Research Article Open Access
Optimization of Operational Conditions for Maximum Biodecolorization ofOrange C2RL Dye
| Muhammad Asif Javaid1*, Sofia Nosheen2, Muhammad Adnan Ayub1, Majid Mustafa1, Adil Naseer1, Amer Iqbal1 and Waheed Arshad3 | |
| 1Department of Chemistry, University of Agriculture, Faisalabad-38040, Pakistan | |
| 2Department of environmental science, Lahore College for Women University, Lahore | |
| 3Government College University Faisalabad, Pakistan | |
| Corresponding Author : | Muhammad Asif Javaid University of Agriculture Faisalabad, Pakistan Tel: 92-41-9200161 E-mail: asifjavaidchemist@gmail.com |
| Received: November 26, 2015 Accepted: December 29, 2015 Published: January 09, 2016 | |
| Citation: Javaid MA, Nosheen S, Ayub MA, Naseer A, Arshad W, et al. (2016) Optimization of Operational Conditions for Maximum Biodecolorization of Orange C2RL Dye. J Bioremed Biodeg 7:324. doi: 10.4172/2155-6199.1000324 | |
| Copyright: © 2016 Javaid MA, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Biodecolorization of Orange C2RL dye was studied with microbial strains isolated from textile dye waste water. In this work optimization process was also examined by changing different parameters like temperature, pH, concentration of dye and time. Biodecolorization process was monitor and corroborated by using spectroscopic techniques like UVVisible and FTIR spectroscopy and by evaluating Chemical oxygen demand (COD) and total organic carbon (TOC) of the dye. The results evidently showed that the decolorization capability decreased with increasing dye concentration, and a marked prohibition outcome was observed when the Orange C2RL concentrations were more than 100 mg/L. The optimum temperature for bacterial decolorization was 35°C. The maximum decolorization activity occurred at pH 7.5. Analysis of dye UV-vis and FTIR spectra after bacterial treatment demonstrated that the decolorization of dye was due to biodegradation.
| Keywords |
| Biodecolorization; Biodegradation; Diazotization; Triplicates |
| Introduction |
| Azo reactive dyes attribute the greater part of entire textile dyes stuff generated and had been the abundantly and often utilized synthetic dye in the textiles, printing of color paper, paper making, cosmetic, leather, and food industries [1,2]. Production of several dyes having azo groups incorporates diazotization of primary amines of aromatic compounds chased by coupling with greater or one nucleophile [3]. Dyes containing azo groups are disobedient xenobiotic and therefore, customary shaking waste water treatments mechanism commonly cannot accurately biodecolorize and biodegrade azo dyes containing effluent to the regulative level [4], sorption [5], Photo catalysis [6] and electrochemical destruction [6]. The potency of bacterial biodecolorization relies upon the flexibility and the potency of elected microorganisms. Huge extent of microorganisms including yeasts, bacteria, algae, fungi and actinomycetes able to biodegrading reactive azo dye has been announced [7]. The process of bioremediation of azo dyes associates the reduction breakdown of azo bond (–N=N–) by the use of azo enzyme called reductase under shaking assays appeared into the production of colorless solution. Biodecolorization with the help of bacteria is more efficient and rapid, but individually bacteria strains generally cannot biodegrade azo reactive dyes entirely and the biodegrading materials are often dangerous amine of aromatic compounds that requires being again biodegrading. It means treatments assay constitutes of collective microbial growths acquire larger amounts of mineralization and remediation because of combined intermediate actions of bacterial society and gives appreciable advantages over the utilization of pure growth mediums in the biodegradation of man-made dyes [8]. In microbes conspiracy the alone strain might attacked the dye molecules over several sites or can use up intermediates developed by the co-existing microbes for again breakdown [9]. |
| Fast urbanization and industrialization culminates in the release of great amounts of unwanted materials into the atmosphere, which consequently produce greater pollutions. Pluralities of colored effluent containing azo dyes, absolved to the environment from textile dyestuff and dyeing industries. Due to color pollution in the environment; problems are amplified [10]. Such kinds of pollutions are related to azo dye compounds, which narrates meaningful proportions of the entire dye market. In recent azo dyeing mechanism, comparatively low levels of dye fiber fixations almost 51.0% if the dyes which exist in the original dye baths are deprived to the waste waters [11]. Those more stable azo reactive dye products, that not biodegraded are by common waste water treatment mechanisms, barge into atmosphere in the form of colored waste effluent [12]. |
| Thus, the chief intention of this research was to affirm that the decolorization of reactive azo dyes in a successive aerobic process using, bacterial strain isolated from textile waste water to decolorize the dyes but also to achieve a good degree of mineralization with low running and maintenance costs. To investigate the effect of different amendments of nitrogen and carbon source on the biodegradation process were also studied. |
| Materials and Methods |
| Collection of samples |
| Sample of activated sludge was collected from the drain near Local Textile and Processing industry on which many processing units are situated. Samples were taken from drain at different location and sampling sites were selected on the basis of the allocation of outlet from textile units. These industries extensively used the dye under study. |
| Material and methods |
| The azo dye Orange C2RL was kindly provided by the Textile Company sandal dyestuff Faisalabad, Punjab, Pakistan. All other analytical grade chemicals are bought by the Local and trusted Market of Faisalabad. The mineral salts medium (MSM) at pH 7.2 used in all experiments contained: NaCl (1.00 g/L), CaCl2.2H2O (0.10 g/L), MgSO4.7H2O (0.50 g/L), KH2PO4 (1.00 g/L), Na2HPO4 (1.00 g/L), Yeast Extract (4.0 g/L) and Agar (16.0 g/L). Dye was added to sterile MSM from their respective filter sterile stock solutions. |
| Isolation: Primary bacterial strains were grown on the agar and isolated. Prominent isolated cultures then enriched with MSM and Azo dye (100 mg/L) as a source of nitrogen and carbon. The medium bearing 200 ml of MSM broth and dye in 500 ml Erlenmeyer Flasks were inoculated with approximately 10 ml volume of sludge. The flasks were incubated at 32.0°C for 2 days under shaking states. Cells suspension from every flask were dished onto MSM agar culture after the incubation and incubated at 30°C for 24 hour for bacterial colonies. Afterward a second transfer of the cell suspensions onto MSM agar dishes bearing small amount of carbon and nitrogen (yeast extract), 24 energetically flourishing cultures with dissimilar colony growths property were choose from every origin and were purify by smearing twice on medium containing agar. These separated strains were protected in refrigerator for subsequent study [13]. |
| Screening: Screening was done to find out the efficient bacterial strains capable of decolorizing the Orange C2RL dye using modified MSM. Decolorization ability of each isolate was tested in liquid medium and the medium was inoculated with the respective inoculate and incubated at 35°C for 48 hours. After 48 hours centrifugation of the medium was done at 10000 rpm for 12 minutes. Then decolorization was determined with the help of spectrometer at 492 nm. Also Uninoculated blanks were run to determine abiotic decolorization. Percent decolorization was calculated and the three most effective bacterial isolates (D1, D12, and D15) exhibiting maximum decolorization ability were selected from the last screening and again investigated for their decolorizing properties in test tubes. Autoclaved MSM Broth (10 mm) bearing Orange C2RL (100 mg/L) was added to sterilized test tubes enriched with yeast extract (carbon source) as a co substrate. The tubes were inoculated with relevant microbial cultures and were firmly closed, then were placed under shaking condition over 32.0°C. Uninoculated test tubes with mineral salt medium bearing azo dye plus yeast extract was also incubated in like condition to study for abiotic biodecolorization of sample. The decolorization was measured at 492 nm by spectrophotometer as described above. |
| Selection of optimum dye concentration |
| A Two day experiment was conducted with six different concentrations (i.e., 20, 40, 60, 80, 100 and 120 ppm) of Orange C2RL dye. 100 ml of same dye solution of different concentrations was taken separately in a set of six 250 ml Erlenmeyer flasks. These flasks were inoculated with the selected strains. All the flasks were kept on a rotary shaker for 2 days at 39 ± 1°C (150 rpm). The solution from each flask was employed for the measurement of color using the above method after two days. |
| Incubation Period |
| Degradation pattern is studied at different time intervals to optimize the temperature conditions for the biodecolorization of selected dye. For degradation samples were drawn at regular intervals of time that is 24, 48, 144 and 296 hours. |
| Effect of pH |
| The effect of pH on the visible absorption was assayed between pH 5.0 to 9.0 at λ max of the Orange C2RL dye. The pH of media was adjusted to various pH values: 6.00, 6.5, 7.00, 7.5, 8.00, 8.5 and 9.0. In order to ensure that decolorization was a function of pH change. The dye concentration was taken 100 mg L-1 separately in a set of seven 250 ml Erlenmeyer flasks and pH were adjusted from 6.0 to 9.0. Flasks were inoculated and were analyzed after two days. |
| Effect of temperature on biodegradation of orange C2RL |
| Three different levels of temperature 30°C, 35°C and 40°C were used to find the optimum temperature level for maximum degradation. Assays were done in order to determine the effect of temperature on biodegradation of Orange C2RL by selected strains. Remaining conditions were kept same. Inoculums in nutrient media was inoculated and incubated for 48 hours at three different temperatures. For degradation analysis, samples were drawn at regular intervals of time that is 24, 48 and 72 h. All the experiments run in triplicates. |
| UV-vis analysis |
| The dye degradation progress was determined by using UV–Vis spectrophotometer. Untreated dye was scanned from the range 200 to 800 nm to check the λ max and measured the absorbance of the culture media at this wavelength. Beer Lambert law was used for calculation |
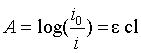 |
| Here; A=absorbance of sample; c=conc. of sample (moles/L); l=path length of sample solution (cm); ε=molar extinction coefficient. Decolorization assay were studied under aerobic conditions containing MSM cultures in the 250 ml Erlenmeyer flask nourished with orange C2RL (100 mg/L) and yeast extract. Yeast extract nourishes the bacterial microbes [14]. The MSM cultures were first inoculated with the selected microbes and placed under static condition at 32°C for 48 hours. These cultures were then aerated by centrifuging at 150 rpm without any addition of the medium in periodically moving incubator. After several times interval, a small portion of culture media was removed and then centrifuged over 5000 rpm for 12 minutes. Floatable material of the centrifugal culture media was taken and checked the absorbance at the 492. The percentage decolorization of the process was estimated by using the following equation. |
 |
| Where Ab is the absorbance of Orange C2RL dye at the λmax before biodecolorization and similarly Aa is the absorbance of the dye after biodecolorization. All degradation assays were performed in four replicates. |
| Degradation assay via FT-IR Spectroscopy |
| To identify the changes in the chromophore of the dye responsible for the change in color, samples were analyzed by Fourier transform infrared (FTIR) microscopy. The controls and samples were dried and mixed with KBr (1:20; 0.02 g of sample with KBr at a final weight of 0.40 gm). These models were then crushed; air is removed at 61oC for all the day and afflicted to get infra-red transpicuous bullets. The FT-IR absorbance spectrum of the samples was noted with the help of FT-IR spectrophotometer. The spectrum was than scanned within a limit of 390–3990 cm-1. For scanning background signal the FT-IR spectrometer was first calibrated with a pure KBr control sample and then the analytical samples was scanned. The control’s FT-IR spectrum of at the end was deducted from the spectrum of the non-decolorized (without) and decolorized dyes [15]. |
| Measurements of water quality parameters |
| The water quality parameters like chemical oxygen demand (COD) and total organic carbons (TOC) was also calculated. Analytical procedure adopted for these parameters will according to standard techniques for the investigation of waste water and water [16]. |
| Chemical oxygen demand (COD): Digestion solution was prepared by adding 2.60 g K2Cr2O7, 8.30 g HgSO4 in 42.0 ml H2SO4 (98% concentrated) and then diluting it to 250 ml with deionized water digestion solution was prepared. Similarly Catalyst solution was prepared 5.06 g Ag2SO4 was dissolved in 500 ml concentrated H2SO4 and placed it for two days to confirm that the complete Ag2SO4 was dissolve. A standard solution of Potassium hydrogen phthalate (KHP) was prepared from a stock solution (1g KHP/1L) of 425 ppm concentration in deionized water. 425 ppm solution of KHP gives 500 mg/L COD. For chemical oxygen demand analysis in accordance with approved methods 3.5 ml of catalytic solution was poured to clean screw-cap Lovibond COD vials containing 1.50 ml digestion solution and allowed to ambient temperature. Then 2.50 ml of sample was added. COD samples were incubated over 140°C for 2 hours in a dry incubator. After allowing the COD tubes to cool ambient temperature and COD values were noted by calculating the absorbance of digested assay solutions at λ=600 nm on UV-Visible recording spectrophotometer. All these samples were analyzed in triplicate [17]. COD was determined by using following calculation. |
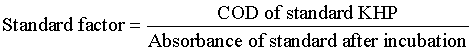 |
| COD of sample (mg/L)=standard factor × absorbance of sample after incubation |
| Total organic carbons (TOC): 2N potassium dichromate solution (K2Cr2O7) was prepared by dissolving 98.06 gram per litter. 250 ppm solution of glucose was prepared by dilution method from a stock solution of 1000 ppm solution. 25 ml of stock solution was taken and diluted to 100ml in volumetric flask. Analytical grade sulphuric acid (98%) was used. 1.6 ml of sulphuric acid was taken in clean screw-cap vials and then 1 ml of 2N potassium dichromate (K2Cr2O7) was also added and mixed. At the end 4ml sample added and incubate at 110°C for 1.5 hours. After incubation absorbance were taken at λ=590 nm using UV-Visible recording spectrophotometer [17]. |
| TOC of sample (mg/L)=Standard factor × absorbance of sample after incubation |
| Results and Discussions |
| Isolation of bacterial strains |
| Sludge samples were collected from the effluent channel of local textile mill. Numerous colonies were obtained through serial dilution and streaking method. Isolated colonies were then obtained through serial streaking method on nutrient agar. Each strain was then inoculated into nutrient broth and incubated for 24 h at room temperature on a platform shaker at 150 rpm. A 10% (v/v) inoculum was transferred into 250 ml flask containing 100 ml nutrient media and incubated similarly. After 24 h, 10% (v/v) samples were sub-cultured into fresh nutrient media containing the azo dye and further incubated as described above. 24 strains capable of utilizing Orange C2RL dye as a nutrient source were plated on agar plates and incubated at 32°C for 24h. Isolated colonies were taken and repeatedly streaked on nutrient agar to obtain pure cultures. The pure bacterial cultures were subsequently transferred into nutrient broth. Efficiency of bacterial strains was examined by checking the optical density of the liquid medium based upon relative decolorization efficiency of different bacterial strains. Three (1D, 12D and 15D) out of 24 of the most efficient bacterial isolates were used for evaluating the potential to decolorize an azo dye Orange C2RL in liquid medium. The most efficient bacterial strain in decolorizing the Orange C2RL was 1D with 89% color removal followed by 12D and 15D with 85% and 80% decolorization respectively. Several authors have reported that bacteria can degrade azo dyes efficiently in water [18]. Decolorization of Orange C2RL was studied under aerobic conditions (150 rpm) with an initial dye concentration of 100 mg/L by all the three strains. |
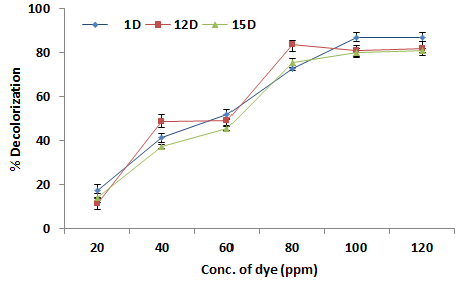
| Effect of concentration of dye on % decolorization |
| The effect of different concentration of Orange C2RL azo dye from 2-12 mg/L was studied for maximum decolorization. It is evident from the figure that Orange C2RL azo dye decolorization was sharply increase up to 100 ppm of substrate concentration, then after there was a sharp decline1D was the most efficient azo dye decolorizing strain with 87.13% decolorization at 100 ppm and its minimum decolorization was recorded at 20 ppm, 12D was the second at the rank with almost 81.97% decolorization at 100 ppm and l5D same trend was followed in case of 15D, which showed almost decolorization up to 81.09 percent at 100 ppm. Both strains showed there minimum decolorization at 20 ppm that was approximately 15% by both strains (Figure 1). It is observed that when concentration was increased up to 120 ppm all the isolates showed decline in decolorization potential. Azo dyes are considered recalcitrant xenobiotic compounds due the presence of nitrogen (– N=N–) nitrogen double bond Results showed that the increase in substrate concentration from its optimum had negative effect over bacterial decolorization. This inhibition in decolorization potential may be due to saturation of the cells with dye products, inactivation of transport system of the dye or the blockage of the active sites of azoreductase enzyme by the dye [19,20]. |
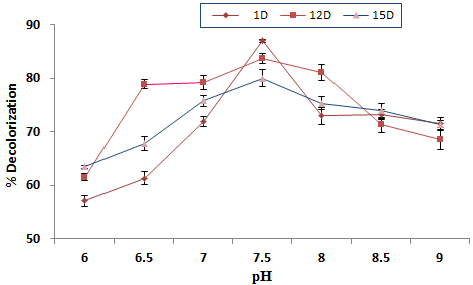
| The effect of pH of media on % decolorization |
| The pH of the medium played an important role in decolorization process. For the optimization of pH, 6-9 pH of the medium was used. It is cleared from the graph with increasing pH decolorization grew up to 7.5 than fell down up to 9 for all the strains. ID showed maximum 87 percent decolorization at pH 7.5 then suddenly decrease at 8.0 pH and remained constant afterwards. 12D showed minimum 60% decolorization at pH 6.0 then went up at 6.5 and approximately remained stable up to pH 8.0 and dropped afterwards. Same trend was observed with 15D isolate. 15D showed maximum 77.9% Decolorization at pH7.5 and minimum 63.12% at pH 6 (Figure 2). |
| These results are in good agreement with those claimed by [21]. The difference in pH effects for the azo dye decolorizing bacteria may be due to the difference in genetic potential responsible for decolorization (e.g. the origin of azo reductase) or in bacterial physiology (e.g., mechanism for transport of dye molecules) [1]. In fact, researchers have found that molecular weight of azoreductase varies from species to species [22] indicating the diversity in decolorization related gene products. |
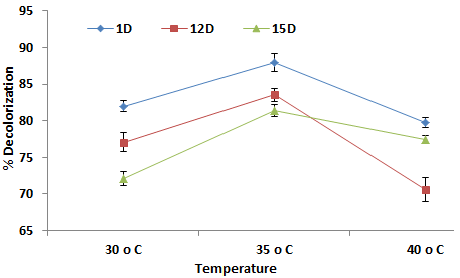
| Effect of incubation temperature on % decolorization |
| To optimize the temperature for maximum decolorization, medium was incubated at three different temperatures 30, 35 and 40°C (Figure 3). Maximum decolorization by 1D was observed at 35°C with 87.93 % decolorization and minimum was observed at 30°C. Similarly the decolorization rate by 12D increased up to temperature 35°C and showed 83.56% decolorization at 35°C. The maximum decolorization by 15 D was 81.37 % at 35°C and minimum decolorization was 72.12% at 30°C. It is clear from the figure as the temperature increased from 35°C up to 40°C there was a sharp decline in decolorization with all isolates. It as also observed that with rise in temperature biotic decolorization also increased up to 35°C. Decline in decolorization activity at higher temperature can be attributed to the loss of cell viability or to the denaturation of the azoreductase enzyme [18]. Therefore all selected isolates were mesophilic bacteria because they all showed better decolorization in the temperature range of 25 to 35°C. The obtained results were in coincidence with earlier studies reported by different authors [9] also reported the maximum decolorization activity at temperature between 23 to 37°C. . |
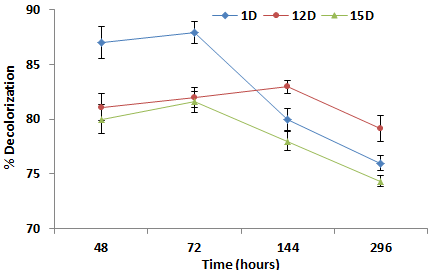
| Effect of time on % decolorization of orange C2RL |
| It was observed that decolorization of the azo dye increased with time by all the three strains, after 48 hours approximately remained constant, but with further increase in time, biodecolorization behavior of all the selected strain decreased. Maximum decolorization with 1D was 87.0% after 48 hours and minimum was 76.06 % after 296 hour. 12D Isolate showed maximum decolorization of the azo dye 81.01% after 48 hours. 15 d isolate had maximum decolorization at 48 hours was 81.57 % and minimum was 74.35% after 296 hours. Such kinds of findings are reported by [23,24]. This decrease in decolorization potential may due to death of microbes (Figure 4). |
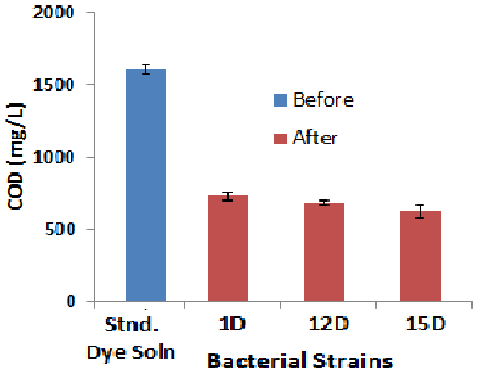
| Chemical oxygen demand (COD) |
| The initial COD level in the medium was 1608 mg/L. The COD level decreased up to 55% (1D) from 1608 mg/L to 724 mg/L under the optimum conditions. Reductions in COD with other strains were 58% (12D), 62% (14D). These results also indicate the detoxification of the dye and dye mixtures [25] observed similar reductions in COD during the study of biodecolorization and detoxification of a dye in textile effluent by a newly isolated strain. Reductions in COD level indicate the formation of new metabolites during the process of decolorization (Figure 5). |
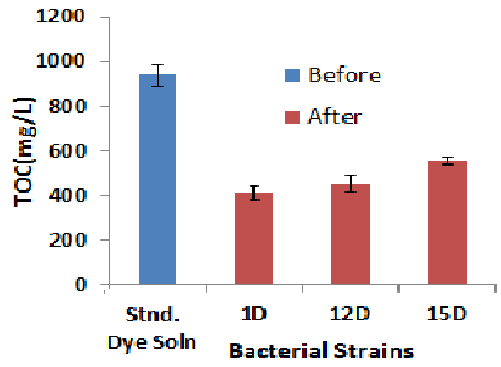
| Total organic carbon (TOC) |
| The TOC removal during the Biodegradation was monitored to assess the mineralization extent of Orange C2RL azo dye. The initial TOC level in the medium was 936 mg/L. TOC level decreased after the treatment of 1D was 412 mg/L. It means 56% TOC level was decreased after the treatment with 1D. TOC level after the treatment of 12 D was decreased up to 452 mg/L, which showed that carbon content was decreased up to 51%. 15D showed TOC value 492 mg/L, from the figure it is cleared that 15D has ability to decreased the carbon content of Orange C2RL dye up to 42% (Figure-6). |
| UV-Visible analysis of the biodecolorization |
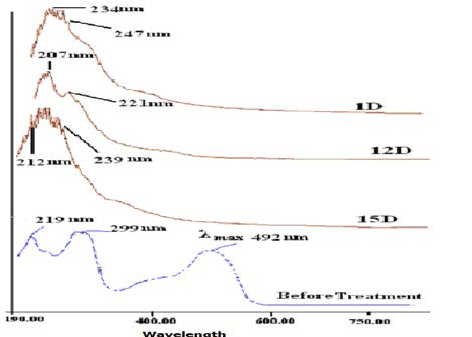
| The correlation of UV-visible absorption spectra (210-790 nm) before and after treatment (1D, 12D and15D) of samples of dye showed complete vanishing of peak at 492 nm representing to λmax of Orange C2RL after the treatment with all strains (1D, 12D and 15D) is shown in the figure. It indicated complete decolorization and breakdown of chromophoric group. In the UV spectra, the peaks at 219 and 299 nm disappeared and were replaced by new peaks in range of 240-300 nm. It was confirmed by the spectroscopic analysis all the isolates have potential to decolorize the Orange C2RL dye (Figure 7). Disappearing of the peaks in the visible region is the confirmation of the biodegradation as well. However formation of new peaks in the UV region may due to the formation of aromatic amine after the treatment. It is observed our isolates have no potential to decolorize the aromatic amines. |
| FT-IR characterization |
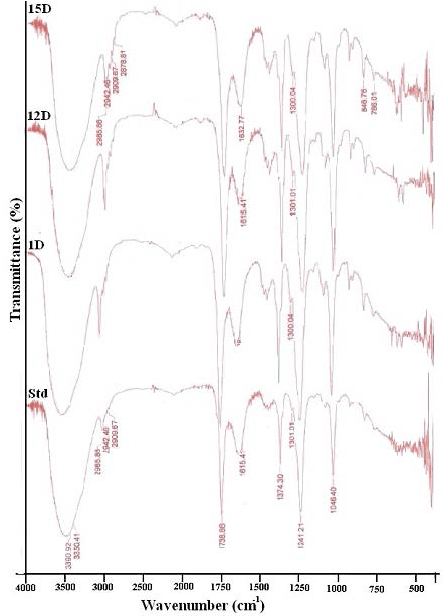
| The FT-IR spectra achieved from the uninoculated azo dye samples displayed various peaks in the region where N–H and O–H stretching is commonly noticed (3250–3510 cm-1). Peaks near 1738-1650 cm-1 due to ketonic group(C=O) and C=C (aromatic) starching or ring vibration respectively. This ketonic group may be due to tautomeric structures. Peaks due to azo group (-N=N-) are observed near 1550 to 1625 cm-1. Peaks near 1200-1350 cm-1 indicates the presence of Sulphonic group (Figure 8). After aerobic treatments significant reduction in absorption was observed all the peaks. The spectra obtained after the microbial treatment (1D, 12D, 15D) are shown in the figure. It is cleared from the spectra peak due to azo group (1550 to 1610 cm−1) is disappeared with all isolates, which indicate the biodecolorization of azo dye. It is observed that most of the peaks are not diminished but there intensity is decreased up to 70%. The intensity of the peak observing at 3390 cm−1 (due to hydrogen bonding) is decreased from 15.25 to 7.023, 9.163 and 8.087 with 1D, 12D and 15D respectively. Similarly intensity of sulphonic groups after the aerobic treatment is decreased up to 70%, 65.12% and 67.13% with 1D, 12D and 15D respectively. Similarly all peaks decreased approximately from 65 to 75% after the aerobic treatment. The obtained variations in IR spectra of the dyes could be attributed to the induction of azo dye reductase enzyme and cleavage of azo linkage of Orange C2RL and the subsequent formation of aromatic amine. Additionally, the obtained differences in spectral intensity and the occurrence of vibration in some IR figures of biomass of isolated strains treated with some dyes also manifest possible biosorption besides the Bacterial degradation activities. Possible genotoxic effects of textile dyes are discussed most often with respect to certain azo dyes, which contain an azo group (–N=N–) able to split off genotoxic and carcinogenic amines (e.g., Acid Red 85, which releases benzidine). |
| The elucidation of degradation pathways is of special interest regarding health and environmental priorities. The FTIR spectral comparison between the control dye and samples extracted at different time intervals and pH values detected the presence of different peaks, which expressed the breakdown of the dye into various compounds, such as primary and secondary amines and an absence of benzene rings [26]. |
| Effect of additional carbon sources on % decolorization |
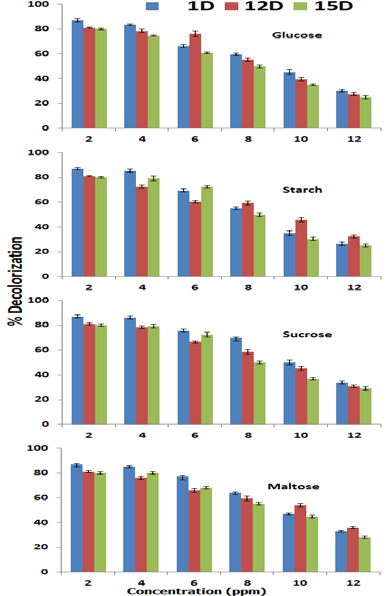
| Effect of different carbon sources such as glucose, starch, sucrose and maltose (2-12 mg/100 ml) were evaluated on Orange C2RL degradation by different bacterial strains is illustrated below. It was found that overall with increasing concentration of glucose, sucrose and starch showed inhibitory effect on the % decolorization of the azo dye. With the addition of 2 mg/100 ml glucose source % decolorization reached to 83.45% (1D), 78.1% (12D) and 74.59% for 15D. With enlargement of sucrose 2mg/100ml) the percent decolorization was depressed to 84.12% (1D), 76.12% (12D) and 81.65% for 15D. Similarly with the addition of starch (2mg/100mls) percent decolorization decreased from 87.01 to 85.23% (1D), 81.1 to 77.36% (12D) and 80 to for 75.23% 15D. But with the addition of sucrose and maltose positive effect was observed by all the strains but only at 2mg/100 ml (Figure 9). The % decolorization potential of the dye reached to a level of 87.15% (1D), 83.32% (12D) and 81.98% for 15D with maltose (2 mg/100 ml). With further addition all strains show inhibitory effect and reached to the bottom at 10 mg/100 ml. In short, all the bacterial strain showed negative effect on decreasing the color potential of azo dye with the addition of carbon sources. Bacterial growth even at higher concentration confirmed that these carbon sources had no toxic effects. It is similar to the findings of [27]. But higher concentration of carbon source inhibited decolorization ability. Chen et al. (2003) reported that the inhibition of cell growth and bacterial decolorization of azo dye by carbon source is attributed to the reduced pH in the surrounding medium through a biological conversation to organic acids. |
| Effect of additional nitrogen sources on % decolorization |
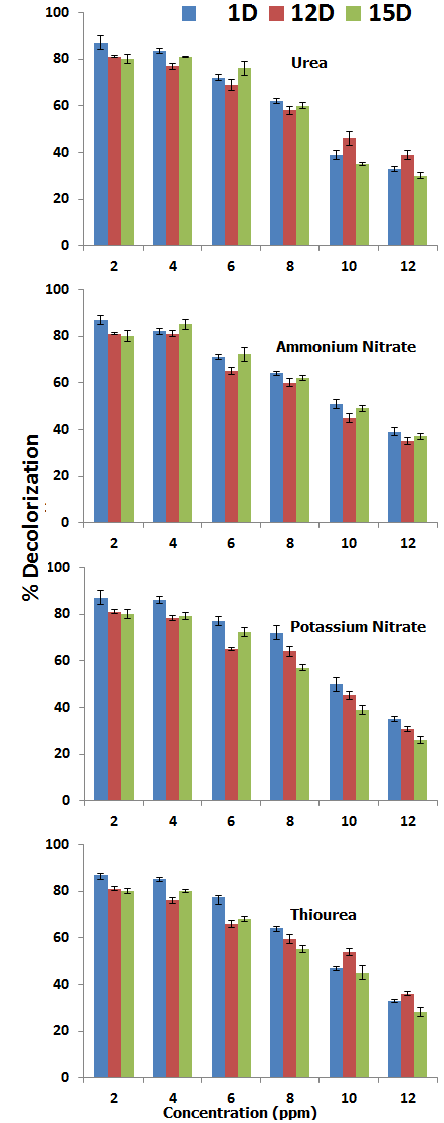
| Four different nitrogen sources (Ammonium Nitrate, Urea, Potassium nitrate and Thiourea) were studied with different levels of concentration(0, 2, 4, 6, 8, 10 and 12 mg/100 ml) to check the effect of nutrients on bioremediation. It is observed that almost all strains showed inhibitory effect over biodegradation even at low concentrations. By adding ammonium nitrate (2 mg/100 ml) decolorization potential increased lightly and % decolorization reached 87.68% (1D), 81.23% (12D) and 80.64% for15D. With further addition decolorization potential declined and showed its minimum value at 10mg/100ml. Urea could not show marked biodecolorization even at low amounts. In spite of this urea caused depression in decolorization capability of all the selected isolates under study (Figure 10). Potassium nitrate showed negative effect with 1D and 12D bacterial strains but 15D strain showed an increase in decolorization potential. But at higher concentration all isolate exhibit decrease in biodecolorization. Similar results obtained in case of thiourea as shown in the figure. Decolorization ability of all the bacterial strains decreased with the addition of thiourea. The additions of inorganic nutrient are not always effective to stimulate the degradation because many other factors can decrease the biodegradation [28]. Also the addition of inorganic nutrient can change the pH, and it is evident from the above study micro flora act on the optimum pH. Similar findings are reported by the previous authors [27] also observed such kind of results [29-31]. |
| Summary |
| All the 24 bacterial strains were capable of decolorizing orange C2RL azo dye more than 70%. But three best strains 1D, 12D and 15D were used for biodecolorization potential. Rate of decolorization was increased with increasing concentration of substrate upto 100 ppm, further increased in concentration had inhibitory effect on decolorization of the dye. Sleeted strains showed the maximum decolorization at 35°C and with increase in temperature all the isolates show decline in decolorization. In the same way acidic to neutral pH favored the decolorization of orange C2RL dye and at pH 7.5, decolorization was maximum. More acidic or alkaline conditions inhibited the decolorizing ability of microbes. All the strains showed good results for decreasing COD and TOC value. 1D showed more than 50% decreased in COD and TOC results.12D followed the same trend and showed more than 45% less TOC and COD value. 15D was at the last in the results. It was observed that with addition of carbon sources (glucose, starch, sucrose and maltose) and nitrogen sources (urea, ammonium nitrate, potassium nitrate and thiourea); some strains increase in concentration at 2ppm but with further increase in concentration all the bacterial strains showed decrease in decolorization potential. Decrease in the intensity of all peaks in the FTIR spectrum and appearance of new peaks in UV-Visible spectrum confirmed the decolorization of orange C2RL azo dye. |
References
|
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
 |
 |
 |
 |
 |
| Figure 6 | Figure 7 | Figure 8 | Figure 9 | Figure 10 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 10446
- [From(publication date):
January-2016 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 9561
- PDF downloads : 885
