Osteoid Osteoma like Osteoblastoma in Proximal Humerus of a Dog
Received: 26-Apr-2017 / Accepted Date: 03-May-2017 / Published Date: 05-May-2017 DOI: 10.4172/2161-0681.1000308
Abstract
Osteoid osteoma and osteoblastoma are uncommon benign bone tumors which originate from osteoblasts in animals. Both tumors have similar characteristics. Osteoid osteoma is well described in humans, but there known to be only two cases in domestic animal species. Differentiating the diagnosis of osteoid osteoma from osteoblastoma by using immunohistochemistry was aimed in this study. A 12-year-old mix breed female dog was brought to the clinic with complaints of swelling in proximal forelimb and progressive loss of motion. Animal was died during surgery. In necropsy, tissue samples were taken from the mass and fixed in %10 formalin. Samples were stained with hematoxylin and eosin, Masson’s trichrome and Alizarin Red S. For revealing of cell differentiation, ABC-P method was applicated to adhesive section by using BMP6, S100, vimentin ve p53 markers. Macroscopically, proximal humerus was swollen. Cut section was generally lytic within bone residues. Soft tissue was haemorrhagic and edematouse. Microscopically, osteoblasts and hypervascular nidus were surrounded by large sclerotic and mineralized bone tissue. Large lytic areas were also observed. Masson’s trichrome revealed sclerosis and Alizarin Red S revealed osteoid matrix. Immunohistochemically, BMP6 was moderately reacted in osteoid matrix and S100 was moderately reacted with nerve fibers in nidus. Vimentin had strictly positive reaction in fibrocytes. P53 was negative in osteoblasts.
In conclusion, this case is the third reported in domestic animals and the second in dogs. Also osteoid osteoma is evaluated for the first time by immunohistochemistry in animals.
Keywords: Dog; Immunohistochemistry; Osteoblastoma; Osteoid osteoma: Pathomorphology
315706Case Description
Osteoid osteoma and osteoblastoma have been variously regarded as both similar and distinct entities in the past [1]. It is suggested that these two entities should be classified as a single entity in the future [1]. Currently, WHO classifies these tumors separately [2].
Osteoid osteoma was firstly described by Jaffe in 1935. Osteoid osteomas are benign, painful, small sized osteogenic tumors of young patients [3-5]. Typically, long bones are affected, and it is primarily located within the cortex [3,4]. It is composed of a small “nidus (lytic lesion)” measuring from a few millimeters to 1.5-2 cm, which exhibits various portions of calcification and is surrounded by reactive sclerosis and periostal reaction [4,6]. Histopathologically, osteoid osteoma is composed of anastomosing bony trabeculae of various widths and bony trabecule is rimmed by a single layer of osteoblasts, sclerotic bone and vascular osteoid tissue [4,5,7]. Cartilage tissue and mitotic figures are usually absent [4,7]. Osteoblastoma has identical histological and immunoprofiles to osteoid ostoma [1]. On the other hand, osteoblastoma often extends to extraskeletal soft tissue structures, mostly recures after surgery, may even metastasize and has more extensive lytic and sclerotic areas than osteoid osteoma [7,8]. Additionally, osteoid osteoma has a smaller size of 2 cm in diameter while osteoblastoma is 2 to 10 cm [3,6]. In veterinary medicine, there were two reports of osteoid osteoma; affecting the mandible of a cat and the humerus of a dog [8,9]. In this case report, we aimed differentiating the diagnosis of osteoid osteoma from osteoblastoma by using immunohistochemistry in a dog.
A 12-year-old mix breed, female dog was brought to the clinic with complaints of swelling in proximal forelimb and progressive loss of motion. The swelling was firmness in palpation and it was thought to be a tumorigenic mass. Surgery was decided. During operation, animal was died following pulmonary and cardiac arrest. In necropsy, tissue samples were taken from the mass and fixed in 10% formalin. Tissue samples were processed routinely and embedded in paraffin. Paraffin blocks were sectioned at 4 μm. Samples were stained with hematoxylin and eosin, Masson’s trichrome and Alizarin Red S. Immunohistochemistry was performed according to a standard avidinbiotin- complex peroxidase (ABC-P, Dako) method by using BMP6 (1:50 dilution, Santa Cruz Biotechnology), S100(1:200 dilution, Dako), vimentin (Sigma, 1:200) and p53 (1: 500, Dako) markers as primary antibodies. As chromogen, 3,3’-diaminobenzidine (DAB, NovaCastro) was selected and Harris’s haematoxylin were used for counterstaining. Control sections were stained by normal mouse sera. Clinically, the health condition of the animal was normal in apperance. However, there was a painful swelling detected in right forelimb during clinical examination. At radiography, there were no prominent extra osteoid structures except structure of regional bones. Macroscopically, periphery of proximal humerus was swollen. This swelling was 12 cm in diameter (Figure 1).
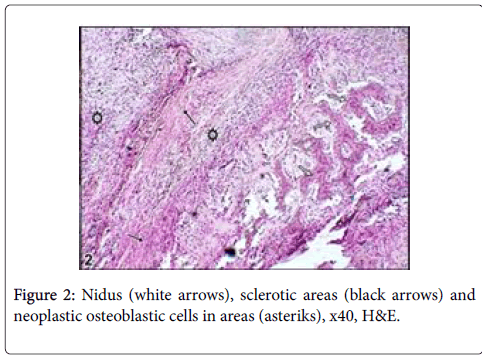
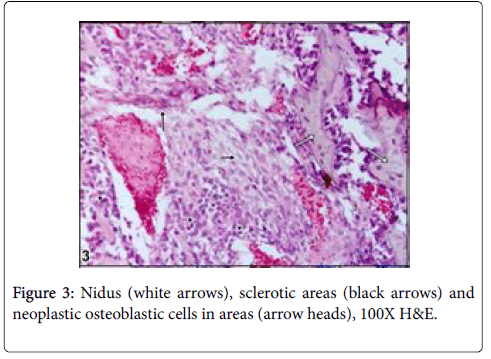
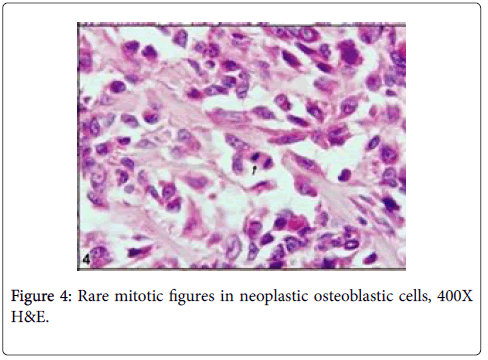
Cut section was generally seen as lytic within bone residues. Soft tissue was haemorrhagic and edematouse. Microscopically, neoplastic osteoblasts and hypervascular nidus were surrounded by largely sclerotic and mineralized bone with very rare mitotic figures (Figures 2-4).
Figure 2: HPLC chromatogram of the nine reference compounds in 50% aqueous methanol, measured at 370nm. Retention times for rutin, sutherlandin A, sutherlandin B, kaempferol-3-O-rutinoside, sutherlandin C, sutherlandin D, quercitrin, quercetin and kaempferol were 11.9, 12.7, 13.8, 15.3, 16.2, 17.0, 18.0, 26.2 and 28.1 minutes, respectively.
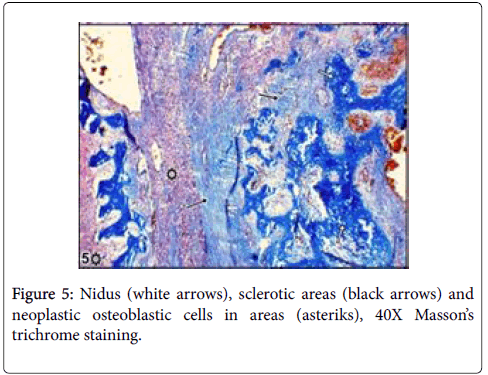
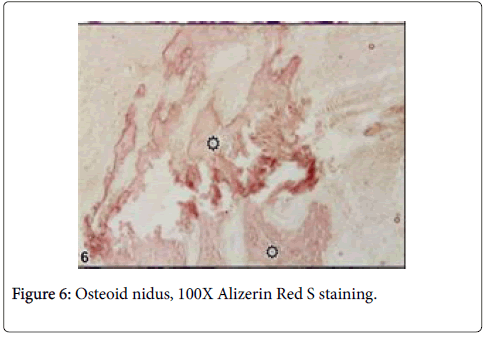
Large lytic areas were also attended. Masson’s trichrome staining differentiated sclerotic areas in tumors and Alizerin Red S differentiated osteoid matrix (Figures 5 and 6).
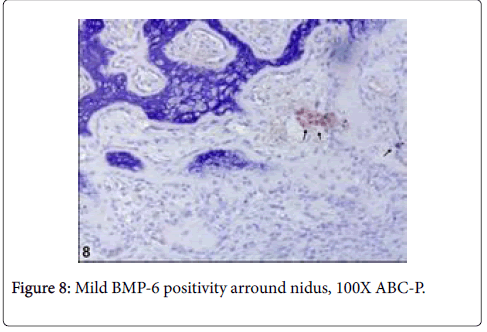
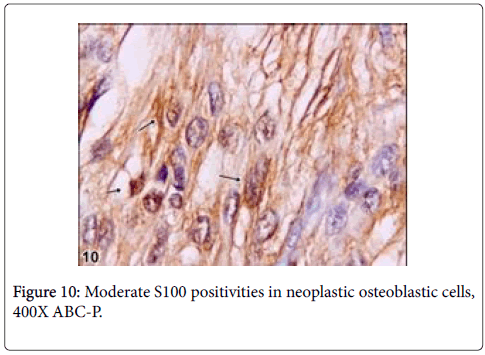
Immunohistochemically, BMP6 was moderately reacted in osteoid matrix and S100 was moderately reacted with nerve fibres (Figures 7 and 8).
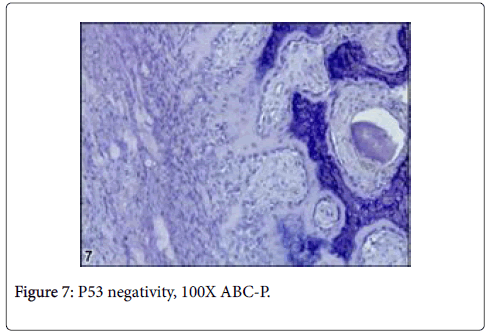
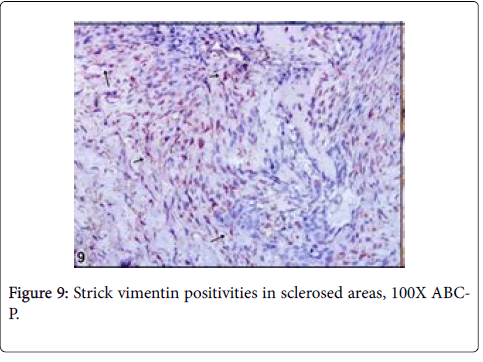
Vimentin gave strictly positive reaction in fibrocytes in especially sclerotic areas of the tumor, P53 was negative in osteoblasts and other cells such as fibrocytes and bone residual cells (Figures 9 and 10).
It has been reported that, nerve fibers that express S100 are present in the reactive zone around the nidus and/or in the nidus itself,in approximately 25% of osteoid osteomas [10]. These fibers have not been observed in any other tumors, which suggests that the nerve supply of osteoid osteoma might serve as a marker in diagnostically difficult cases [10]. However, any documentation could not be found on the basis of veterinary literatures regarding osteoblastoma. In this case, S100 was moderately reacted with nerve fibers within nidus. Due to this reaction, it is thought that the tumor can be pertain with osteoid osteoma even though it is rarely expressed in this kind of tumors. But, some morphological features thought that the tumors can be osteoblastoma. On the other side, among benign bone tumors, bone morphogenetic proteins (BMPs) are expressed in osteoid osteomas and osteoblastomas and effect reactive bone formation [11]. Also in this case, BMP6 was mildly reacted in osteoid matrix and results similar to the previous knowledge were observed. An immunohistochemical assay demonstrated that BMP-2-4 was expressed in the cytoplasm of the osteoblastic cells in osteoid osteoma [12]. However, p53, which is known as malignancy showing marker, was negative in the current case even though there were rare mitotic figures in osteoblastic cells. Thus, it was thought that the tumor had low or no malignancy [13].
In conclusion, the tumor was diagnosed as osteoid osteoma because it was smaller than 2 cm in diameter, rarely shown mitotic figures, had p53 negativity showing no malignancy in neoplastic cells. Also, the case showed that BMP6 and S100 were useful for revealing presence of osteoid matrix and differentiation [13,14]. Osteoid osteoma was evaluated before only in a dog to best of our knowledge [5]. But, immunohistocehmistry has not been used for describing in such kind of cases. Thus, we believe that immunohistochemistry has been immensely recommended to the researchers for providing differentiation of the dilemma in such kind of cases.
References
- Barlow E, Davies AM, Cool WP, Barlow D, Mangham DC et al. (2013) Osteoid osteoma and osteoblastoma: novel histological and immunohistochemical observations as evidence for a single entity. J Clin Pathol 66: 768-774.
- Fletcher CDM, Bridge JA, Hogendoorn P (2013) Wold Health Organisation Classification of Tumors. Pathology and Genetics. Tumours of soft tissue and bone. 4th ed. ARC,5, 277-280
- Chotel F, Franck F, Solla F, Dijoudb F, Kohlera R, et al. (2012) Osteoid osteoma transformation into osteoblastoma: Fact or fiction? Orthop TraumatolSurg Res 98: 98-104.
- Papathanassiou ZG, Megas P, Petsas T, Papachristou DJ, Nilas J, et al. (2008) Osteoid osteoma: diagnosis and treatment. Orthopedics 31: 1118.
- Zenmyo M, Yamamoto T, Ishidou Y, Komiya S, Ijiri K, et al. (2011) Osteoid osteoma near the intervertebral foramen may induce radiculopathy through tumorous inflammation. Diagn Pathol 6: 10.
- Ciftdemir M, Tuncel SA, Usta U (2015) Atypical osteoid osteomas. Eur J Orthop SurgTraumatol 25: 17-27.
- Klein MH, Shankman S (1992) Osteoid Osteoma-radiologic and pathological correlation. Skeletal Radiol 21: 23-31.
- Gorra M, Burk RL, Greenlee P, Weeren FR (2002) Osteoid osteoma in a dog. Vet Radiol Ultrasound 43: 28-30.
- Kapatkin AS,Marretta SM, Patnaik AK (1991) Mandibular swelling in cats: Prospective study of 24 cats. J AmerAnimHospAssoc 27: 575-530.
- Muro-Cacho CA (1998) The role of immunohistochemistry in the differential diagnosis of soft-tıssue tumors. Cancer Control 5: 53-63.
- Yoshikawa H, Nakase T, Myoui A, Ueda T, (2004) Bone morphogenetic proteins in bone tumors. J Orthop Sci 9: 334-340.
- Okuda S, Myoui A, Nakase T, WadaKazuo E, Yonenobu K, et al. (2001) Ossification of the ligamentumflavum associated with osteoblastoma: a report of three cases. Skeletal Radiol 30: 402-406.
- Jaffe HL (1935) Osteoid-osteoma a benign osteoblastic tumor composed of osteoid and atypical bone. Arch Surg 31: 709-728.
- O’Connel JX,Nanthakumar SS, Nielsen GP, Rosenberg AE (1998) Osteoid osteoma: uniquely innervated bone tumor. Mod Pathol 11: 175-180.
Citation: Kutlu T, Alcigir ME, Ergin I (2017) Osteoid Osteoma like Osteoblastoma in Proximal Humerus of a Dog. J Clin Exp Pathol 7:308. DOI: 10.4172/2161-0681.1000308
Copyright: © 2017 Kutlu T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 6642
- [From(publication date): 0-2017 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 5684
- PDF downloads: 958