Organic Ectopy of Immunocompetent Cells
Received: 24-Oct-2017 / Accepted Date: 11-Nov-2017 / Published Date: 20-Nov-2017
Abstract
This review summarizes contemporary researches about the role of the resident immune cells in different organs – pineal gland, placenta, ovary, endometrium, liver, skin, gastro-intestinal tract, bronchial system. Mechanisms of the immune response realization reflects the relationship between different cells, tissues and organs, and thus support the notion of the unity of the nervous, immune and endocrine systems. It was established that the interaction of these systems is complex. Populations of various immune cells have common structural and functional features what is the foundation of a safety mechanism of the immune system. Reduction in the correlation of different subpopulations immune cells number in the organism is the foundation of various pathological processes.
Keywords: Organs ectopia; Immune cells; Pathology; Neuroimmunoendocrine system
Abbreviations
CD: Cluster of Differentiation; CGRP: Calcitonin Gene Related Peptide; CTACK: Cutaneous T-cell Attracting Chemokine: DCs: Dendritic Cells; GIT: Gastro Intestinal Tract; HLA: Human Leukocyte Antigen; HSC: Hepatic Stellate Cell; ICAM: Intra Cellular Adhesion Molecule; IgA: Immunoglobulin of class A; IL: Interleukins; INF: Interferon; KGCs: Kashchenko-Gofbauer Cells; KIR: Killer Inhibitory Receptor; KC: Kuepfer Cell; LC - Langerhans Cell; LFA-1: Leucocyte Function Associated-1 protein; LP – α: Lipoprotein; LSEC: Liver Sinusoidal Endothelium Cell; MALT: Mucosa Associated Lymphoid Tissue; MDSC: Myeloid Derived Suppressor Cells; MMP: Matrix MetalloProteinase; mRNA – matrix RiboNucleic Acid; NK cells: Natural Killers Cells; NKT: Natural killer T-cells; NO: Nitric Oxide; pDC - plasmacytoid Dendritic Cells; SP: Substance P; TNF: Tumor Necrosis Factor; VEGF: Vascular Endothelial Growth Factor
Introduction
The functional community of the nervous, immune and endocrine systems is expressed in maintaining the body's homeostasis at the molecular-cellular level. When analyzing the similarities in the organization of the nervous and immune systems, first of all, attention is paid to the fact that all of these systems consist of a large number of phenotypically different cells organized into complex networks. Within the network, cells are interconnected and function on the principle of feedback. As early as the 1970s, it was shown that the administration of supernatants from immune cell cultures to animal models induced an increase in the level of corticosterone (a pituitary hormone) in the blood plasma and reduced the synthesis of norepinephrine in the rat brain [1]. Currently, information on the relationship of the immune system cells with the tissues of various organs is constantly expanding, which indicates the importance of the functional activity of immunocompetent cells at the level of signal molecules in the tissues of the nervous, endocrine, reproductive and other systems. For example, Ridder K et al. supposed, that leukocytes enter the brain and extracellular vesicles released in short distance to the target cell are able to bind and release their content. This direct signaling of immune cells to the brain is independent of microglia [2]. The aim of this review is to summarize the data on ectopic organs of immunocompetent cells.
Pineal gland resident immune cells
It is shown that participation of similar elements of neuroimmunnendocrine regulation interactions reduces the risk of various disorders, compensating for the deficiency or hyperproduction of some regulatory molecules due to the corresponding changes in the others. The pineal gland (epiphysis) is a prominent example of this phenomenon. It is known that the lymphoid component occupies 20% of the epiphysis tissue, this fact allows to consider the pineal gland as a possible peripheral part of the immune system, which value in the organism increases with aging [3-6]. The lymphoid component of the epiphysis is represented by mature T and B lymphocytes, but most part of the organ is occupied by low-grade lymphocyte precursors. With age-related thymus involution, its functions are delegated to lymphoid tissue of other organs, one of which can be the pineal gland [7]. There are 4 main subpopulations of immune cells in the human and animal epiphysis: CD4+ (T-helpers), CD8+ (cytotoxic T cells), CD5+ (activated low-grade lymphocytes) and CD20+ (B-lymphocytes) [5,8].
Macrophages are one of the cells populations in the pineal gland involved as a mediator of immune modulating effects of pinealocytes. These cells regulate the morphofunctional state of the pinealocytes and the serotonin maintenance in vitro, as well as increase the expression of cytokines and components of the main complex of the class II histocompatibility system.
Periventricular structures of the brain located outside the bloodbrain barrier are affected by various biologically active substances. One of these structures of the brain is the pineal gland, which has been studied in the aspect of its immunoregulatory functions. The epiphysis secretes some hormones, including melatonin, that stimulate the immune system to produce certain cytokines. A large number of lymphoid tissues in the pineal gland allow assuming the existence of new mechanisms of neuroimmunoendocrine interactions in this part of the brain. The presence of lymphoid tissue suggests the existence of interaction mechanisms between lymphocytes and other types of pineal gland cells, including mutual influence on circadian rhythms in both systems: the immune and endocrine. Since the synthesis of melatonin in the pineal gland is associated with the time of day, it is assumed that the activity rhythms and dividing immune cells also have a temporal dependence. Evidence pineal gland effects on periodic changes in the immune system have been identified for avifauna and mammals [9-11]. The interactions between the neuroendocrine and immune systems suggest that there are feedback mechanisms between them, implemented through a variety of immune factors, although the cellular and molecular mechanisms of this feedback are not currently studied enough [12,13].
Female reproductive system resident immune cells
Placenta resident immune cells: The placenta is a unique organ that simultaneously performs 2 multidirectional functions: on the one hand, it ensures the fetus's safety from the mother`s immune system, and on the other - shows high immune activity and protects the fetus from the pathogenic effect of heterogeneous antigens of viral and infectious nature permeating the maternal organism.
Macrophages, dendritic cells (DCs), natural killers (NK cells), and cytotoxic T-lymphocytes were verified in the villous chorion of the full-term placenta [14-16]. In the late placenta of young women, the ratio of immune cells in the chorionic villus is 5:1:1:0.2, where NK cells are the most numerous subpopulation, followed by cytotoxic T cells and placental macrophages in the equal proportions, and the smallest subpopulation is DCs [17]. Possibly, the ratio of different subpopulations of immune chorionic villi cells optimally provides an immune response, the major components of which are destruction of damaged and transformed cells carried by NK-cells, control of heterogeneous agents provided by placental macrophages chorionic villi and protection semi allogenic fetus from maternal immune aggression supported by T - suppressors. In this case, DCs are the link between placental macrophages of chorionic villi and T-lymphocytes, providing activation of T suppressors and participating in the presentation of antigens.
According to some authors, placental macrophages of chorionic villi - Kashchenko-Gofbauer cells (KGCs) account for up to 40% of all non-fibrotic placenta cells and are practically the only population of immunocompetent cells in chorionic villus. CD14, CD68 and CD206 are specific markers for macrophages which were detected not only in placental macrophages of chorionic villi, but also in cells similar to DCs and fibroblasts [18]. Placental macrophages participate in the development of the immune response and the activation of the inflammatory response by the synthesis of TNFα tumor necrosis factor [19]. It is possible that an increase in their number may be a prerequisite for the development of the pathological course of pregnancy.
DCs related macrophages derived from bone marrow hematopoietic stem cells, being a branch of the myeloid-monocytic hematopoietic lineage. Their number in the villous chorion of the placenta is negligible. However, they play an important role in the formation of the immune status of the placenta. In contrast to macrophages, DCs have not only an antigen presenting function with respect to mature T lymphocytes, but also are able to activate T-cell differentiation. The similarity of DCs and macrophages suggests the existence of a close interaction between them, in which the properties of one cell population are supplemented by the properties of another. For example, DCs, unlike macrophages, have a set of co-stimulating molecules (CD80, CD86) inducing a primary immune response but incapable of phagocytosis. There is evidence that macrophages are able to differentiate into DCs [20]. Currently, the marker of inactive mature DCs is CD83, whereas the activation of DCs is indicated by the CD35 marker.
According to our data, single CD35+ cells are localized predominantly in the stromal component of stem and intermediate villi, as well as in lumina stromal channels [15]. As well as for KGCs, an increase in the number of DC in the chorionic villi of the terminated placenta is correlated with the age of women [21].
NK cells are a subpopulation of immune cells lacking T- and B-lymphocyte markers, and constitute 10-15% of the total number of lymphocytes. The origin of NK cells is still unclear. Apparently, NK cells belong to an independent line of differentiation of lymphocyte precursors. Morphologically, NK-cells are large granular lymphocytes. In some cases, the functions of this type of cells can perform T- and B-lymphocytes and macrophages. NK cells are one of the most numerous subpopulations of immune cells in the placenta in the early stages of its development [22]. A significant place among the NK cells is the K-cell subpopulation, which carries out antibody-dependent cellular cytolysis. Another specific for NK-cells is killer inhibitory receptor (KIR) suppressing cytotoxicity allowing NK-cells recognize normal and transformed cells.
T-lymphocytes are another subpopulation of immune cells of the villous chorion of the placenta. The main function of T-lymphocytes is the recognition of peptide fragments of foreign proteins embedded in autologous molecules of histocompatibility. T cells, unlike B lymphocytes, do not recognize the foreign cells and the modified cells own organism.
T-lymphocytes are another subpopulation of immune cells of the villous chorion of the placenta. The main function of T-lymphocytes is the recognition of peptide fragments of heterogeneous proteins embedded in autologous molecules of histocompatibility. T-cells, in contrast to B-lymphocytes, recognize not heterogeneous cells, but altered cells of the organism. During recognition T-lymphocytes activated and differentiated into cytotoxic T cells and regulatory T-helper cells. CD4 and CD8 molecules increase the bond with the antigen receptor by the action of it. T-lymphocytes are not numerous subpopulations of immune cells in a full-term placenta compared to the NK-cells and macrophages. According to some reports [23], the presence of T-lymphocytes in the chorionic villi during inflammation associated with their migration from the blood, but normally they are not detected in the placenta, it means that T lymphocytes are not resident cells of the placenta. According to other data [24], cytotoxic T-cells were verified in the chorionic villus of the mature placenta in young primiparous women, but T-helper was not detected. Furthermore, it was shown that with aging the quantity of cytotoxic T-lymphocytes increase in placenta.
Studies on the verification of various subpopulations of immune cells in the chorionic villi of the mature placenta suggest a mechanism according to which the immune cells of the villous chorion, on the one hand, perform a protective function and, on the other hand, restrain the immune aggression of the maternal organism against the semiallogenic fetus. Probably, this duality is achieved by a difference in the functions that perform four basic subpopulations of mature immunocompetent cells in the placenta.
It is known that NK cells are able to synthesize some cytokines and granulocyte-macrophage stimulating factor, regulating the activity and proliferation of placental macrophages of chorionic villi and participating in the early phase of the immune response [25]. In turn, placental macrophages of chorionic villi are able to regulate the activity of the T-cell link.
The described interactions between immune cells of the placenta have not been studied enough but evidence suggests a complex cascade of molecular reactions resulting in the functional integrity of placenta immune cells, aimed at maintaining of normal pregnancy development.
The importance of immunocompetent cells of the villous chorion of the placenta in the mechanisms of immune defense against heterogeneous agents and the preservation of tolerance between the tissues of the maternal and fetus organisms are confirmed by a wide spectrum of their subpopulations, verified in the placenta at various stages of its development. Violation of complex molecular interactions between different types of immune cells of the placenta at different stages of its development might be one of the causes of the pathology of pregnancy.
Endometrium resident immune cells: It is shown that the immune system is actively involved in the process of implantation, adhesion and invasion of trophoblast during normal pregnancy [26].
Some authors have shown that immune cells can induce differentiation of endometrium in preimplantation period regardless of the endocrine system [27].
The endometrium is a highly tissue system consisting of specialized cells of the parenchyma and stroma multicomponent. Gland epitheliocytes are specialized cells of the parenchyma. The stroma matrix is represented by a set of immunocompetent and specific endometrial stromal cells, vessels and interstitial substance. The stroma contains a small amount of lymphocytes, representing 10-25% of all endometrial cells.
The amount of lymphocytes, macrophages and mast cells dynamically vary during the menstrual cycle in the endometrial tissue diffusely infiltrating the stroma. Macrophages are present throughout the menstrual cycle. Their number increased by the end of the cycle, accounting for about 20-30% of the total population of lymphoid cells. Macrophages have phenotypic differences, and their subtypes express different metalloproteinases (MMP-9, membrane MMP-MT1- MMP) - the enzymes involved in the process of extracellular matrix reconstruction.
Macrophages are the source of vascular endothelial growth factor (VEGF), as well as produce a wide range of regulatory molecules that are able to stimulate the production of metalloproteinases and proinflammatory cytokines in the endometrium. Interleukins IL-1, IL- 2, IL-12 are the main cytokines-regulators of the local cellular antigenspecific immunity synthesized by macrophages and interferon INF-α. Their activation occurs during development of inflammation with the subsequent production of a high level of prostaglandin E2 and tumor necrosis factor. The functions of macrophages are inhibited by high doses of progesterone.
NK-cells are phenotypically unique population of immune cells in the endometrium, which form a significant part of the total population of lymphocytes in the stroma of the endometrium, and are different from those in the peripheral blood. Cytokines IL-10 and IL-15 are expressed in normal endometrium and able to stimulate the production and subsequent production of INF-γ and IL-10.
Disorders of the immune status in women with endometriosis (increased activity of B-lymphocytes, reduced activity of T cells, the appearance of autoantibodies in the substrates of endometriosis lesions), along with the previously known etiopathogenic mechanisms indicate immunological determinacy of the disease. Recent years among the immunological causes responsible for the decline in reproductive function the activation of B-lymphocytes (CD19+CD5+) cells are emphasized, the main function of which is related to the production of autoantibodies to hormones important for the normal development of pregnancy: estradiol, progesterone, chorionic gonadotropin. Normal levels CD19+СD5+ cells in peripheral blood is from 2 to 10%, the number of more than 10% is considered pathological [28]. Changing the immune status of endometriosis is local and is expressed in increasing number of NK-cells with cytotoxic activity (CD3+CD8+ CD16+), capable of producing the cytokines IL-4 and IL- 10, which play an important role in the regulation of autoimmunity. It is assumed that NK cells can exercise self-aggression in relation to the endometrium in endometriosis. The smallest population in the endometrium is T-lymphocytes (approximately 10% of endometrial cells). Thus, different types of immunocompetent cells are verified in the endometrium, the number of which varies in normal and pathological conditions [29]. Normally, the maternal organism should maintain immunological tolerance to the fetus, however an inflammation foci is formed associated with endometriosis, inside which there are complex of dynamic processes that are a signal for inclusion in the inflammatory response of the immune system. This process is accompanied by an increase in the number of T-lymphocytes, macrophages, CD16+ NK cells and activated cytotoxic CD57+ NK cells producing embryotoxic cytokines, the increase in which leads to abortion in early stages or to infertility.
Ovaries resident immune cells: Locally disposed immunocompetent cells play an important role in the regulation of ovarian function [30]. Normally, leukocytes (eosinophils, neutrophils, macrophages-monocytes and lymphocytes) are present in the ovarian tissue and act as modulators of their function through various secreted factors. Conditions for immune-endocrine interactions in the ovaries are conducive due to their anatomical structure and type of vascularization, which allows free migration of leukocytes. During the middle follicular phase of the menstrual cycle, a large number of macrophages and single neutrophils are found in the ovary stroma. There is an increased amount of neutrophils and macrophages before ovulation at the maximum level of luteinizing hormone in the tissue of the ovaries. A cluster of neutrophils forms around the site of the follicle rupture immediately after ovulation.
The number of macrophages varies during the menstrual cycle. Small amounts of B-lymphocytes and NK-cells are determined in the ovaries, which density does not change before ovulation. Cytotoxic T-lymphocytes are detected in the follicular before ovulation. The granules and atresial body follicle contains a small number of T-cells. A small number of T-cells was determined in the forming corpus luteum and increase significantly at its regression. CD45RO+ - memory T cells are detected in all structures where T-lymphocytes are present [31].
Leukocytes in the ovaries synthesize numerous cytokines that are important regulators of steroidogenesis and maturation of gametes. IL-1 and TNFα. are the major cytokines produced by the ovaries. Both molecules stimulate ovulation with the participation of a luteinizing hormone [32].
Nitric oxide (NO) regulates IL-1 action in the ovary, as a potential vasodilator and the main mediator of the antitumor and bactericidal activity of macrophages. NO stimulates the anti-apoptotic effect of IL-1 in follicle cell culture. The concentration in the serum of TNFα and the factor of the colony stimulating factor of granulocytes and macrophages also depends on the phase of the menstrual cycle.
Various cell types, including monocytes, endothelial cells, fibroblasts, mesothelial cells and endometrial stromal cells are able to produce IL-8, which has a pronounced angiogenic effect and promotes neutrophil migration. It is considered that IL-8 determines the neutrophil migration to follicles immediately before ovulation. IL-8 is expressed by neutrophils and endothelial vascular theca cells carrying autocrine-paracrine mechanism of regulation of neutrophils migration to the ovary.
It was shown that a variety of signaling molecules involved in the cascade of inflammatory reactions - prostaglandins, leukotrienes, bradykinin, histamine and cytokines play an important role in the ovulation process [33]. Cytokines that are secreted by the constantly presented leukocytes and endothelial cells in the ovaries promote activation and migration of leukocytes circulating in the blood to it. Macrophages and lymphocytes within the processes of folliculogenesis, the formation of the corpus luteum and its degradation have a paracrine effect on the surrounding endocrine ovarian cells.
Thus, immunocompetent cells consist in close connection with the cells of female reproductive system, so the maturation of oocytes occurs not only under the influence of hormones, but also under the control of the immune cells, but immunocompetent cells express receptors to sex hormones [34]. Violation of such a relationship is a prerequisite to the formation of pathological processes.
Bronchopulmonary system resident immune cells
Alveolar macrophages account for 80-90% of all cells of the Broncho alveolar fluid. Neutrophil leukocytes constitute 2-3% of the total number of all cells of bronchopulmonary system. Macrophages are antigen presenting cells, capable for reacting with T lymphocytes. Lung macrophages actively participate in the formation of specific immunity by synthesis of cytokines and mediators that play a role the immune response regulators [35]. These substances induce proliferation, differentiation and effector function of lymphocytes. At the same time, influence predominates that allows avoiding uncontrolled growth of the inflammation reaction. Another important function of macrophages is the presentation of antigens. Antigens after processing in macrophages, together with class I/II antigens of the histocompatibility complex via the T-cell receptors, are presented on СD4+ Т-lymphocytes. That leads to the activation of СD4+ Т-lymphocytes. They start to produce cytokines interacting with B-cells leading to the synthesis of specific antibodies [36].
In addition to alveolar macrophages, macrophages located in the respiratory tract, tissue macrophages, intravascular and pleural ones are present in the lungs. The macrophages of the respiratory tract phagocytose pathogens caught with inhaled air. In combination with the mucociliary protective mechanism, they form the most important protective barrier [37].
Tissue macrophages are located in the interstitial space. Their number approximately corresponds to the number of alveolar macrophages. Pulmonary intravascular macrophages are mature tissue macrophages penetrating the pulmonary circulation. They express leukocyte integrin LFA-1 (leucocyte function associated-1), which is able to bind to the endothelial adhesion molecule ICAM-1 (intracellular adhesion molecule) [38]. Intravascular macrophages phagocytize microorganisms, penetrated into the bloodstream of lungs. Pleural macrophages are under anaerobic conditions and resemble peritoneal ones, but they have been studied much less than other types of macrophages.
Thus, alveolar macrophages provide the first line of defense of the body against the introduction of heterogeneous microorganisms through their ability to phagocytize, recruit and activate other cells, as well as to maintain and restore the lung parenchyma. At all these stages, the functional state of alveolar macrophages is exerted by modulating regulatory peptides synthesized by endocrine cells in the lungs, allowing the regulation of in situ.
Gastrointestinal tract resident immune cells
The inductive and the effector zone may be isolated in the immune system of the gastrointestinal tract (GIT) [39,40]. The first consists of Peyer's patches, appendix, and regional lymph nodes, the second - from the lamina propria and epithelial cells of the intestinal mucosa. Recognition, antigen presentation and formation of a population of antigen-specific T- and B-lymphocytes occur in the inductive zone, and the synthesis of immunoglobulins by B-lymphocytes, cytokines, T-lymphocytes and NK cells by T-cytotoxic cells i.e., their effector functions occur in the effector zone.
It has been shown that Peyer's plaques play an extremely important role in the immune system of the digestive tract [41]. Peyer's plaques like all lymphoid formations consist of T- and B-zones with the presence of germinal centers in the B-zone. Their cellular composition is not significantly different from that of any peripheral lymph node. They are characterized by a unique morphological structure - the follicularassociated epithelium, which the main feature is the so-called M-cell. This cell has short cytoplasmic processes and forms an intraepithelial pocket, wherein there are macrophages, DCs, T-and B-lymphocytes in addition to the M-cells. The main function of M-cells is selective absorption of antigens.
It is established that Peyer's plaques of the small intestine are the source of plasmatocytes synthesizing immunoglobulins of class A (IgA). This provided a basis for the allocation of a special, relatively autonomous body immunity - associated lymphoid tissue with mucous membranes (mucosa-associated lymphoid tissue - MALT). MALT-lymphocytes migrate, populating characteristic for each population habitats with inclusion of the immune response to antigenic stimulation in all mucous membranes of the body. This allows you to make a fundamentally important conclusion that MALT stimulation can activate the immune system of any of the mucous membranes - pulmonary, gastrointestinal, genitourinary tract and other organs [42]. MALT is a powerful system for the protection of the digestive tract. It should be noted that the lymphoid tissue is 25% of the total mass of the digestive tract mucosa. Three types of lymphoid tissue are distinguished in the wall of the stomach and intestine: intraepithelial lymphocytes, diffusely located lymphocytes and plasma cells of the lamina propria. MALT is functionally similar to the peripheral lymphoid tissue of the spleen and lymph nodes. However, it should be noted that the local immune system is less developed in the stomach than in the intestine, so antigens not only penetrate it, but are also actively absorbed by the blood through the mucous membrane [43].
Despite the fact that MALT can be regarded as an independent system, there is a multifaceted interaction between it and the general immune system of the body. Since the intestine is the entrance gate to numerous pathogenic pathogens and, in addition, constantly interacts with antigens contained in food, the early contact of the MALT system with antigens has a decisive influence on the further response development of the entire immune system. When analyzing the interaction between the MALT system and the general immune system of the body, it must also be borne in mind that lymphocytes circulating between the regional immune system and the general immune system of the organism are involve in this interaction.
Lymphocytes of the efferent link of the intestine immune system targeted to participate in immune responses of two basic types. Firstly, they must provide immune protection of the organism from pathogens threatening it using the generation of antigen-specific immune response, and secondly, suppress the local as well as the systemic immune response to ingesting food antigens.
Cytokines are directly involved in the regulation of the immune response. The main role in pathogenesis is given to imbalance of cytokines with pro-inflammatory and anti-inflammatory (regulatory) action with inflammatory and immunoinflammatory (autoimmune) diseases, including ulcer disease and inflammatory bowel diseases. Imbalance of proinflammatory and anti-inflammatory cytokines is a consequence of a violation of the ratio of different cell populations in the mucosa of the gastrointestinal tract and their pathological antigenic activation. In the normal mucosa of the gastrointestinal tract, the content of T-cell subpopulations, and accordingly, the ratio of proinflammatory and anti-inflammatory cytokines is balanced, which provides an adequate immune response to antigenic irritation [44].
The synthesis profile of cytokines is under genetic and hormonal control. Development of immune inflammation in the inflammatorydestructive lesion of the gastrointestinal tract is associated, first of all, with a violation of the balance of interleukins in the direction of the predominance of the production of pro-inflammatory cytokines. The reasons for this dysregulation are not completely known, but it can be supposed, this is due to an abnormal immune response in the persistence of one or more antigens and impaired interaction within the MALT system.
The presence of powerful local immunity is a reliable barrier to the penetration of pathogenic microbes into the internal environment of the organism and the development of the infectious process. Therefore, a high degree of organization of the immune system is particularly important in the digestive tract, as it is the first line of defense against invasion of microbes and allergens. Activation of different immune cellular subpopulations that is so widely represented in the digestive tract with the synthesis of the corresponding cytokines can occur depending on the nature of the antigen and on the genetic determinacy of the immune response, which defines different variants of the immune response of the organism.
Liver resident immune cells
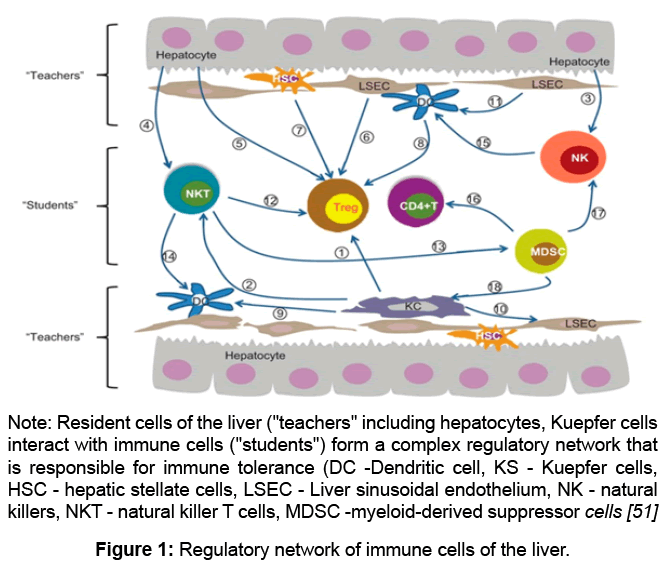
The liver is the source of pluripotent stem cells capable of self-maintenance and differentiation, as well as immature T- and B-lymphocytes during embryogenesis. Some populations of immune cells mature and undergo differentiation in the liver. Close linkage between the liver and the immune system is maintained in the adult body (Figure 1).
The leading role of the liver is to maintain optimal level of vitamins, microelements, proteins, amino acids, lipotropic factors, excess or deficiency of which affects the content and the activity of T-lymphocyte and is the cause of disorders of both cellular and humoral immune responses, and determines the effect of liver on the violation of immunological reactivity [45]. For example, a high content of cholesterol and low density lipoprotein in the serum inhibits the transformation of lymphocytes in response to the presence of mitogen, reduces the phagocytic activity of polymorphonuclear leukocytes.
Liver is the main source of some complement system components, so one of the special cases of reducing protein-synthetic liver function is the decrease in the intensity of antigen-antibody responses, as well as antibody-dependent cell-mediated cytotoxicity [46,47].
Recently it has been shown that hepatocytes and other liver cells are capable of activating T-lymphocytes. Among the cellular populations that induce T-cell tolerance, sinusoidal endotheliocytes have a special role, since they involve the functions of macrophages and antigen presenting cells. In this respect, sinusoidal endotheliocytes resemble immature DC. Moreover, Kuepfer cells (KS) which constitute 20-30% of the lining hepatic sinusoids are typical macrophages.
The liver has a set of different types of antigen presenting cells able to interact with CD4+ T-cells [48]. It consists of dendritic cells, KS, sinusoidal endothelial cells, stellate cells, and possibly hepatocytes at least in the areas of inflammation. It has been reported that stimulation of CD4 T-cells by these hepatic antigen presenting cells provides immune tolerance of the liver [49].
The functional state of granular cells determines the intensity of the immune response to many antigens. The lack of immune reactions in the territory of a healthy liver is provided by the inhibiting factors of the microenvironment (humoral immunosuppressive factors - thermolabile proteins with different molecular weights). If the structure of the organ is damaged, which is accompanied by various diseases, it becomes possible to contact antigen-presenting cells with T-lymphocytes and develop immune responses [50] (Figure 1).
With many diseases of the liver and age-related dysfunction, the composition of blood lipoproteins is significantly impaired. Reduction of α-lipoprotein (LP) and the increase in β-lipoprotein and pre-β-lipoprotein contributes to the formation of antibodies to the β-lipoprotein and the appearance of autoimmune-antibody complexes LP, which triggers the autoimmune process in the liver.
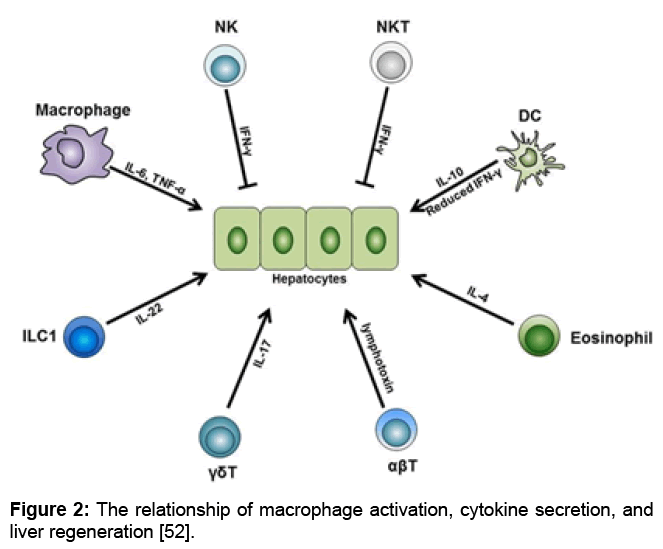
Different subsets of the innate and adaptive immune cells are indispensable for normal liver regeneration after partial hepatectomy. Among these cells, liver macrophages produce IL-6 and TNF-α and initiate the regeneration process after partial hepatectomy. Besides, liver DCs upregulate their IL-10 expression level while downregulate their IFN-γ level, thus facilitate liver regeneration. In addition, liver eosinophil-derived IL-4 also promotes the regeneration process. Furthermore, γδT cell-derived IL-17 and ILC1-derived IL-22 are both necessary for normal regeneration. On the other side, NK and NKT cells play inhibitory roles in liver regeneration, and this is mainly dependent on the IFN-γ they secrete. Besides these innate immune cells, conventional αβT cells can secrete lymphotoxin and stimulate liver regeneration. Abbreviations: TNF-α, tumor necrosis factor-α; IFN-γ, interferon-γ; ILC, innate lymphoid cell (Figure 2).
Figure 2: The relationship of macrophage activation, cytokine secretion, and liver regeneration [52].
Features of immunological reactions of the liver play an important role in the development and metastasis of tumors in it, which often complicate the development of age-related pathology [51-53]. Study of different populations of liver cells showed that hepatocytes can inhibit the supervision of the tumor cells from nonparenchymal cells. There are other ways of influencing the liver for immunological reactivity associated with changes in its functions - secretory, excretory, detoxification, and others. For example, it has been established that bilirubin and bile acids can alter the functional activity of immunocompetent cells and affect the overall reactivity of the organism, and the involvement of the liver in IgA transport largely determines the intensity of local immunological reactivity in the mucosa of the gastrointestinal tract and other organs.
Skin resident immune cells
Skin cells are able to initiate a systemic response to received antigens. As an immune organ, the skin is capable of isolation, pressing and presentation of antigens and the development of a local immune response. The skin contains immunocompetent cells of bone marrow origin, e.g. resident histiocytes, mast cells, Langerhans cells, lymphocytes and granulocytes, capable of generating regulatory peptides identical to those in the central nervous, immune and endocrine systems.
Keratinocytes are the main cells of skin with direct action of damaging agents, bacterial factors and products of inflammation which activated and acquire the features of immunocompetent cells. Activated keratinocytes secrete IL-8 and CTACK (Cutaneous T-cell - Attracting Chemokine), projecting attractants for T-lymphocytes, epithelial origin factors ENA-78, and GRO, activating neutrophil and chemotactic protein monocytes MCP-1, pro-inflammatory IL-1 and -6, TNF-α, colony-stimulating factors, such as granulocytemacrophage, granulocyte and monocyte-macrophage, as well as IL- 7. Signal molecules increase the inflammatory response acting on endothelial cells of the skin. Thus, keratinocytes trigger the primary "antigen-independent" inflammation, transforming external signals into the secretion of cytokines and chemotactic factors that exert an auto- and paracrine effect, and changing the expression of adhesion molecules. The initial phase of inflammation can be transformed into an amplification phase and the "antigen-dependent" associations of keratinocytes with T-lymphocytes and antigen-presenting cells. At this stage, activated keratinocytes acquire the ability to present antigens due to the appearance on their surface of MHC class II molecules and costimulatory molecules of group B7 - CD80, CD86 and CD40. However, keratinocytes do not always express the complete set of molecules required for the presentation of the peptide antigen even in the conditions of activation.
Immune functions of mast cells of the skin according to modern concepts are manifested in hypersensitivity reactions. Skin mast cells capable of recognizing antigens of certain microorganisms (respiratory viruses, human immunodeficiency virus, mycoplasmas, enterobacteria, mycobacteria tuberculosis) via surface receptors, as well as products of an inflammatory reaction, which develops when their implementation (e.g., nucleotides). Moreover, mast cells can phagocytize bacteria, process and present their antigens (at least antigens of gram-negative bacteria, including Salmonella typhimurium and Escherichia coli).
Particular importance in the modulation of mast cell functions is given to the substance P (SP). This substance is well known as the classic mediator of the triad of skin inflammation symptoms - erythema, edema and itching. The SP source is the end of nerve fibers, as well as some skin cells. SP, like neurokinin A, can exert its effects directly by binding to NK cell receptors expressed by endothelial cells. However, it seems that its main effect is mediated through histamine release and TNF-α mast cells upon binding of NK-cell receptors on their surface. Histamine, in turn, causes vasodilation, acting on the smooth muscle cells of the vessels through the H1 receptor, and TNF-α, enhancing the expression of E-selectin by endotheliocytes, increases the ability of leukocytes to enter the inflammation zone. Moreover, SP modulates cytokine expression by mast cells, selectively stimulating the expression of mRNA and TNF-α and as a consequence, the synthesis of the corresponding protein.
Calcitonin - gene-related peptide (CGRP) is represented in the skin in significant quantities. Although there is no clear data on its role in the interaction of mast cells and endings of nerve fibers, there are prerequisites for such an assumption. First, CGRP is identified immunohistochemically in the unmyelinated nerve fibers of the dermis in contact with mast cells. Secondly, dura mast cells are highly sensitive to this neuropeptide. Therefore, it can be assumed that CGRP has similar effects on dermal mast cells. This assumption is supported by the results of comparatively recent studies in which the CGRP secretion induced by ultraviolet radiation results in the release of histamine, TNF-α, and IL-10, known immunosuppressive effects, by mast cells [54].
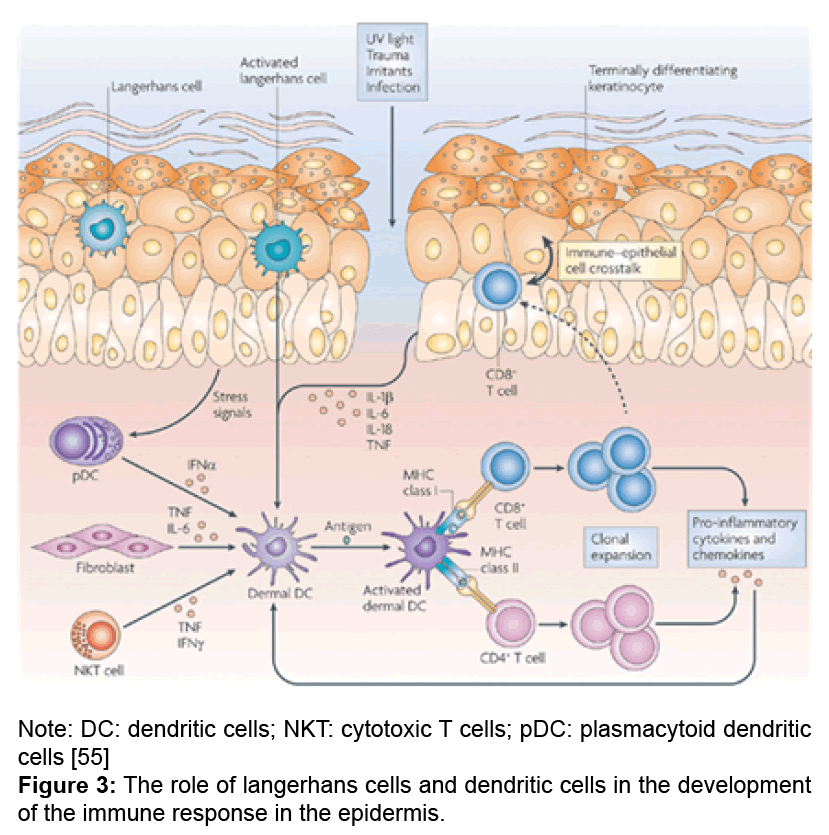
Langerhans cells (LC) is a subpopulation of dendritic cells of bone marrow origin, located in the epidermis and constituting 2 to 8% of all epidermal cells. These cells were described as early as 1868; however, the study of their morphological and functional properties is still an important aspect of understanding the immunological functions of the skin. They belong to the macrophage - monocytic - histiocytic line and play a central role in the initiation of the immune response (Figure 3).
It has been established that the epidermis contains clusters of LC forming a branching system inside the spiny layer, due to the large number of dendritic processes. LC leave the skin through the lymphatic vessels and migrate to the lymph nodes. The action of TNF-α is the impetus for this process. Morphological feature of LC is the presence on their membrane CD1 molecules, considered a marker of immature cortical thymocytes and CD4, characteristic for the T-helper cells. It was established that the skin resting LC actively perceive and process the antigen, but have a poor ability to present this cells to T-helpers and cause their activation, whereas the ratio of activity is the opposite in proliferating LC (and, probably, in the form of DC, which is acquired by LC after admission to the lymph node). In this regard, it is considered that LC perform binding and antigen treatment in the epidermis, whereas the procedure for representing the antigenic peptide to T-helpers is carried out by migrating LC in the lymph node (Figure 3).
Antigens associated with the immune response are expressed only by LC in the normal epidermis. LC induce antigen-specific activation of T-cells and promote the generation of cytotoxic T-lymphocytes. Due to the antigen-presenting capacity, these cells play a crucial role in contact sensitization and immune defense against viruses. LC also take part in the process of skin transplant rejection.
Mast cells of the skin play an important role in the allergic reactions and secretion of vascular-active substances, in vessel reactions, in the migration of T-lymphocytes, in the implementation of immediatetype hypersensitivity reactions. In contrast to the lymphoid organs of the body's immune system and a number of peripheral organs of this system (intestines, lung tissue), where there are extensive scattered lymphoid foci, large clusters of lymphoid tissue are not found in the skin. Only isolated reports have been published about the presence of small accumulations of lymphocytes in areas of skin transitional to the mucous membranes. They are considered as the innate immune centers [55,56].
Lymphocytes perform their functions in the skin by constant recirculation. Most of the lymphocytes in healthy skin are located in 2-3 rows around postcapillary venules of the superficial vascular plexus. Only a small number of lymphocytes reveal in the intact epidermis. T-lymphocytes make up 90% of all lymphocytes of the skin and are located mainly in the epidermis and upper layers of the dermis, a small part of B-lymphocytes is found in the middle and deep layers of the dermis. Some authors did not find B-lymphocytes in normal skin at all [57].
It has been found that the lymphocytes in the perivascular areas consist of almost equal number of T-helpers, T-suppressors and the helper-suppressor index is 0.93-0.96. Most of these cells are found to be activated, as evidenced by the expression on their surface of the immunoassociation antigens: HLA-DR and IL-2 receptors. Intraepidermal T-lymphocytes are referred primarily to the suppressorcytotoxic subpopulation. It is suggested that lymphocytes migrating into the epidermis perform functions identical to intraepithelial GIT lymphocytes [58].
With antigenic stimulation of the skin, the influx of lymphocytes into it sharply increases. It is believed that a small number of antigenspecific T-lymphocytes can remain in the places of the former sensitization for a long period, as the substrate specific immunological memory. Clinically, this is manifested by the reaction of inflammation in allergic contact dermatitis and recurrences of fixed erythema [59].
The skin contains a large number of immunoregulatory mediators. The most studied metabolites of arachidonic acid are leukotrienes and prostaglandins, as well as cytokines, which begin to synthesize keratinocytes in response to damage. Initially, they were thought to be synthesized by T-cells and macrophages, then it was found out that they were also produced by structural skin cells-fibroblasts, keratinocytes and endothelial cells. Cytokines bind keratinocytes to lymphocytes, phagocytes and granulocytes and are critical elements in the skin response to damage or infectious-viral damage. Among cytokines, there are both immunostimulating and immunosuppressive factors, as well as agents that promote T-cells proliferation and differentiation and suppress T-cells responses.
A factor activating NK-cells was isolated as an independent cytokine produced by epidermal cells. It is produced not only by normal keratinocytes, but also by multiple lines of malignant cells. The role of this factor is to stimulate the NK-cells. The activation factor of granulocytes (epidermal granulocyte activating factor), which stimulates the ability of these cells to oxidative processes in them, has also been obtained. Using epidermal cytokine chemotaxis is of immunocompetent cells regulated to the skin, their cooperation, differentiation of a part of T-lymphocytes occurs, their proliferation and cytotoxicity are induced.
The ability of the skin's epithelium to influence the differentiation and proliferation of T-lymphocytes makes it possible to be considered the skin as an organ that performs certain functions similar to those of the thymus. The similarity of epidermal skin cells and thymus epithelium was proved by histochemical, enzymological methods, and also by the immunofluorescence reaction. Common heteroantigens are found in the basal cells of the epidermis and the hormonal epithelium of the thymus. The cell surface antigen was found in Hassall`s corpuscles characteristic of the basal layer cells of the epidermis and antigens specific for spinosum, granular and horny layers were identified in the deeper layers of these cells. Components similar to antigenically components of the intercellular structures of the epidermis were found in extracellular structures located among the cells of the Hassall`s corpuscles. This is no accident that nude mice lack hair and there is an inadequacy of the epidermis. The immune system of the skin has features of both a local immune response, and involves integration into the overall immune system of the body. During the rest period it has minimal activity (keratinocytes does not function as immunocytes in this case). Thus, the skin is the source of a large number of immunocompetent cells, which allows it to carry out a number of important immunological functions, such as the recognition of antigens, differentiation of immature cells into T-lymphocytes beyond thymus and immunological surveillance of tumor cells.
Conclusion
In the literature, there is a sufficient amount of data on immunocompetent cells in various organs and tissues, while their numbers and diversity vary. Any violation of the outer boundaries integrity of the body leads to the activation of immunocompetent cells, the immune system not only generates a local aggression towards a heterogeneous agent, but also contributes to the immunological memory in the whole organism. Immunocompetent cells, as well as various types of cells that are not included in the immune system but perform immunological functions, take part in the synthesis and secretion of numerous biologically active substances into the internal environment of the organism, ensuring the maintenance of homeostasis in the body and acting in unity with the nervous and endocrine systems. Violation of the functional activity of the endocrine and nervous systems causes a change in the composition and activity of the immune cells subpopulation that leads to various pathologies of the immune system.
References
- Paltsev MA, Kvetnoy IM, Ployakova VO, Gurko GI, Mursalov SU (2012) Neuro-immuno-endocrinology mechanisms of aging and age-related diseases. Nauka, Saint Petersburg, Russia.
- Ridder K, Keller S, Dams M, Â Rupp AK, Schlaudraff J, et al. (2014) Extracellular vesicle-mediated transfer of genetic information between the hematopoietic system and the brain in response to inflammation. PLoS Biol 12: e1001874.
- Ivanov SV (2007) Aging morphology of human pineal gland: Intravital study. Advances in Gerontology 20: 60-65.
- Linkova NS (2011) Abilities of reciprocal compensation of pineal gland and thymus function during its ageing. Prophylactics and Clinic Medicine 4: 113-116.
- Polyakova VO, Linkova NS, PichuginSA (2011) Changes in Apoptosis and cell proliferation in human pineal gland during aging. Bull Exp Biol Med 150: 468-470
- Khavinson VK, Golubev AG (2002) Pineal gland ageing. Advances of Gerontology 9: 67-72.
- Linkova NS, Polyakova VO, Trofimov AV, Kvetnoy IM, Khavinson VKh (2011) Peptidergic regulation of thymocyte differentiation, proliferation, and apoptosis during aging of the thymus. Bull Exp Biol Med 151: 239-242
- Paltsev MA, Polyakova VO, Kvetnoy IM, Anderson G, Kvetnaia TV, et al. (2016) Morphofunctional and signaling molecules overlap of pineal gland and thymus: Role and significance in aging. Oncotarget 7: 11972-11983
- Kliger CA, Gehad AE, Hulet RM, Roush WB, Lillehoj HS, et al. (2000) Effects of photoperiod and melatonin on lymphocyte activities in male broiler chickens. Poult Sci 79: 18- 25
- Mosenson J, McNulty J (2006) Characterization of lymphocyte subsets over a 24-hour period in Pineal-Associated Lymphoid Tissue (PALT) in the chicken. BMC Immunol 7: 1.
- McNulty JA, Relfson M, Fox LM, Kus L, Handa RJ, et al. (1990) Circadian analysis of mononuclear cells in the rat following pinealectomy and superior cervical ganglionectomy. Brain Behav Immun 4: 292–307.
- Csaba G (2016) The role of the pineal-thymus system in the regulation of autoimmunity, aging and lifespan. Orv Hetil 157: 1065-1070
- Litvinenko GI, Shurlygina AV, Gritsyk OB, Melnikova EV, Tenditnik MV, et al. (2015) Effects of melatonin on morphological and functional parameters of the pineal gland and organs of immune system in rats during natural light cycle and constant illumination. Bull Exp Biol Med 159:732-735
- Gluchovets BI, Gluchovets NG (2002) Afterbirth pathology. Graal, Saint Petersburg, Russia.
- Kvetnoy IM, Ajlamazian EK, Lapina EA, Kolobov AV (2005) Signal molecules-markers of placentae matures. MEDpress-inform, Moscow, Russia.
- Korzevskij DE, Otellin VA, Neokessarijskij AA (2005) Structural organization of macrophages in the form human placenta. Morphology 128: 60-62.
- Linkova NS, Kostylev AV, Kostuchek IN, Polyakova VO (2009) Expression of CD8 as a risk marker of habitual abortion in women with gesthosis. Vestnik RUDN. Medical series. Obstetrics and Gynecology (Russia) 6: 326-331.
- Nakamura Y, Ohta Y (1990) Immunohistochemical study of human placental stromal cells. Hum Pathol 21: 936-940
- Selkov SA, Pavlov OV (2007) Macrophages of placenta. Tovarishectvo nauchnich izdanij KNK, Moscow, Russia.
- Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, et al. (2003) Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early pregnancy. Am J Pathol 162: 887-896
- Ajlamazian EK, Lapina EK, Kolobov AV (2004) Placenta aging. Journal of Obstetrics and Woman Diseases (Russia) 53: 4-10.
- Moffett A, Loke YW (2004) The immunological paradox of pregnancy: A reappraisal. Placenta 25: 1-8.
- Nelson DM (1996) Apoptotic changes occur in syncytiotrophoblast of human placental villi where fibrin type fibrinoid is deposited at discontinuities in the villous trophoblast. Placenta 17: 387-391.
- Ajlamazian EK, Polyakova VO, Linkova NS, Durnova OA, Kvetnoy IM (2008) The role of resident immunocompetent cells in placenta development in normal and pathology. Journal of Obstetrics and Woman Diseases (Russia) 59: 8-14.
- Chaouat G, Ledee-Batallie N, Dubanchet S (2005) Immunological similarities between implantation and pre-eclampsia. Am J Reprod Immunol 53: 222-229
- Yoshioka S, Fujiwara H, Nikayama Т, Kosaka K, Mori T, et al. (2006) Intrauterine administration of autologies peripheral blood mononuclear cells promoter implantation in patients with repeated failure of IVF–embryo transfer. Hum Reproductol 21: 3290-3294
- Takabatake K, Fujiwara H, Goto Y, Nakayama T, Higuchi T, et al. (1997) Intravenous administration of splenocytes in early pregnancy changed implantation window in mice. Hum Reproductol 12: 583–585
- Sbracia M, McKinnon B, Scarpellini F, Marconi D, Rossi G, et al. (2017) PreImplantation factor in endometriosis: A potential role in inducing immune privilege for ectopic endometrium. PLoS One 12: e0184399
- Hamilton KJ, Hewitt SC, Arao Y, Korach KS (2017) Estrogen hormone biology. Curr Top Dev Biol 125:109-146
- Hong H, Stastny M, Brown C, Chang WC, Ostberg JR, et al. (2014) Diverse solid tumors expressing a restricted epitope of L1-CAM can be targeted by chimeric antigen receptor redirected T lymphocytes. J Immunother 37: 93-104
- Taghavi SA, Ashrafi M, Mehdizadeh M, Karimian L, Joghataie MT, et al (2014) Toll-like receptors expression in follicular cells of patients with poor ovarian response. Int J Fertil Steril 8: 183-192.
- Wang S, Li F, Hu L, Liu S, Li H, et al. (2014) Structural and functional characterization of a TGFβ molecule from amphioxus reveals an ancient origin of both immune-enhancing and inhibitory functions. Dev Comp Immunology 45: 219-226
- Zhang J, Zhou L, Tang L, Xu L (2014) Plasma visfatin levels and mRNA expression of visfatin in periph eral blood mononuclear cells and peripheral blood monocyte-derived macrophages from normal weight females with polycystic ovary syndrome. Exp Ther Medicine 7: 1215-1220
- Lambrecht BN, Hammad H (2013) Asthma: the importance of dysregulated barrier immunity. Eur J Immunol 43: 3125-3137
- Seiler F, Hellberg J, Lepper PM, Kamyschnikow A, Herr C, et al. (2013) FOXO transcription factors regulate innate immune mechanisms in respiratory epithelial cells. J Immunol 190: 1603-1613
- Schuijs MJ, Willart MA, Hammad H, Lambrecht BN (2013) Cytokine targets in airway inflammation. Curr Opin Pharmacol 13: 351-361
- Chang DH, Rutledge JR, Patel AA, Heerdt BG, Augenlicht LH, et al. (2013) The effect of lung cancer on cytokine expression in peripheral blood mononuclear cells. PLoS One 8: e64456
- Liu Q, Jin LH (2017) Tissue-resident stem cell activity: a view from the adult drosophila gastrointestinal tract. Cell Commun Signal 15: 33
- David BA, Rubino S, Moreira TG, Freitas-Lopes MA, Araújo AM (2017) Isolation and high-dimensional phenotyping of gastrointestinal immune cells. Immunology 151: 56-70
- Konorev MR, Konevalova NJ (2010) Modern performance about immune system, which associated with mucosa cover of intestine. Immunopathology, allergology, infectology 2: 40-46.
- Sotnikova NJu (2009) Immune system of mucosal coat and microflora. Russian Immunology Journal 3: 111-120.
- Sena TML, Attygalle DA, Wotherspoon CA (2014) Helicobacter pylori infection in gastric extranodal marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT) lymphoma: A re-evaluation. Gut 63: 1526-1527.
- Brucklacher-Waldert V, Carr EJ, Linterman MA, Veldhoen M (2014) Cellular plasticity of CD4+ T cells in the intestine. Front Immunol 5:488.
- Qu W, Sun LY, Zhu ZJ, Liu Y, Wei L, et al. (2014) Correlation analysis of survival period and CD4+ Tcell-iATP levels in liver transplant recipients. Zhonghua Gan Zang Bing Za Zhi 22: 693-697
- Sprinzl MF, Galle PR (2014) Immune control in hepatocellular carcinoma development and progression: Role of stromal cells. Semin Liver Dis 34: 376-388
- Maricic I, Girardi E, Zajonc DM, Kumar V (2014) Recognition of lysophosphatidylcholine by Type II NKT cells and protection from an inflammatory liver disease. J Immunol 193: 4580-4589
- Thomson AW, Knolle PA (2010) Antigen-presenting cell function in the tolerogenic liver environment. Nat Rev Immunol 10: 753-766.
- Willimsky G, Schmidt K, Loddenkemper C, Gellermann J, Blankenstein T (2013) Virus-induced hepatocellular carcinomas cause antigen-specific local tolerance. J Clin Invest 123: 1032–1043.
- Ibrahim MA, Mostafa AA, El-Said HW, Mohab AM, Hebah HA (2014) Study of peripheral blood natural killer cells, T-cell helper/T-cell suppressor ratio and intercurrent infection frequency in hepatitis C seropositive prevalent hemodialysis patients. Hemodial Int 18: S23-31.
- Li F, Tian Z (2013) The liver works as a school to educate regulatory immune cells. Cell Mol Immunol 10: 292-302
- Li N, Hua J (2017) Immune cells in liver regeneration. Oncotarget 8: 3628–3639
- Sun C, Sun H, Zhang C, Tian Z (2015) NK cell receptor imbalance and NK cell dysfunction in HBV infection and hepatocellular carcinoma. Cell Mol Immunol 12: 292-302
- Kim BS (2014) Innate lymphoid cells in the skin. J Invest Dermatol 135: 673-678
- Nestle FO, Di PM, Qin JZ, Nickoloff BJ (2009) Skin immune sentinels in health and disease. Nat Rev Immunol 9: 679-691
- Bos JD, Kapsenberg ML (1986) The skin immune system. Its cellular constituents and their interactions. Immunol Today 7: 235-240.
- EIbe A, Kilgus O, Strohal R, Payer E, Schreiber S, et al. (1992) Fetal skin: A site of dendritic epidermal T cell development. J Immunol 149: 1694-1701.
- Kozlova NN, Prokopenko VD (2006) Skin as an immune organ. Immunopathology, Allergology, Infectology 4: 34-40.
- Rachael AC (2010) Skin resident T cells: The ups and downs of onsite immunity. J Invest Dermatolo 130: 362–370.
Citation: Paltsev MA, Polyakova VO, Kvetnoy IM, Paltseva EM, Linkova NS, et al. (2017) Organic Ektopy of Immunocompetent Cells. Immunol Curr Res 1: 102.
Copyright: © 2017 Paltsev MA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 6933
- [From(publication date): 0-2017 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 5904
- PDF downloads: 1029