3: 71-82.
- Nelson JM, Vos MB, Walsh SM, O’Brien LA, Welsh JA (2015) Weight management-related assessment and counseling by primary care providers in an area of high childhood obesity prevalence: current practices and areas of opportunity. Child Obes 11: 194-201.
- Anhê FF, Varin TV, Schertzer JD, Marette A (2017) The gut microbiota as a mediator of metabolic benefits after bariatric surgery. Can J Diabetes 41: 439-447.
- Bouchard C (1991) Current understanding of the etiology of obesity: genetic and nongenetic factors, Am J Clin Nutr 53: 1561S-1565S.
- Sjöström L, Peltonen M, Jacobson P, Sjöström CD, Karason K, et al. (2012) Bariatric surgery and long-term cardiovascular events. JAMA 307: 56-65.
- Brethauer SA (2011) Sleeve gastrectomy. Surg Clin North Am 91: 1265-1279.
- Miras AD, le Roux CW (2013) Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol 10: 575-584.
- No authors (1992) Gastrointestinal surgery for severe obesity: National Institutes of Health Consensus Development Conference Statement. Am J Clin Nutr 55 Suppl: 615S-619S.
- Sağlam F, Güven H (2014) Obezitenin Cerrahi Tedavisi. Okmeydanı Tıp Dergisi 30: 60-65.
- Angrisani L, Santonicola A, Iovino P, Formisano G, Buchwald H, et al. (2015) Bariatric surgery worldwide 2013. Obes Surg 25: 1822-1832.
- Nguyen NT, Varela JE (2017) Bariatric surgery for obesity and metabolic disorders: state of the art. Nat Rev Gastroenterol Hepatol 14: 160-169.
- Kumar R, Lieske JC, Collazo-Clavell ML, Sarr MG, Olson ER, et al. (2011) Fat malabsorption and increased intestinal oxalate absorption are common after Roux-en-Y gastric bypass surgery. Surgery 149: 654-661.
- Regan JP, Inabnet WB, Gagner M, Pomp A (2003) Early experience with two-stage laparoscopic Roux-en-Y gastric bypass as an alternative in the super-super obese patient. Obes Surg 13: 861-864.
- Karamanakos SN, Vagenas K, Kalfarentzos F, Alexandrides TK (2008) Weight loss, appetite suppression, and changes in fasting and postprandial ghrelin and peptide-YY levels after Roux-en-Y gastric bypass and sleeve gastrectomy: a prospective, double blind study. Ann Surg 247: 401-407.
- Wang Y, Liu J (2009) Plasma ghrelin modulation in gastric band operation and sleeve gastrectomy. Obes Surg 19: 357-362.
- Dixon JB, O’Brien PE, Playfair J, Chapman L, Schachter LM, et al. (2008) Adjustable gastric banding and conventional therapy for type 2 diabetes: a randomized controlled trial. JAMA 299: 316-323.
- Jain-Spangler K, Portenier D, Torquati A, Sudan R (2013) Conversion of vertical banded gastroplasty to stand-alone sleeve gastrectomy or biliopancreatic diversion with duodenal switch. J Gastrointest Surg 17: 805-808.
- Dixon JB, Lambert EA, Lambert GW (2015) Neuroendocrine adaptations to bariatric surgery. Mol Cell Endocrinol 418: 143-152.
- Aron-Wisnewsky J, Doré J, Clement K (2012) The importance of the gut microbiota after bariatric surgery. Nat Rev Gastroenterol Hepatol 9: 590-598.
- Rautava S (2016) Early microbial contact, the breast milk microbiome and child health. J Dev Orig Health Dis 7: 5-14.
- Mueller NT, Bakacs E, Combellick J, Grigoryan Z, Dominguez-Bello MG (2015) The infant microbiome development: mom matters. Trends Mol Med 21: 109-117.
- Nicholson JK, Holmes E, Kinross J, Burcelin R, Gibson G, et al. (2012) Host-gut microbiota metabolic interactions. Science 336: 1262-1267.
- Spor A, Koren O, Ley R (2011) Unravelling the effects of the environment and host genotype on the gut microbiome. Nat Rev Microbiol 9: 279-290.
- Korem T, Zeevi D, Zmora N, Weissbrod O, Bar N, et al. (2017) Bread affects clinical parameters and induces gut microbiome-associated personal glycemic responses. Cell Metabolism 25: 1243-1253.e5.
- Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, et al. (2006) An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 444: 1027-1031.
- Ottman N, Smidt H, de Vos WM, Belzer C (2012) The function of our microbiota: who is out there and what do they do? Front Cell Infect Microbiol 2: 104.
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R (2012) Diversity, stability and resilience of the human gut microbiota. Nature 489: 220-230.
- Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, et al. (2005) Diversity of the human intestinal microbial flora. Science 308: 1635-1638.
- Patel RM, Denning PW (2015) Intestinal microbiota and its relationship with necrotizing enterocolitis. Pediatr Res 78: 232-238.
- Karlsson F, Tremaroli V, Nielsen J, Bäckhed F (2013) Assessing the human gut microbiota in metabolic diseases. Diabetes 62: 3341-3349.
- Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, et al. (2009) A core gut microbiome in obese and lean twins. Nature 457: 480-484.
- den Besten G, van Eunen K, Groen AK, Venema K, Reijngoud DJ, et al. (2013) The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J Lipid Res 54: 2325-2340.
- Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, et al. (2005) Obesity alters gut microbial ecology. Proc Natl Acad Sci USA 102: 11070-11075.
- Ley RE, Turnbaugh PJ, Klein S, Gordon JI (2006) Microbial ecology: human gut microbes associated with obesity. Nature 444: 1022-1023.
- Mai V, McCrary QM, Sinha R, Glei M (2009) Associations between dietary habits and body mass index with gut microbiota composition and fecal water genotoxicity: an observational study in African American and Caucasian American volunteers. Nutr J 8: 49.
- Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, et al. (2007) Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol 73: 1073-1078.
- Rivière A, Selak M, Lantin D, Leroy F, De Vuyst L (2016) Bifidobacteria and butyrate-producing colon bacteria: importance and strategies for their stimulation in the human gut. Front Microbiol 7: 979.
- Santacruz A, Marcos A, Wärnberg J, Martí A, Martin-Matillas M, et al. (2009) Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity (Silver Spring) 17: 1906-1915.
- Faizo AA, Qashqari FS, El-Kafrawy SA, Barasheed O, Almashjary MN, et al. (2023) A potential association between obesity and reduced effectiveness of COVID-19 vaccine-induced neutralizing humoral immunity. J Med Virol 95: e28130.
- Kooistra EJ, de Nooijer AH, Claassen WJ, Grondman I, Janssen NAF, et al. (2021) A higher BMI is not associated with a different immune response and disease course in critically ill COVID-19 patients. Int J Obes (Lond) 45: 687-694.
- Gosai F, Covut F, Alomari M, Hitawala A, Singh A, et al. (2020) Obesity Is Associated with Decreased Risk of Clostridium difficile Infection in Hospitalized Patients with Pouchitis. Dig Dis Sci 65: 1423-1428.
- Peat CM, Kleiman SC, Bulik CM, Carroll IM (2015) The intestinal microbiome in bariatric surgery patients. Eur Eat Disord Rev 23: 496-503.
- Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, et al. (2013) The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes 37: 216-223.
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, et al. (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505: 559-563.
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B (2009) Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89: 147-191.
- Ahmad NN, Pfalzer A, Kaplan LM (2013) Roux-en-Y gastric bypass normalizes the blunted postprandial bile acid excursion associated with obesity. Int J Obes 37: 1553-1559.
- Islam KB, Fukiya S, Hagio M, Fujii N, Ishizuka S, et al. (2011) Bile acid is a host factor that regulates the composition of the cecal microbiota in rats. Gastroenterology 141: 1773-1781.
- Ravussin Y, Koren O, Spor A, LeDuc C, Gutman R, et al. (2012) Responses of gut microbiota to diet composition and weight loss in lean and obese mice. Obesity (Silver Spring) 20: 738-747.
- Cani PD, Delzenne NM (2009) The role of the gut microbiota in energy metabolism and metabolic disease. Curr Pharm Des 15: 1546-1558.
- Queipo-Ortuño MI, Seoane LM, Murri M, Pardo M, Gomez-Zumaquero JM, et al. (2013) Gut microbiota composition in male rat models under different nutritional status and physical activity and its association with serum leptin and ghrelin levels. PLoS One 8: e65465.
- Khan MJ, Gerasimidis K, Edwards CA, Shaikh MG (2016) Role of gut microbiota in the aetiology of obesity: proposed mechanisms and review of the literature. J Obes 2016: 7353642.
- Bruce-Keller AJ, Salbaum JM, Luo M, Blanchard E, 4th, Taylor CM, et al. (2015) Obese-type gut microbiota induce neurobehavioral changes in the absence of obesity. Biol Psychiatry 77: 607-615.
- Arrieta MC, Stiemsma LT, Amenyogbe N, Brown EM, Finlay B (2014) The intestinal microbiome in early life: health and disease. Front Immunol 5: 427.
- Murphy R, Tsai P, Jüllig M, Liu A, Plank L, et al. (2017) Differential changes in gut microbiota after gastric bypass and sleeve gastrectomy bariatric surgery vary according to diabetes remission. Obes Surg 27: 917-925.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Pellegrino G (2023) Obesity and Microbes: The Role of Bariatric Surgery.J Obes Weight Loss Ther 13: 541. DOI: 10.4172/2165-7904.1000541
Copyright: © 2023 Pellegrino G. This is an open-access article distributed underthe terms of the Creative Commons Attribution License, which permits unrestricteduse, distribution, and reproduction in any medium, provided the original author andsource are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 2336
- [From(publication date): 0-2023 - Nov 27, 2025]
- Breakdown by view type
- HTML page views: 1952
- PDF downloads: 384