Research Article Open Access
Periodontal Intrabony Defects and the Treatment with Enamel Matrix Derivative and a Synthetic Bone Substitute in Humans: A Clinical and Radiographic Case Series
Patricia Garani Fernandes1,2, Idiberto José Zotarelli Filho3, Gustavo Henrique Apolinário Vieira1, Arthur B. Novaes Jr1, Sergio Luís Scombatti de Souza4, Mario Taba Jr4, Daniela Bazan Palioto4 and Márcio Fernando de Moraes Grisi4*1Department of Periodontology, University of São Paulo, Brazil
2Unorp - University Center North Paulista, São José do Rio Preto, Brazil
3Unipos - Post graduate and continuing education, Brazil
4Department of Oral Surgery and Periodontology, University of São Paulo, Brazil
- Corresponding Author:
- Márcio Fernando de Moraes Grisi
Associate Professor
Department of Oral Surgery and Periodontology
Ribeirão Preto School of Dentistry
University of São Paulo
Avenida do Café - s/n, 14040-904
Ribeirão Preto, SP, Brazil
Tel: +55-16-602-3981
Fax: +55-16-3602-4788
E-mail: mgrisi@forp.usp.br
Received date: July 20, 2015; Accepted date: August 13, 2015; Published date: August 18, 2015
Citation: Fernandes PG, Zotarelli Filho IJ, Apolinário Vieira GH, Novaes Jr AB, Scombatti de Souza SL, et al. (2015) Periodontal Intrabony Defects and the Treatment with Enamel Matrix Derivative and a Synthetic Bone Substitute in Humans: A Clinical and Radiographic Case Series. J Interdiscipl Med Dent Sci 3:185. doi: 10.4172/2376-032X.1000185
Copyright: © 2015 Fernandes PG, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Objective: The aim of this study was to analyze by clinical and radiographic parameters the use of enamel matrix derivative (EMD) with or without a synthetic bone substitute (Bone Ceramic) in the treatment of periodontal intrabony defects in humans.
Methods: Nine patients and eighteen defects in need of treatment for periodontal intrabony defects were selected and assigned to the treatment with EMD or EMD plus Bone Ceramic assessing clinical and radiographic measurements.
Results: At one year, mean probing depth (PD) reduction demonstrated improvements in all groups when compared to the baseline (P<0.01). At 1 year PD reduction ≥ 2mm was measured in 57% of the defects (i.e., in 4 of the 7) when EMD + BC was used; in 36% of the defects (i.e., 4 of the 11) when EMD was used. There were no statistically significant differences when standardized radiographic subtractions were compared radiographic.
Conclusion: In conclusion, results from the present cases series confirm that a regenerative procedure based on EMD plus Bone Ceramic had better results in defect fill, although there was a variation in the numbers of defects.
Keywords
Biocompatible materials; Bone grafting; Hydroxyapatite; Enamel matrix derivative; Periodontal intrabony defects
Introduction
The intrabony defects are considered the main consequence of periodontal disease, presenting itself as a challenge to the clinician. These represent locations that, if untreated, are at an increased risk for disease progression. Although resection surgery has been used a treatment option to their elimination, the treatment of choice is periodontal regeneration [1].
Reports of guided tissue regeneration (GTR) as a periodontal procedure appeared more than 20 years ago [2,3]. The rationale behind this therapy is to achieve regeneration of tooth supporting structures, or cementum, periodontal ligament and alveolar bone. This is different from conventional periodontal surgery that achieves healing by repair. Another way to approach periodontal regeneration is to mimic the process that occurs during development of periodontal tissues [1,3-6].
After the discovery of the presence of a layer of enamel matrix between the peripheral dentin and cementum under development, along with the ability of this protein to induce the formation of acellular cementum, periodontal ligament, and alveolar bone, provided the fundamental concept for use tissue derivatives of enamel matrix in periodontal regenerative therapies. The results of several clinical studies indicated that topical application on the root surface of the enamel matrix proteins commercially available (EMD, Straumann, Basel, Switzerland) during access flap surgery promoted clinically significant gains in clinical attachment and bone formation in intrabony defects. Moreover, prospective, controlled clinical trials have shown that these benefits are significantly greater than those obtained by the access alone. The formation of new attachment after treatment of intrabony periodontal defects with EMD has been demonstrated in both experimental animals and humans [7-11].
Combination of bone grafts with EMD has the potential to result in a synergistic effect of both materials. This assumption is based on the fact that two distinct procedures for resolution of the wound may occur together in a given vertical bone defect: while the graft material can act as an osteoconductive material, also acting in the space maintenance defect, the EMD can work at the root level, promoting new cementum and new formation of the attachment apparatus [8,12-14].
Standardized intra-oral radiographs provide an acceptable diagnostic test for the evaluation and monitoring of periodontal bone status. By measuring distances between anatomical landmarks (e.g., cemento-enamel junction [CEJ], alveolar ridge), the extent of bone loss and by comparison of these measures over time, changes in bone level can be assessed. Moreover digital radiographs are shown to be useful for clinical correlation between clinical attachment and bone fill in furcation defects and intrabony defects [15].
The aim of this study was to analyze clinical and radiographic parameters for the use of enamel matrix derivative (EMD), with or without a synthetic bone substitute (Bone Ceramic) in the treatment of intrabony periodontal defects in humans.
Methodology
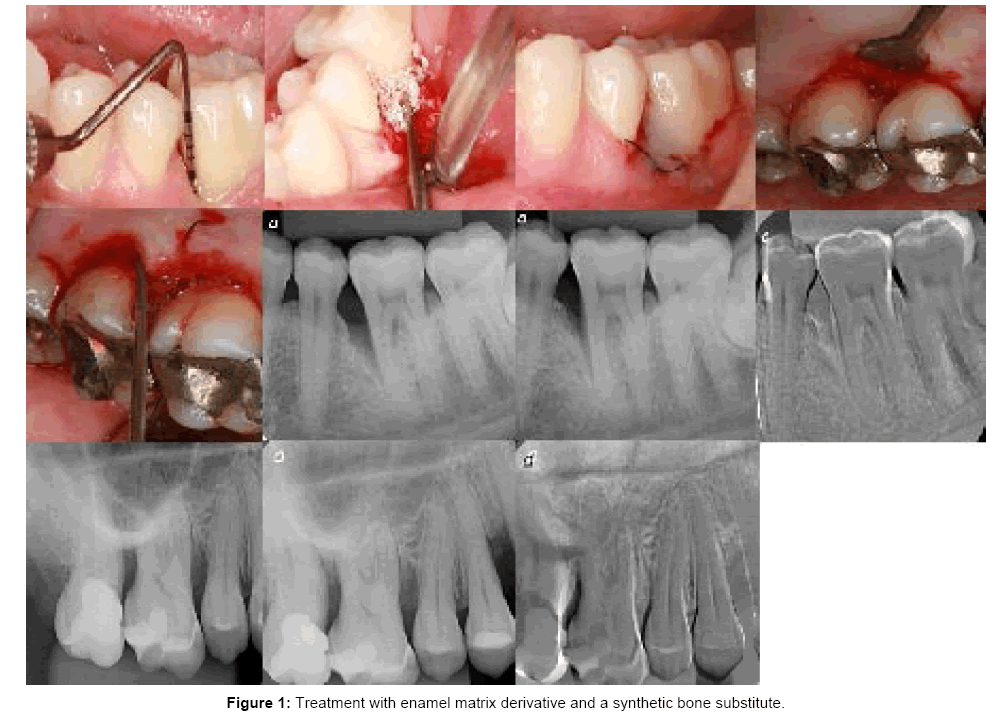
This case series report was conducted according to the principles outlined in the Declaration of Helsinki (1975, as revised in 2000) on experimentation involving human subjects. The present study was performed at the University of São Paulo, School of Dentistry of Ribeirão Preto, São Paulo, Brazil, between June 2010 and December 2013. Nine patients aged 22 to 53 were selected from those requiring periodontal treatment at the Department of Periodontology. The entry Inclusion and exclusion criteria were: non-compromised systemic health and no contraindications for periodontal surgery; any previous periodontal surgical treatment on the involved sites; non-smokers, nonpregnants and each patient had at least 2 intrabony periodontal defects as detected on radiographs and clinical findings. At the screening visit, patients were enrolled after satisfying admission criteria, completing a medical history, and providing informed consent. They had completed full-mouth scaling and root planing and home care instructions prior to surgical therapy. A total of 21 intrabony defects with a probing depth of at least 5 mm were included in this study. The following clinical parameters were assessed at the moment and at 1 year after the surgical procedure using the same periodontal probe (UNC 15, Hu-Friedy, Chicago IL, USA): plaque index (PI), bleeding on probing (BOP) and pocket depth (PD). The cemento-enamel junction (CEJ) was used as the reference point (Figure 1).
Surgical Procedure
Following local anesthesia, intracrevicular incisions and fullthickness (mucoperiosteal) buccal and lingual access flaps were raised. Vertical releasing incisions were performed if deemed necessary for a better access to the surgical site or to achieve better closure. All granulation tissue was removed from the defects and the roots were thoroughly scaled and planed by means of hand and ultrasonic instruments. Granulation tissue adherent to the alveolar bone of the associated intrabony defects was removed to provide full access and visibility to the root surfaces. After defect debridement, in both groups, the root surfaces adjacent to the defects were conditioned for 2 minutes with ETDA gel (Straumann PrefGel™, Straumann, Basel, Switzerland) to remove the smear layer.
In control sites, EMD (Straumann™ Emdogain, Straumann, Basel, Switzerland) was immediately applied to the exposed root surface, starting at the most apical portion of the defect and covering the entire root surface. In the test group, the defects were additionally filled up with the mixture of EMD+BC (Straumann™ BoneCeramic, Straumann, Basel, Switzerland). During surgery, the following measurement was made: distance from the CEJ to the bottom of the defect. Finally, the mucoperiosteal flaps were replaced and sutured appropriately with 5-0 monofilament vertical or horizontal mattress sutures. Both test and control sites were treated during the same surgical session and all surgical procedures were performed by one and the same surgeon.
All patients received antibiotics for 1 week (3×500 mg amoxicillin per day) starting 1 h preoperatively. In the first 4 weeks post-surgery, the patients were instructed to rinse twice daily with chlorhexidine 0.2% solution and to use modified oral hygiene procedures, avoiding brushing and flossing. Sutures were removed 14 days after the surgery. Recall appointments in which subjects received hygiene instructions and professional prophylaxis were scheduled weekly during the first month after surgery and every 2 months following the rest of the observation period of one year. At baseline, probing pocket depth (PPD) and standardized radiographic were taken, and the same parameters were also registered at 1 year post-surgery. All clinical measurements were taken with a periodontal calibrated probe by the same operator (P.G.F).
Statistical Analyses
The statistical analysis (using statistical software Bioestat version 5.3) was performed to evaluate the long-term benefits of the therapy. For the statistical evaluation of the changes from baseline to 1 year, the paired t test was used. There was a critical level of significance and Pearson's correlation (p<0.01).
Results
Only adverse events were minor and limited to usual postoperative discomfort, swelling and pain during the days following surgery phase. None of the patients presented any adverse reaction against the regenerative materials used.
There were no differences in terms of defect distribution and configuration between the groups. The baseline defects characteristics are presented in Table 1. No statistically significant difference in the initial depth of the intrabony component was found between the groups.
| EMD + BC (n=7) | EMD (n=11) | BC (n=3) | |
|---|---|---|---|
| Maxilla | 2 | 7 | 1 |
| Mandible | 5 | 4 | 2 |
Table 1: Distribution of the treated defects.
Mean PD reduction (Tables 2 and 3) demonstrated statistically significant improvements in both groups at 1 year compared to the baseline (P<0.01). At 1 year PD reduction ≥ 2mm was measured in 57% of the defects (i.e., in 4 of the 7) in the EMD + BC group; in 36% of the defects (i.e., 4 of the 11) in the EMD group. EMD + BC (7.17 ± 1.46) and EMD (5.91 ± 0.94).
| Group | PD initial | PD final | p intragroup | p intergroup |
|---|---|---|---|---|
| EMD + BC | 7.17 ± 1.46 | 5.17 ± 1.34 | 0.001* | 0.05* |
| EMD | 5.91 ± 0.94 | 1.27 ± 2.00 | 0.017* | 0.33 |
*Statistically significant difference between the groups (p<0.05).
EMD: Enamel Matrix Derivative; BC: Bone Ceramic; PD: Probing Depth.
Table 2: Mean + SD (mm) of the clinical parameters initially and after 12 months for the three groups.
| Groups | Δ | p |
|---|---|---|
| EMD + BC | 2.00 ± 1.15 | 0.04* |
| EMD | 1.27 ± 2.00 | 0.15 |
Table 3: Mean, standard deviations (mm) and statistical correlation for the differences between initial measurements and 12 months.
Table 4 presents the comparison of radiographic subtraction within the groups. After 1 year the mean were 124.00 ± 28.85 for EMD and 125.30 ± 25.20 for EMD + BC (P>0.32) there were no statistical difference between the groups.
| Groups | Subtraction mean |
|---|---|
| EMD | 124.00 ± 28.85 |
| EMD + BC | 125.30 ± 25.20 |
| p | 0.32 |
Statistically significant difference between the groups (p <0.05).
EMD: Enamel Matrix Derivative; BC: Bone Ceramic.
Table 4: Mean ± standard deviation of radiographic subtraction in pixels.
Discussion
The results of this study have shown that treatment of intrabony defects with both, a combination of EMD+BC and EMD alone may lead to statistically significant PD reductions, which can be maintained over a period of 1 year. However, the statistical analysis has failed to reveal significant differences between the two treatment modalities in any of the investigated clinical parameters at 1 year. Compared to baseline, at 1 year, a maxim PD reduction of 4 mm was measured in the defects of the test group and 6 mm of the defects in the control group. The test group has shown a better distribution of PD reduction, 4 of 7 (57%) defects presented a PD reduction ≥ 2mm. In the control group only 36% (4 of 11 defects) had the reduction.
It should, however, be emphasized that the study does not have the statistical power to rule out the possibility of a difference between the two groups. Further studies, with a higher number of patients and defects would be needed to detect an eventual difference between the treatments [16]. Furthermore, the present results are in line with findings from previous controlled clinical studies which have failed to show significant differences in the clinical outcomes following regenerative surgery using EMD alone or combined with different alloplastic materials such as bioactive glass, beta tricalcium phosphate graft [17,18].
Those results are even support by a recent meta-analysis of studies comparing the effect of enamel matrix derivatives when used alone or in combination with bone grafts and/or membranes in the treatment of intrabony periodontal defects Twenty-eight randomized controlled trials (RCTs) were included in the review. For study inclusion, the patients (or the periodontal defects) had to be randomly assigned to the test or control group and there had to have been follow-up for a minimum of 6 months. As result the analysis shows that adding membranes and/or bone grafts to the EMDs does not provide significant benefits in the treatment of periodontal defects [19].
In a recent multicenter, randomized, controlled clinical study comparing treatment with EMD+BCP (test) to EMD alone (control), the obtained mean CAL gain measured at 1 year after therapy 1.7±2.1 mm in the test and 1.9±1.7 mm in the control group, respectively. To close to the results shown in our case of series. These slight differences may, on one hand, be related to differences in the initial depth of the defects and, on the other, to differences in defect configuration. It is well documented that in deeper defects, a greater Cal gain may be achieved [18]. In the present study, baseline PD was 7.17±1.46 mm in the test group and 7.0± 2.11mm in the control group, while in the study referred to, the corresponding values measured 6.9±1.8 mm and 7.1± 1.5 mm, respectively [11].
Other key factors, which have been shown to profoundly affect wound healing following conventional and regenerative periodontal surgery, are infection control and smoking [20,21]. The pivotal role of careful patient selection and strict maintenance program is further supported by the present findings where the patient population did not include any smokers, while in groups, the plaque and bleeding values remained unchanged throughout the entire observation period of 12 months.
Despite the absence of statistical differences in clinical parameters, when comparing the two treatment, it needs to be mentioned that findings from preclinical that seems to favor the use of bone substitutes together with the enamel matrix proteins. In a histomorphometric and immunohistochemical study in canine, immunohistochemical evaluation showed that the defects treated with a combination of Emdogain and Bone Ceramic had the most intense staining, indicating more extracellular osteopontin (OPN) expression in these defects in comparison with the other treatments.
OPN is a noncollagenous phosphorylated acidic glycoprotein that resides in the extracellular matrix of mineralized tissues and is produced by osteoblasts, osteoclasts, osteocytes, preosteoblasts, some bone marrow cells, and many nonbone cells. It has been shown that OPN can bond to HA and calcium ions with its arginine-glycine-aspartate sequence. OPN acts as an important factor in bone remodeling, wound repair, angiogenesis, cell survival, immune function, and several pathophysiological processes. In mineralized tissue, OPN is secreted by both osteoblasts and osteoclasts, and its concentration in areas of newly formed bone should be increased. Some previous immunohistochemical studies have reported a progressive increase, either in OPN detected in maturing membranous bone matrix or in OPN expression by preosteoblasts and osteoblasts in developing mandibular bone [22].
When interpreting the present results, it should be pointed to the results from controlled clinical studies comparing various combination protocols of EMD with others biomaterials, to EMD alone and have shown higher clinical improvements following the combination approach. On the other hand, in most other studies where EMD was combined with different alloplastic grafts, no additional benefits were detected [7,23,24]. Thus, all these findings appear to suggest that the grafting material itself may also influence the healing process and, subsequently, the clinical outcomes. However, further research is necessary to elucidate the exact biological mechanisms behind these combinations and the use of the proposed regenerative strategy resulted in large amounts of clinical probing depth reduction at 1 year in all of the treated population. It is, therefore, possible to suggest to clinicians wishing to optimize clinical outcomes of periodontal regeneration in intrabony defects to incorporate the operative strategies described in this study in their clinical conduct.
Most authors found no statistically significant difference when evaluating the initial and final radiographs. The works cited are linear measurements on radiographs, our study made the subtraction radiography through Emago® (2015 Oral Diagnostic Systems) [25-27]. Although there is no statistically significant difference between EMD and EMD more BC groups, our study showed that the group where the particulate material was used showed higher radiopacity, suggesting that the biomaterial has filling capacity of intraosseous defects.
Conclusion
In conclusion, results from the present cases series confirm that a regenerative procedure based on EMD plus Bone Ceramic had better results in defect fill, although there was a variation in the numbers of defects.
Acknowledgements
We appreciate the support of the University of São Paulo, Ribeirão Preto, School of Dentistry, the University Center North Paulista - Sao Jose do Rio Preto - SP, Brazil and UNIPOS - Post graduate and continuing education of Sao Jose do Rio Preto / SP.
References
- Wang HL, Greenwell H, Fiorellini J, Giannobile W, Offenbacher S, et al. (2005) Periodontal regeneration. J Periodontol 76: 1601-1622.
- Nyman S, Gottlow J, Karring T, Lindhe J (1982) The regenerative potential of the periodontal ligament. An experimental study in the monkey. J ClinPeriodontol 9: 257-265.
- Gottlow J, Nyman S, Karring T, Lindhe J (1984) New attachment formation as the result of controlled tissue regeneration. J ClinPeriodontol 11: 494-503.
- Cortellini P, Bowers GM (1995) Periodontal regeneration of intrabony defects: an evidence-based treatment approach. Int J Periodontics Restorative Dent 15: 128-145.
- Lekovic V, Camargo PM, Weinlaender M, Nedic M, Aleksic Z, et al. (2000) A comparison between enamel matrix proteins used alone or in combination with bovine porous bone mineral in the treatment of intrabony periodontal defects in humans. J Periodontol 71: 1110-1116.
- Hammarström L (1997) Enamel matrix, cementum development and regeneration. J ClinPeriodontol 24: 658-668.
- Zucchelli G, Bernardi F, Montebugnoli L, De SM (2002) Enamel matrix proteins and guided tissue regeneration with titanium-reinforced expanded polytetrafluoroethylene membranes in the treatment of infrabony defects: a comparative controlled clinical trial. J Periodontol73:3-12.
- Zucchelli G, Amore C, Montebugnoli L, De Sanctis M (2003) Enamel matrix proteins and bovine porous bone mineral in the treatment of intrabony defects: a comparative controlled clinical trial. J Periodontol74:1725-1735.
- Sculean A, Donos N, Blaes A, Lauermann M, Reich E, et al. (1999) Comparison of enamel matrix proteins and bioabsorbable membranes in the treatment of intrabony periodontal defects. A split-mouth study. J Periodontol 70: 255-262.
- Gestrelius S, Andersson C, Lidström D, Hammarström L, Somerman M (1997) In vitro studies on periodontal ligament cells and enamel matrix derivative. J ClinPeriodontol 24: 685-692.
- Gestrelius S, Andersson C, Johansson AC, Persson E, Brodin A, et al. (1997) Formulation of enamel matrix derivative for surface coating. Kinetics and cell colonization. J ClinPeriodontol 24: 678-684.
- Yukna RA, Mellonig JT (2000) Histologic evaluation of periodontal healing in humans following regenerative therapy with enamel matrix derivative. A 10-case series. J Periodontol 71: 752-759.
- Yilmaz S, Kuru B, Altuna-Kiraç E (2003) Enamel matrix proteins in the treatment of periodontal sites with horizontal type of bone loss. J ClinPeriodontol 30: 197-206.
- Sculean A, Windisch P, Chiantella GC, Donos N, Brecx M, et al. (2001) Treatment of intrabony defects with enamel matrix proteins and guided tissue regeneration. A prospective controlled clinical study. J ClinPeriodontol 28: 397-403.
- Windisch P, Sculean A, Klein F, Tóth V, Gera I, et al. (2002) Comparison of clinical, radiographic, and histometric measurements following treatment with guided tissue regeneration or enamel matrix proteins in human periodontal defects. J Periodontol73:409-417.
- Gunsolley JC, Elswick RK, Davenport JM (1998) Equivalence and superiority testing in regeneration clinical trials. J Periodontol 69: 521-527.
- Jepsen S, Topoll H, Rengers H, Heinz B, Teich M, et al. (2008) Clinical outcomes after treatment of intra-bony defects with an EMD/synthetic bone graft or EMD alone: a multicentre randomized-controlled clinical trial. J ClinPeriodontol 35: 420-428.
- Meyle J, Hoffmann T, Topoll H, Heinz B, Al-Machot E, et al. (2011) A multi-centre randomized controlled clinical trial on the treatment of intra-bony defects with enamel matrix derivatives/synthetic bone graft or enamel matrix derivatives alone: results after 12 months. J ClinPeriodontol 38: 652-660.
- Verardi S (2012) The use of a membrane and/or a bone graft may not improve the effects of enamel matrix derivatives in infrabony defects. J Evid Based Dent Pract12:127-128.
- Trombelli L, Kim CK, Zimmerman GJ, Wikesjö UM (1997) Retrospective analysis of factors related to clinical outcome of guided tissue regeneration procedures in intrabony defects. J ClinPeriodontol 24: 366-371.
- Tonetti MS, Prato GP, Cortellini P (1996) Factors affecting the healing response of intrabony defects following guided tissue regeneration and access flap surgery. J ClinPeriodontol 23: 548-556.
- Birang R, Abouei MS, Razavi SM, Zia P, Soolari A (2012) The effect of an enamel matrix derivative (Emdogain) combined with bone ceramic on bone formation in mandibular defects: a histomorphometric and immunohistochemical study in the canine. ScientificWorldJournal2012:196791.
- Zetterström O, Andersson C, Eriksson L, Fredriksson A, Friskopp J, et al. (1997) Clinical safety of enamel matrix derivative (EMDOGAIN) in the treatment of periodontal defects. J ClinPeriodontol 24: 697-704.
- Eickholz P, Röllke L, Schacher B, Wohlfeil M, Dannewitz B, et al. (2014) Enamel matrix derivative in propylene glycol alginate for treatment of infrabony defects with or without systemic doxycycline: 12- and 24-month results. J Periodontol 85: 669-675.
- Cury PR, Araujo NS, Bowie J, Sallum EA, Jeffcoat MK (2004) Comparison between subtraction radiography and conventional radiographic interpretation during long-term evaluation of periodontal therapy in class II furcation defects. J Periodontol75:1145-1149.
- Eickholz P, Krigar DM, Kim TS, Reitmeir P, Rawlinson A (2007) Stability of clinical and radiographic results after guided tissue regeneration in infrabony defects. J Periodontol 78: 37-46.
- Mardas N, D'Aiuto F, Mezzomo L, Arzoumanidi M, Donos N (2011) Radiographic alveolar bone changes following ridge preservation with two different biomaterials. Clin Oral Implants Res 22: 416-423.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 15958
- [From(publication date):
August-2015 - Aug 24, 2025] - Breakdown by view type
- HTML page views : 11197
- PDF downloads : 4761