Research Article Open Access
Physiologic Behavioural Changes of Precancerous Leukoplakia Cell lines Exerted to Extremely Low Frequency Electric Field
Ahmed Korraah1, Margarete Odenthal2, Marion Kopp3, Nadarajah Vigneswaran4, Peter G Sacks5, Hans Peter Dienes2, Hartmut Stützer6 and Wilhelm Niedermeier3*
1Department of Oral Pathology, Faculty of Dentistry, Suez Canal University, Egypt
2Institute for Pathology, University Hospital, Cologne, Germany
3Department of Prosthetic Dentistry, Dental School, University of Cologne, Germany
4Department of Diagnostic Sciences, School of Dentistry, University of Texas, USA
5Department of Basic Science and Craniofacial Biology, College of Dentistry, New York University, USA
6Institute for Medical Statistics, Informatics and Epidemiology, University of Cologne, Germany
- Corresponding Author:
- Wilhelm Niedermeier
Department of Prosthetic Dentistry
Dental School, University of Cologne
Kerpenerstr. 32, 50931 Cologne, Germany,
Tel: 0049-478-6337
Fax: 0049-478-5982
E-mail: wilhelm.niedermeier@uk-koeln.de
Received Date: November18, 2014, Accepted Date: December 26, 2014, Published Date: December 31, 2014
Citation:Ahmed Korraah, Margarete Odenthal, Marion Kopp, Nadarajah Vigneswaran, Peter G Sacks, et al. (2015) Physiologic Behavioural Changes of Precancerous Leukoplakia Cell lines Exerted to Extremely Low Frequency Electric Field. J Interdiscipl Med Dent Sci 3:165. doi: 10.4172/2376-032X.1000165
Copyright: © 2015 Korraah A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Objectives: Oral galvanism arising from the presence of two different metallic fillings in the mouth that may reach up to 16V/m, induces several changes in oral environment such as gingival swelling and erythema, mucosal pain, lichenoid reactions and leukoplakia.
Study design: To investigate in vitro the physiologic reactions of oral leukoplakia caused by electrostatic corrosion potentials, human leukoplakia cell lines (MSK-LEUK1), cultivated in KGM-2 supplemented with bullet kit in 5% CO2 humidified air at 37°C, were exposed to electric field strength of 1-20 V/m for 24 hours in a pseudo realistic apparatus called “Pulse chamber”. Following this, the cells were analysed for proliferation using BrdU assay, and for apoptosis using TUNEL assay. Findings were assessed utilizing fluorescent microscopy. Ultra structural changes were studied by TEM. Data was evaluated statistically using non parametric Chi-square test.
Results: Electric field strength of 1-10V/m led to upregulation of cell proliferation rate between 10.64% and 44.06% (p=0.0001). The apoptotic index increased significantly (p=0.0001) from 20.03% at 1V/m to 46.56% at 10V/m. Leukoplakia cells treated with 16V/m show individual cell keratinization.
Conclusion: Oral galvanism induces subcellular changes in oral precancerous leukoplakia cells in vitro that resemble some of the histopathologic features of oral squamous cell carcinoma cells in vivo.
Keywords
Oral galvanism (OG); Apoptosis; Pulse chamber; MSK-LEUK1 cell lines
Abbrevations
A=Ampere; Ac/Me=Acetone/Methanol fixative; AI=Apoptotic index; ANCC=Apoptotic negative control cells; ATP=Adenosine triphosphate; BrdU=5-Bromo-2´-deoxyuridine; BSA=Bovine Serum Antigen; C=Celsius; Ca2+=Calcium ions; CO2=Carbon dioxide, dm=decimetre; DAPI=4',6-diamidino-2-phenylindole; DC=Direct current; DCV=Direct Current Voltage; DNA=Desoxyribonucleic acid; EDTA=Ethylenediaminetetraacetic acid; EF=Electric Field; EFS=Electric field strength; EFSC=Electric field stimulated cells; ELSC=Electrode stimulated cells; EMF=Electromagnetic Field; FBS=Foetal bovine serum; H2O2= Hydrogen peroxide; HUH-7=Hepatoma cell lines; Ig=Immunoglobulin; KGM-2=Keratinocytes growth medium-2; ml=millilitre; mS/m=millisiemens/meter; mV=millivolt; MSK-LEUK1=Leukoplakia cell lines; n=number of carried out experiments; Nm=nanometer; NADPH=Nicotinamide adenine dinucleotide hydrogen; OG=Oral galvanism; OPM=Oral premalignant; OSCC=Oral squamous cell carcinoma; pH=Power of hydrogen; PBS=Phosphate buffered saline; PCC=Positive Control Cells; PFA=Paraformaldehyde; PI=Proliferation index; PNCC=Proliferating negative control cells; Redox=reduction- Oxidation; Rpm=revolution per minute; ROS=Reactive oxygen species; S=seconds; T1N0M0=Tumor stage 1, no lymph node involvement and no Metastasis; TMR=Tetramethylrhodamine; TUNEL=Terminal uridine deoxy nucleotidyl transferase dUTP nick end labelling; V/m=Volt per meter
Introduction
In dental fields, gold, cobalt, palladium, silver, chromium, copper, zinc, tin and nickel are used. When there are two or more different dental metallic restorations in the presence of saliva, an electrical field is induced that is called oral galvanism (OG), in which saliva plays the role of the conducting solution (the electrolyte) as it contains inorganic ions as a major component [1]. The generated electric field may reach 950 mV between an aluminium splint and a gold crown [2].
Oral Leukoplakia is defined clinically in 1968 as a white patch, not less than 5 mm in diameter, which cannot be rubbed off with a blunt instrument and which cannot be classified as any other diagnosable disease [3]. It appears clinically white but histopathologically varies between simple hyperkeratosis to severe epithelial dysplasia or carcinoma in situ. Recently, Warnakulasuriya defined oral leukoplakia as white plaques of questionable risk having excluded (other) known diseases or disorders that carry no increased risk for cancer [4]. It is one of the most frequent types of oral precancerous lesions and shows a variable rate of malignant transformation to oral squamous cell carcinoma (OSCC) [5]. Pindborg mentioned that oral squamous cell carcinoma (OSCC) is frequently preceded by oral leukoplakia, which has a malignant transformation rate between 0.13-6% [6,7]. OSCC genesis passes through many genetic and non-genetic steps that have two clinical forms: (1) oral premalignant (OPM) lesion, which will transform into (2) OSCC [8,9]. OPM lesions usually appear either white (leukoplakia) or red (erythroplakia). Leukoplakia represents 85% of such lesions and has a prevalence rate of 2–8% in people over the age of 70 [10]. The basis of our approach was that oral squamous carcinoma is very frequently preceded by a precancerous and that established squamous carcinomas of the mouth are invariably surrounded by leukoplakia, erythroplakia or both lesions [6].
There has been much controversy regarding whether oral galvanism (OG) really plays a role in the development of oral cancer, lichen planus-like lesions, and various other oral diseases. Previous studies indicate that oral galvanism could lead to the development of oral leukoplakia and the removal of the metallic restorations resulted in the resolution of these lesions [11-13]. In addition, a remarkable recovery of diseases of the oral mucosa was noted when harmful potentials were eliminated [14-16].
In the wake of the pioneering work of Kerr, the importance of cell loss through apoptosis (active cell death) and accidental cell death (passive cell death) and the role of apoptosis in tumorigenesis has been increasingly recognized [17]. Although apoptosis is a naturally occurring cellular event that helps maintain organism homeostasis, its role in the field of oncology, especially in tumorigenesis erupts recently and current researches are now focused on the role of the unbalance between cell proliferation and cell apoptosis in tumorigenesis [17,18].
In the present study, we examined the physiologic behaviour of oral precancerous leukoplakia cell lines regarding its proliferative and apoptotic activities when exerted to extremely low frequency electric field treatment.
Material and Methods
Cell lines
Leukoplakia cell lines (MSK-LEUK1) derived from dysplastic leukoplakia adjacent to an early invasive OSCC (T1N0M0) involving the tongue of a 47-year-old female was used in our research. These cells showed loss of response to calcium and have no terminal differentiation (characteristics of premalignant keratinocytes) [7,19].
A serum-free keratinocyte growth medium (KGM-2) supplemented with growth factors (Bullet kit; Cat#CC-3103; Lot#01119272; LONZA Walkersville, Inc, Chicago, IL, USA) was used for the growth of MSK-LEUK1 cells.It was changed every 2 days and the cells were trypsinized every week with a sequential method. Trypsin 2.5%/ EDTA 0.03% was used for trypsinization. The cells were plated onto 6cm cell culture Petri dishes. Experiments were performed with sub-confluent cell cultures. All cell lines were incubated under standard conditions with 5% CO2 at 37°C in a humidified atmosphere.
Enzymatic dissociation of cell lines with trypsin/EDTA (Sequential trypsinization)
The adherent growing cells were enzymatically dissociated using 37°C warm trypsin/EDTA.
First, the trypsin/EDTA mixture was heated in water bath at 37°C. After the cell culture medium was removed from the monolayer, the cells were briefly washed with 5 ml PBS to remove any static medium radicals, which can affect the action of trypsin. The cells then incubated in a 5 ml trypsin/EDTA mixture for 5 minutes in an incubator. After this period, the majority of the cells resolved from the surface of the monolayer. A stopping medium was prepared in a 25 ml tube. It consists of 4 ml KGM-2 and 1 ml of foetal bovine serum. The detached cell suspension was transported into the stopping medium. The still attached cells were then incubated for another 5 minutes with Trypsin/EDTA. This step was repeated until all the monolayers were detached. The Trypsin/EDTA cell suspension was centrifuged with 700 rpm for 5 minutes in 15°C. The supernatant was discarded and 2 ml of KGM-2 was added. Lastly, the cell suspension was distributed evenly in Petri dishes and incubated.
In order to have several vials of MSK-LEUK1 cells, vials of cells are frozen under -70°C in liquid nitrogen. Thawing of cells should be in water bath in 37°C till the water crystals disappear.
Cell cultures treatment with electrical field (pulse chamber)
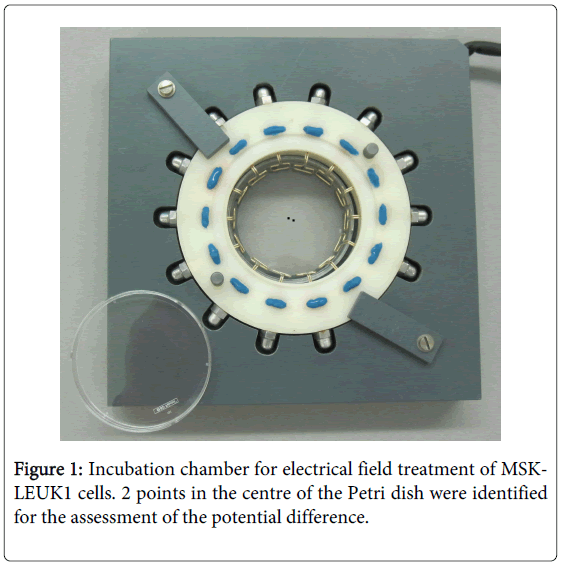
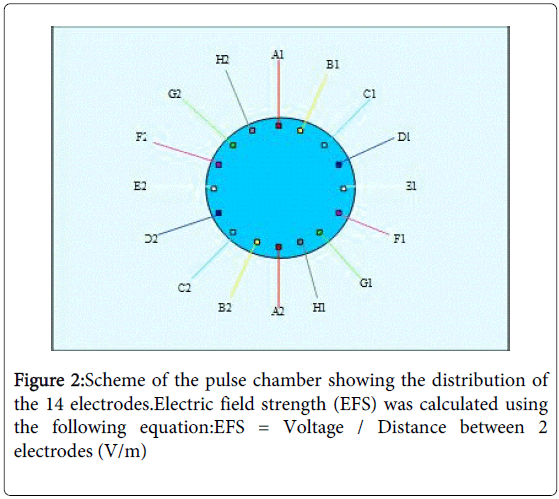
Petri dishes with sub-confluent cell cultures were transferred to a custom-made electropulse apparatus (Figures 1 and 2), which allowed rotating electrical fields to avoid decomposition and pH gradients in the cell culture medium and polarization of cells [20]. The pulse chamber setup consisted of 14 electrodes formed with an alloy called “BiOcclus 4” (DeguDent GmbH, P.O.Box 1364, D-63403 Hanau, Germany) which consists of gold and platinum. The distance between each two opposite electrodes was 5 cm and they were immersed at the border of the Petri dish into the cell culture medium, which has a specific conductivity of 15.75 mS/m. The electrodes were connected to a direct current supply, which generated direct current (DC) voltage pulses with duration of 0.9s. After an interval of 0.1s, the voltage supply automatically coupled to the next electrode pair to initiate the subsequent voltage pulse. During the experiments voltage pulses ranging from 50 to 1000 mV were applied, which resulted in electrical field strength (EFS) of 1 to 20V/m respectively. Before and during each experiment, it was assured that the EFS was in agreement with the input DC voltage in the centre of the Petri dish.The exposure device was placed in an incubator with 5% CO2 at 37oC in a humidified atmosphere. The temperature, osmolarity and pH of the cell cultures treated with electrical fields were controlled before, during and after the experiments. For controls, MSK-LEUK1 cells were treated in a comparable manner in a second pulse chamber that contained electrodes immersed into the culture medium but were not connected to a voltage supply. Both the experimental and the control pulse chambers were placed in the same incubator during the experiments. The MSK-LEUK1 cells were prepared before the experiments by allowing them to grow on 1 cm in diameter cover slips that were placed in the centre of the cell culture dish (the area of optimum field homogeneity). The confluence of cells on the cover slip must be 50%.
Experimental conditions were prepared in such a way as to avoid undesirable effects that might interfere with the specificity and the sensitivity of results. For example, effects that might arise from corrosion of electrodes, decomposition of cell culture medium and change of temperature and pH were all controlled in preliminary experiments (data not shown).
The voltage generated from electrodes without any connection with DC supply
In order to get accurate results through the avoidance of external voltages, a preliminary experiment was conducted to measure the voltage generated from the 14 electrodes when they were not connected to the DC supply. Electrodes were placed into a 6 cm Petri dish containing 4 ml KGM-2. To assess field homogeneity from the site where cells were taken for analysis, the potential difference between two points in the middle of the Petri dish with a distance of 5 mm (Figure 1) was measured every hour for 24 hours using a sensitive voltmeter (KEITHLEY, 2000 Multimeter, ID-NR:20009867, Cleveland, Ohio, USA).
Determination of the best fixative for 5-Bromo-2´-deoxyuridine (BrdU) assay:
Many fixatives (ethanol, paraformaldehyde and acetone/methanol) were used in BrdU assay. In order to determine the best fixative which gives the best results, BrdU assay was done on hepatoma cell lines (HUH-7) exerted to 200 mV for 24 hours. The experiment was done 3 times and each time the fixation of the cells was done using a different fixative. Each experiment was repeated 2 times (n=2). A negative control and cells exerted to electrodes without EMF were included in the experiment.
5-Bromo-2´-deoxyuridine (BrdU) labeling and detection kit I for the proliferation assessment
In order to study the proliferation rate of MSK-LEUK1 cells, BrdU labeling and detection kit I (cat#11296736001; Lot#13410600; ROCHE, Basel, Switzerland) was chosen. BrdU detection kit can detect the entrance of cells in the cell cycle through the detection of DNA replication, and this is by the detection of the uptake of a synthetic labelled nucleotide called 5-bromo-2´-deoxy-uridine by the cellular DNA.
EFS of 1 to 20V/m was applied on MSK-LEUK1 cells, each for 24 hours. Afterwards, cells were incubated with BrdU labeling medium for 1 hour at 37°C in a humidified atmosphere and fixed with ethanol for 20 minutes at -20°C. Cells were covered with anti-BrdU working solution for 30 minutes at 37°C, then with anti-mouse-Ig-fluorescein working solution for 30 minutes (37°C). The counter stain was 4',6-diamidino-2-phenylindole (DAPI) for 1 minute (DAPI enters readily in living cells, binds to DNA and appears blue with fluorescence microscopy). Plates were rinsed three times between each step with washing buffer. Cover slips were mounted on glass slides and examined with a fluorescence microscope with an excitation wavelength in the range of 450-500 nm; the detection was in the range of 515-565 nm (green). Each experiment was performed using the same generation of MSK-LEUK1 cells, i.e. three Petri dishes were prepared from the same passage of MSK-LEUK1 cells:
a) EF- stimulated MSK-LEUK1 cells (EFSC), b) cells that were exposed to electrodes without EFS (electrode-stimulated cells) (ELSC) and c) proliferating negative control cells (PNCC) (cells that were subjected neither to EFS nor to electrodes).
Results were evaluated by calculating the proliferation index (PI) of cells. PI was calculated using the equation:
PI = (Number of proliferating cells / Total number of cells) X 100 (%)
Proliferating MSK-LEUK1 cells were counted by certifying each green (proliferating) cell with the light aperture of the fluorescence microscope to identify normal cells. The experiment was done 2 times (n=2).
Although all reserves were taken to standardize the conditions of experiments, the problem of different generations of MSK-LEUK1 cells still evolved. Every generation had its own proliferation activity which differs from a cell passage to the other i.e. the proliferation activity of the 6th passage differs from that of 5th passage. Therefore, to overcome this, we calculated the corrected mean of PI as follows:
Corrected mean= value of experiment of EFSC X factor (PNCC).
Factor (PNCC) = value of experiment of PNCC /mean of all experiments of PNCC.
Apoptosis detection using Terminal deoxynucleotidyltransferase-mediated dUTP nick end labeling (TUNEL) immunohistochemistry
The apoptotic rate of MSK-LEUK1 cells was studied by using the in situ cell death detection kit, TMR red (cat#12156792910; Lot#13754100; Roche, Basel, Switzerland). This technique relies on the use of exogenous enzyme called terminal deoxy-nucleotidyltransferase (TdT) to incorporate labelled nucleotides into the 3’-hydroxyl (3’OH) recessed termini of DNA breaks that distinguish apoptosis from accidental cell death.
EFS of 1 to 20V/m were applied on MSK-LEUK1 cells, each for 24 hours. Afterwards cells were fixed with paraformaldehyde 4% in PBS (pH 7.4) for 1 hour at 15 to 25°C and incubated on ice with permeabilization solution (0.1% triton X-100 in 0.1% sodium citrate) for 2 minutes. Cells were then covered with 50µl TUNEL reaction mixture (mixture between enzyme solution and labeling solution in a ratio of 1:9) for 1 hour at 37°C in the dark. The counter stain was DAPI for 1 minute. Phosphate buffer solution (PBS) was used to wash the cells 3 times between each step. Cover slips were mounted on glass slides. TMR red labeled nucleotides, incorporated in nucleotide polymers, were detected and quantified by fluorescence microscopy with an excitation wavelength in the range of 520-560 nm; the detection was in the range of 570-620nm (maximum 580nm, red). Each experiment was performed using the same generation of MSK-LEUK1 cells, i.e. four Petri dishes were prepared from the same passage of MSK-LEUK1 cells:
a) EFSC, b) ELSC, c) positive control (PCC) (treatment of fixed and permeabilized MSK-LEUK1 cells with DNase I recombinant (3000 U/ml– 3 U/ml in 50 mM Tris-HCl, pH 7.5, 1 mg/ml BSA) for 10 min at 15 to 25°C to induce DNA strand breaks, prior to labeling procedures, and d) apoptotic negative control cells (ANCC) (treatment of fixed and permeabilized MSK-LEUK1 cells in 50 µl /cover slip in labeling solution without terminal transferase instead of TUNEL reaction mixture).
Results were evaluated by calculating the apoptotic index (AI) of cells. AI was calculated using the equation:
AI = Number of apoptotic cells / Total number of cells) X 100 (%)
Apoptotic MSK-LEUK1 cells were counted by certifying each red (apoptotic) cell with the light aperture of the fluorescence microscope to identify normal cells. The experiment was done 2 times (n=2).
Although all reserves were taken to standardize conditions of experiments, the problem of different generations of MSK-LEUK1 cells still evolved. Every generation had its own apoptotic activity. Therefore, to overcome this, we calculated the corrected mean of PI as follows:
Corrected mean= value of experiment of EFSC X factor (ANCC).
Factor (ANCC) = value of experiment of ANCC /mean of all experiments of ANCC.
Transmission electron microscopy (TEM)
For the determination of ultrastructural changes that occur to MSK-LEUK1 cells during apoptosis, three experiments were conducted. The exposure of cells to 12V/m, 16V/m and 20V/m, each for 24 hours. A negative control was also performed through the non-exposure of MSK-LEUK1 cells to any DCV. MSK-LEUK1 cells were trypsinized, centrifuged for 5 minutes, fixed using gluteraldehyde 2.5% overnight, and then washed. Next, osmium acid was applied for 2 hours and cells were dehydrated using ascending concentrations of ethanol. The soft araldite was poured in gelatin capsules and capsules were turned upon cells until the araldite hardened. After 48 hours, blocks were ultrasectioned and sections were placed on cupper grids to be examined with TEM.
Statistical Methods
Laboratory protocols were registered and analyzed in the data module of the Statistical Package for Social Science (SPSS-16) for Windows. The chi-square test was used to ascertain differences between results obtained from the effect of applied EFS on MSK-LEUK1 cells and those obtained without EFS. A value of P<0.05 was considered significant.
Results
Measurement of the voltage evolving from electrodes without any connection with DC supply. The voltage generated by the electrodes in culture medium, but without any connection to a DC supply was 41.47 mV ± 11.31 (n=2) (Table 1).
| Time | 1st exp. Voltage(mV) | 2nd exp. Voltage(mV) | Mean (mV) | Mean (V/m) |
|---|---|---|---|---|
| 1h | 37 | 53 | 45 | 0.9 |
| 2h | 37 | 46 | 41.5 | 0.83 |
| 3h | 37 | 29 | 33 | 0.66 |
| 4h | 29 | 53 | 41 | 0.82 |
| 5h | 32 | 60 | 46 | 0.92 |
| 6h | 37 | 59 | 48 | 0.96 |
| 7h | 39 | 23 | 31 | 0.62 |
| 8h | 39 | 19 | 29 | 0.58 |
| 9h | 40 | 13 | 26.5 | 0.53 |
| 19h | 50 | 39 | 44.5 | 0.89 |
| 20h | 45 | 23 | 34 | 0.68 |
| 21h | 35 | 28 | 31.5 | 0.63 |
| 22h | 52 | 88 | 70 | 1.4 |
| 23h | 45 | 61 | 53 | 1.1 |
| 24h | 53 | 43 | 48 | 0.96 |
| Mean | 40.47 | 42.47 | 41.47 | 0.83 |
Table 1: Potential difference between 2 points in the KGM-2 medium.These are the potential differences between 2 identified points in the middle of the petri dish. The measurements were taken every hour for 24 hours (n=2).
Determination of the best fixative for BrDU assay
With the usage of ethanol fixative, the mean of PI of HUH-7 cells was approximately 27%. In case of paraformaldehyde (PFA), the mean of PI of HUH-7 cells was approximately 33%, while it was 34% with the usage of acetone/methanol fixative (Ac/Me) (Table 2).
| Ethanol | PFA | Ac/Me | |
|---|---|---|---|
| PI (1) | 26.73% | 32.87% | 32.54% |
| PI (2) | 28.48% | 34.40% | 35% |
Table 2: BrdU assay for HUH-7 cells with different fixatives (PI: the percentage of the proliferation rate of HUH-7 cells; PFA:paraformaldehyde fixative; Ac/Me: acetone/methanol fixative).
From the above results, it was found that paraformaldehyde and Ac/Me were the best fixatives for BrDU assay. But ethanol was used as a fixative in the experiments because it gave best results with negative control and with cells exerted to electrodes without EMF.
Oral mucosa leukoplakia cells proliferation after electrical field treatment
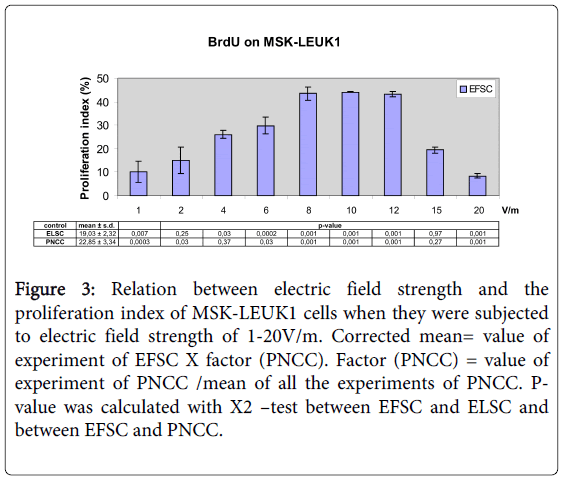
Electrical field treatment (1V/m to 10V/m) of MSK-LEUK1 cells led to an increase of the proliferation index (PI) from 10.64% to 44.06%; PI was 43.48% for 12V/m, 19.39% for 15V/m and 8.44% for 20V/m (Figures 3 and 4). For ELSC, PI varied between 14.16% and 22.54% whereas the cumulative mean was 19.03% (Figure 3). PI of PNCC was between 16.56% and 27.26% and its cumulative mean was 22.85% (Figure 3) (Table 3).
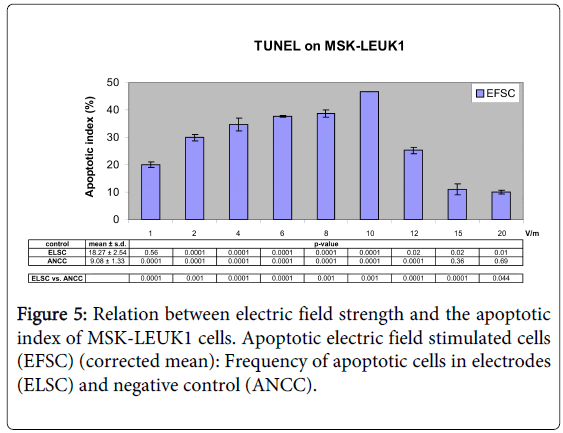
Figure 3: Relation between electric field strength and the proliferation index of MSK-LEUK1 cells when they were subjected to electric field strength of 1-20V/m. Corrected mean= value of experiment of EFSC X factor (PNCC). Factor (PNCC) = value of experiment of PNCC /mean of all the experiments of PNCC. Pvalue was calculated with X2 –test between EFSC and ELSC and between EFSC and PNCC.
| EFS (V/m) | EP (mV) | PI (EFSC) (%) | ||
|---|---|---|---|---|
| 1st exp. | 2nd exp. | C. mean | ||
| 1 | 50 | 6.57 | 12.95 | 10.64 ± 4.51 |
| 2 | 100 | 25.49 | 17.47 | 15.47 ± 5.67 |
| 4 | 200 | 28.79 | 26.31 | 25.90 ± 1.75 |
| 6 | 300 | 28.78 | 33.9 | 30.40 ± 3.62 |
| 8 | 400 | 34.17 | 38.06 | 42.98 ± 2.75 |
| 10 | 500 | 39.29 | 39.38 | 44.06 ± 0.06 |
| 12 | 600 | 45.58 | 44.07 | 43.48 ± 1.07 |
| 15 | 750 | 17.93 | 16.28 | 19.16 ± 1.17 |
| 20 | 1000 | 9.09 | 10.35 | 8.46 ± 0.89 |
Table 3: BrdU assay after the application of different EFS on MSKLEUK1 (EFS: Electric field strength (V/m) acting on MSK-LEUK1 cells; EP: Electric potential (mV); PI (EFSC): Proliferation index in EFstimulated cells; 1st exp.: PI of the first experiment; 2nd exp.: PI of the second experiment; C. mean: Corrected mean ± standard deviation).
Upregulation of apoptosis of oral mucosa leukoplakia cells by electrical field treatment
Electrical field treatment (1V/m to 10V/m) of MSK-LEUK1 cells increased the apoptotic index (AI) from 20.03% to 46.56%; AI was 25.19% for 12V/m, 11.01% for 15V/m and 9.94% for 20 V/m (Figures 5 and 6). AI of ELSC ranged between 13.7% and 20.39% whereas its cumulative mean was 18.27% (Figure 5). AI of ANCC ranged between 6.3% and 10.47% and its cumulative mean was 9.08% (Figure 5). AI of PCC varied between 60.51% and 74.36% (Table 4).
| EFS (V/m) | EP (mV) | AI (EFSC) (%) | ||
|---|---|---|---|---|
| 1st exp. | 2nd exp. | (C.mean) | ||
| 1 | 50 | 21.82 | 20.58 | 20.03 ± 0.88 |
| 2 | 100 | 28.77 | 27.29 | 29.85 ± 1.05 |
| 4 | 200 | 32.61 | 29.38 | 34.71 ± 2.28 |
| 6 | 300 | 33.88 | 34.34 | 37.75 ± 0.33 |
| 8 | 400 | 36.1 | 37.98 | 38.55 ± 1.33 |
| 10 | 500 | 40.37 | 40.39 | 46.56 ± 0.01 |
| 12 | 600 | 28.49 | 30.16 | 25.19 ± 1.18 |
| 15 | 750 | 17.23 | 14.52 | 11.01 ± 1.91 |
| 20 | 1000 | 10.19 | 9.35 | 9.94 ± 0.60 |
Table 4: TUNEL assay after the application of different EFS on MSKLEUK1 (EFS: Electric field strength (V/m) acting on MSK-LEUK1 cells; EP: Electric potential (mV); AI (EFSC): Apoptotic index in stimulated cells; C. mean: Corrected mean ± standard deviation (S.d.))
Transmission electron Microscopy
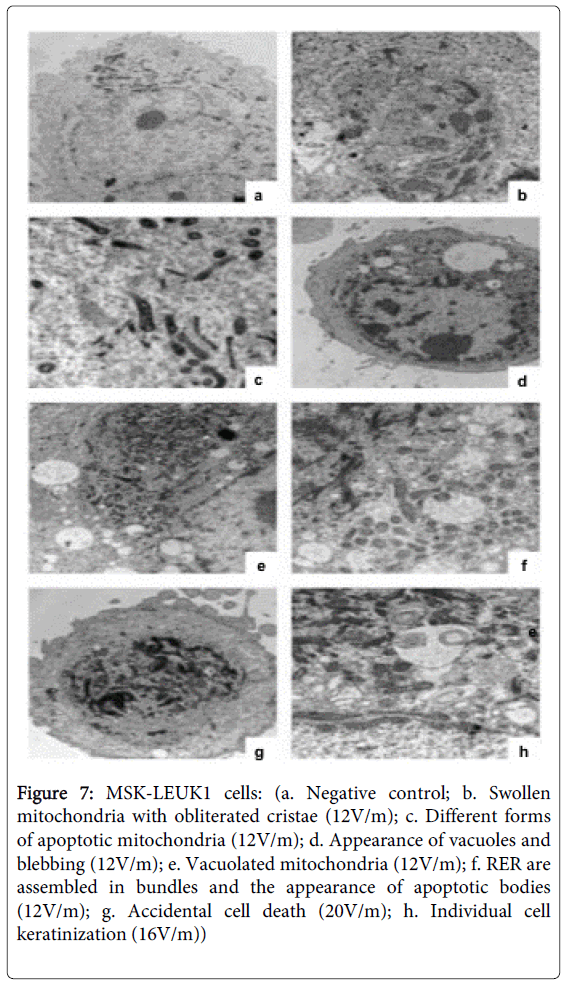
MSK-LEUK1 cells exposed to 12V/m, when compared with the negative control sample (Figure 7a), appeared abnormal in form with disturbed cytoskeleton. Mitochondria were numerous, denser and shrunk with swollen obliterated cristae (Figures 7b and 7c). Pseudopodia (blebbing) were observed on the cell membrane (Figure 7d). Abnormal forms of mitochondria could be seen, some of which were vacuolated (Figure 7e). The nucleus was pyknotic and denser with aggregation of chromatin on the nuclear membrane. The cytoplasm was condensed and vacuolated (Figure 7d) and contained apoptotic bodies (Figure 7f). The rough endoplasmic reticulum (RER) assembled and arranged in aberrant directions (Figure 7f). MSK-LEUK1 cells exposed to 16 and 20V/m shrank and showed a loss of cell membrane integrity, swelling of mitochondria, lysis of internal organelles and the appearance of many vacuoles and lysosomes (Figures 7g and 7h). The cytoplasm was not homogenous. No apoptotic bodies were seen. It should be noted that the percentage of accidental cell death features shown in MSK-LEUK1 cells was more in 20V/m than in 16V/m (Figure 7g). Individual cell keratinization was seen in MSK-LEUK1 cells exposed to 16V/m (Figure 7h).
Figure 7: MSK-LEUK1 cells: (a. Negative control; b. Swollen mitochondria with obliterated cristae (12V/m); c. Different forms of apoptotic mitochondria (12V/m); d. Appearance of vacuoles and blebbing (12V/m); e. Vacuolated mitochondria (12V/m); f. RER are assembled in bundles and the appearance of apoptotic bodies (12V/m); g. Accidental cell death (20V/m); h. Individual cell keratinization (16V/m))
Statistical analysis
For EFS of 1, 4, 6, 8, 10, 12 and 20V/m, the ratio between EFSC and ELSC was significantly increased (P=0.0001).
Chi-square test in apoptotic assay showed the following results:
• EF-stimulation of 1 to 12V/m increased significantly (P<0.05) the apoptotic EFSC/non-apoptotic ANCC ratio.
• The ratio between EFSC and ELSC significantly increased (P<0.05) for EFS 2 to 20V/m.
• The ratio between ELSC and ANCC significantly increased (P<0.05) for EFS 1 to 20V/m.
Discussion
The current study investigated effects of extremely low frequency electric field treatment on the physiologic behaviour of oral premalignant cells from the point of view of its proliferative and apoptotic activity.
It has been previously discussed that OG could lead to a change in taste, burning sensation in the oral mucosa and tongue, xerostomia and erythema, headache and pain in eyes, arms, legs, back, neck, ears and joints [21]. Also itching, nausea, tinnitus and associated diseases like multiple sclerosis, myasthenia, rheumatoid arthritis and asthma could be symptoms of OG [22]. Corrosion currents coming from OG cause teeth destruction [23,24], bone resorption [25] and several oral inflammatory disorders through the high acidity and high concentration of dissolved metal ions formed [26]. Oral leukoplakia may also develop in the oral mucosa due to the chronic irritation caused by OG [11-13]. Inovay (1961) mentioned that OG could cause oral leukoplakia and lichen planus and he noted a remarkable resolution of leukoplakia when harmful potentials were eliminated [14,16]. Considering the need for well-controlled scientific experiments to provide a definitive proof of cause and effect for OG on various human diseases including oral precancers and cancers, we undertook this study to evaluate the effect of OG on precancer cells in vitro.
Normally, electric currents arise by unidirectional ion transport in epithelial cell layers and generate an essentially direct current (DC) voltage across the layer [27].Bergman (1986) said the basis forgalvanic cell formation is always a redox reaction:oxidationat anode (loss of electron) and reduction at cathode (gain of electron) [28]. In the oral cavity, there are two reasons for galvanic cell formation: different metals and different concentrations of the same metal [28]. Sutow (2004) found that galvanic currents for couples such as amalgam-amalgam and amalgam-gold are generally below 15 µA. He mentioned that due to mutual polarization, the anodic corrosion rate of the more negative metal (anode) will be accelerated, while the corrosion rate of the more positive metal (cathode) will be reduced [29].200µA/dm2 was the maximum current density obtained between the conventional amalgam and type III gold alloy, while no current densities were obtained between gold and cobalt-chromium alloys [30].In our research, the intensity of current was measured in all experiments and the mean electrical current was between 5 and 20µA (data not shown).
Nowadays electromagnetic fields (EMF) are the concern of many scientific investigators as they could potentially cause cancer [31]. They suggest that if EMF have any effect on the process of carcinogenesis, they are more likely to act as promoters that influence the progression of cancer than initiators of the disease. In this role, fields would enhance the proliferation of genetically altered cells rather than causing the initial lesion in DNA or chromatin [31]. Valberg in 1997 supposed that for electric or magnetic fields to initiate or promote inconvenient health effects in biological systems, they must have changed the size, shape, charge, chemical state, or energy of biological molecules or structures. The biological system would have an actual effect when it felt the amplified change [32].
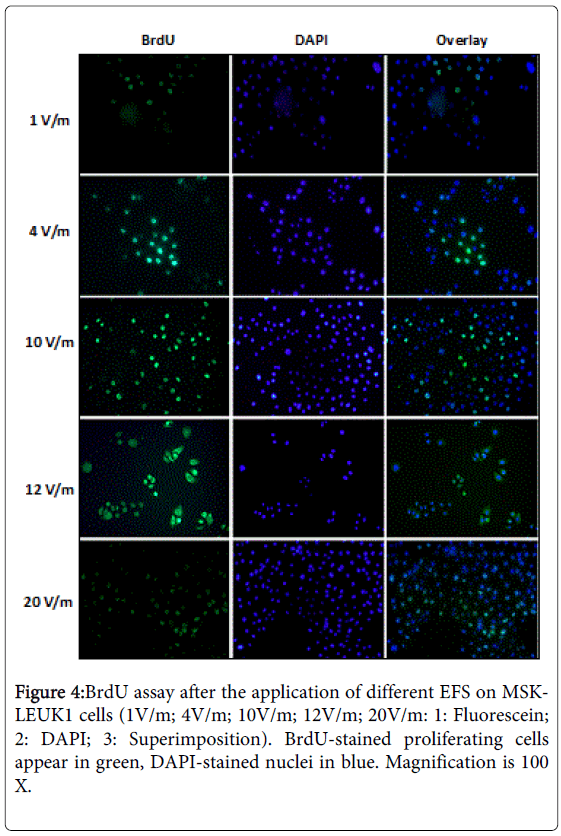
The utilization of BrdU labeling and detection assay in the study of cellular proliferation allowed differentiation between viable and proliferating cells. BrdU can be incorporated into the newly synthesized DNA of replicating cells during the S-phase of the cell cycle, substituting for thymidine. Antibodies specific for BrdU can be used to detect the incorporated chemical, thus indicating DNA synthesizing and proliferating cells. DNA replication, which is indicated by the renewed BrdU assay, is the indicator of the entrance of cells in cell cycle and their proliferation. In our study, it was proved that EFS from 2 to 10V/m could upregulate the proliferation index (PI) of oral leukoplakia cell lines from 15% to approximately 44% (Figures 3 and 4).
In the present research, the voltage generated by electrodes in culture medium but without any connection to a DC supply was measured at approximately 41 ± 11mV (n=2). This indicates the presence of individual potentials for every metal restoration, even if the amount of material is small.
The effect of electrodes with no connection to a DC supply on MSK-LEUK1 cells, which is similar to amalgam-amalgam coupling in the oral cavity, was measured to identify the effect of the presence of metal in an electrolyte. For EFS of 12V/m in the proliferation assay, PI was 43.48% in EF-stimulated MSK-LEUK1 cells (EFSC), 22.54% in electrode-stimulated cells (ELSC) and 22.17% in negative control cells (PNCC). From the above results, we concluded that electrodes alone had little proliferative effect on OPM cell lines, while DC voltage stimulation nearly doubled the proliferation of these cell lines. At 1 V/m, PI was 10.64% in EFSC while it was 18.89% in ELSC. This may be due to inherent variability in MSK-LEUK1. The effect of the current on MSK-LEUK1 cells is, similar to that occurring in the oral cavity, in contrast to the effect of electrodes alone. It could be attributed to the fact that the latter produced steady current on one side of the cells whereas the former applied rotating currents that hit the cells from different sides and thus had a greater impact.
Apoptosis, or programmed cell death, is a normal physiologic process for maintaining tissue homeostasis [17]. There are two types of apoptosis: extracellular (extrinsic inducers) or intracellular (intrinsic inducers). Extracellular signals may include hormones, growth factors, nitric oxideor cytokines. Intracellular apoptotic signalling is a reaction initiated by a cell in response to stress [33,34]. The apoptotic process in our experiments could be of intracellular type where DC voltage is considered as chronic stress to OPM cells. An increasing number of investigators favourin situ labeling of apoptotic cells through the TUNEL assay, which allows both high sensitivity and precise identification of the cell population involved [35]. TUNEL reaction preferentially labels DNA strand breaks generated during apoptosis. This allows discrimination of apoptosis from accidental cell death and from primary DNA strand breaks induced by cytostatic drugs or irradiation. In the present study, it was proved that EFS from 1V/m to 10V/m could up-regulate the apoptosis of OPM lesions in vitro from 21% to 47% (Figures 5 and 6). For EFS of 8 V/m, the apoptotic index (AI) was 38.55% in EFSC, 20.39% in ELSC and 9.45% in negative control cells (ANCC). From the above results, we concluded that electrodes alone had an apoptotic effect on leukoplakia cell lines and this effect was accelerated, nearly double, when these electrodes were connected to a DC supply.
In order to explain the increase of both the proliferation and the apoptosis of MSK-LEUK1, findings of Sauer H (2002) and Wartenberg M (2008) were used. OG expresses chronic irritation on leukoplakia cells; this will lead to the increase of ROS concentration in cells. Intracellular ATP will go through anion channels to the extracellular compartment leading to the activation of purinergic receptors, which in turn lead to the opening of calcium channels and the influx of Ca2+ inside cells. Intracellular increase of Ca2+ concentration will activate membrane receptors, which will activate Phospholipase C and generate inositol triphosphate that in turn leads to entrance of quiescent leukoplakia cells into cell cycle and tumour cell proliferation and growth. In the meantime, ROS increases the expression of NADPH oxidase, considered a pro-apoptotic enzyme. Moreover, the increase of ROS lead to intracellular release of apoptotic signals and the formation of apoptotic proteins which target mitochondria and lead to the release of cytochrome C into cytoplasm; that in turn lead to the activation of caspase 3, a protein who plays an important role in the execution of apoptosis and cancer incidence [20,36,37]. Some authors proved that DC voltage gives rise to the generation of ROS in a variety of preparations including multicellular tumor spheroids [38], embryonic stem cells [39], primary human monocytes and lymphocytes [40] as well as human oral mucosa cancer cells [20]. It is also possible that within stimulated MSK-LEUK1 cells, a few cells proliferate more than others do, while other cells by the strong stimulus become apoptotic.
The data of the present study was, in part, inconsistent with findings of Wartenberg in 2008 that cited a decrease in the proliferation rate of oral squamous carcinoma cell lines exposed to a direct current electrical field of field strength between 2 and 16V/m [20]. As the field strength was increased from 2 to 10V/m, there was an increase in the percentage of proliferating MSK-LEUK1 cells. On the other hand, at levels of 12, 15 and 20V/m, the proliferation rate decreased. The latter may be due to death of MSK-LEUK1 cells through either accidental cell death (which are not marked in our study) or apoptosis, considering that the number of cells is constant. The behavioural difference between both cell lines to DC voltage could be due to their differentiation status. The more differentiated MSK-LEUK1 cells proliferate while the less differentiated OSCC cells necrose under the effect of DC.
Other data from the present study was consistent with findings of Wartenberg in 2008 [20]. As field strength was increased from 1 to 10V/m, there was an increase in the percentage of apoptotic MSK-LEUK1 cells. However, it was proved that at fields of 12, 15 and 20V/m, the apoptotic rate decreased. The latter may be due to death of MSK-LEUK1 cells through accidental cell death (which are not marked in our experiments) other than apoptosis, considering that the number of cells is constant.
This study supports the observation that OG arising in vivo in the oral mucosa may disrupt the dynamic balance between proliferation and cell death leading to the development of oral mucosal diseases such as epithelial dysplasia and cancer.
An interesting and important finding in the electron microscopy examination of MSK-LEUK1 cells exposed to EMF was the presence of individual cell keratinization (intraepithelial keratinization), a morphologic feature commonly seen in malignant oral epithelial cells [41,42]. This finding suggested that that oral galvanism-induced subcellular changes in oral precancer cells in vitro closely simulates the morphologic features of oral squamous cell carcinoma cells in vivo.
In conclusion, OG increases the proliferation of leukoplakia cells and induces cell apoptosis. So, the usage of different metallic restorations in the same patient should be avoided, especially when the two restorations are near to each other. Lastly, studies upon electric fields should be directed to test changes in cell signaling, particularly effects on ornithine decarboxylase (ODC) (due to its role in proliferation of cancer as it is upregulated in many types of cancer) and Na+K+-ATPase activity (due to its role in ion transport).
References
- Momoi Y, Asanuma A, Kohno A, Yanagisawa K (1986) A measurement of galvanic current and electrical potential in extracted human teeth. J Dent Res 65: 1441-1444.
- SCHRIEVER W, DIAMOND LE (1952) Electromotive forces and electric currents caused by metallic dental fillings. J Dent Res 31: 205-229.
- Pindborg JJ, Jolst O, Renstrup G, Roed-Petersen B (1968) Studies in oral leukoplakia: a preliminary report on the period pervalence of malignant transformation in leukoplakia based on a follow-up study of 248 patients. J Am Dent Assoc 76: 767-771.
- Warnakulasuriya S, Johnson NW, van der Waal I (2007) Nomenclature and classification of potentially malignant disorders of the oral mucosa. J Oral Pathol Med 36: 575-580.
- López M, Aguirre JM, Cuevas N, Anzola M, Videgain J, et al. (2003) Gene promoter hypermethylation in oral rinses of leukoplakia patients--a diagnostic and/or prognostic tool? Eur J Cancer 39: 2306-2309.
- Pindborg JJ, Daftary DK, Mehta FS (1977) A follow-up study of sixty-one oral dysplastic precancerous lesions in Indian villagers. Oral Surg Oral Med Oral Pathol 43: 383-390.
- Pindborg JJ. Oral Cancer and Precancer 1st ed. Bristol: John Wright: 1980.
- Lippman SM, Sudbø J, Hong WK (2005) Oral cancer prevention and the evolution of molecular-targeted drug development. J ClinOncol 23: 346-356.
- Vigneswaran N, Beckers S, Waigel S, Mensah J, Wu J, et al. (2006) Increased EMMPRIN (CD 147) expression during oral carcinogenesis. ExpMolPathol 80: 147-159.
- Neville B, Damm D, Allen C, Bouquot J (2002) Oral and Maxillofacial Pathology: Epithelial Pathology. WB Saunders, Philadelphia, USA. p315-387.
- Ullmann K (1932) Leukoplakia caused by electrogalvanic current generated in the oral cavity. Wien KlinWochenschr 45: 840-844.
- Lain ES (1933) Electrogalvanic lesions of the oral cavity produced by metallic dentures. The Journal of American Medical Association 100: 717-720.
- Muller AW1, Van Loon LA, Davidson CL (1990) Electrical potentials of restorations in subjects without oral complaints. J Oral Rehabil 17: 419-424.
- Inovay J, Banoczy J (1961) The role of electrical potential differences in the etiology of chronic diseases of the oral mucosa. J Dent Res 40: 884-890.
- Bánóczy J, Roed-Petersen B, Pindborg JJ, Inovay J (1979) Clinical and histologic studies on electrogalvanically induced oral white lesions. Oral Surg Oral Med Oral Pathol 48: 319-323.
- Psarras V, Derand T, Nilner K (1994) Effect of selenium on mercury vapour released from dental amalgams: an in vitro study. Swed Dent J 18: 15-23.
- Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26: 239-257.
- Thompson CB1 (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267: 1456-1462.
- Sacks PG (1996) Cell, tissue and organ culture as in vitro models to study the biology of squamous cell carcinomas of the head and neck. Cancer Metastasis Rev 15: 27-51.
- Wartenberg M, Wirtz N, Grob A, Niedermeier W, Hescheler J et al. (2008) Direct current electrical fields induce apoptosis in oral mucosa cancer cells by NADPH oxidase-derived reactive oxygen species. Bioelectromagnetics 29: 47-54.
- Johannson BI, Stenman E, Bergman M (1986) Clinical registration of charge transfer between dental metallic materials in patients with disorders and/or discomfort allegedly caused by corrosion. Scand J Dent Res 94: 357-63.
- Schmalz G, Garhammer P (2002) Biological interactions of dental cast alloys with oral tissues. Dent Mater 18: 396-406.
- Chase HS (1879) Oral Electricity. Dent Cosmos 21:205-207.
- Meyer RD, Meyer J, Taloumis LJ (1993) Intraoral galvanic corrosion: literature review and case report. J Prosthet Dent 69: 141-143.
- Olmedo D, Fernández MM, Guglielmotti MB, Cabrini RL (2003) Macrophages related to dental implant failure. Implant Dent 12: 75-80.
- Marek M (1992) Interactions between dental amalgams and the oral environment. Adv Dent Res 6: 100-109.
- Jaffe LF, Nuccitelli R (1977) Electrical controls of development. Annu Rev BiophysBioeng 6: 445-476.
- Bergman M (1986) Corrosion in the oral cavity--potential local and systemic effects. Int Dent J 36: 41-44.
- Sutow EJ, Maillet WA, Taylor JC, Hall GC (2004) In vivo galvanic currents of intermittently contacting dental amalgam and other metallic restorations. Dent Mater 20: 823-831.
- Arvidson K, Johansson EG (1985) Galvanic currents between dental alloys in vitro. Scand J Dent Res 93: 467-473.
- Repacholi MH, Greenebaum B (1999) Interaction of static and extremely low frequency electric and magnetic fields with living systems: health effects and research needs. Bioelectromagnetics 20: 133-160.
- Valberg PA, Kavet R, Rafferty CN (1997) Can low-level 50/60 Hz electric and magnetic fields cause biological effects? Radiat Res 148: 2-21.
- Cotran RS, Kumar V, Collins T, editors (1999) Robbins pathologic basis of disease. WB Saunders Co, Philadelphia, USA. p1-29.
- Latenser BA (2008) Apoptotic death in deep partial thickness burns versus normal skin of burned patients. J Surg Res 146: 161-163.
- Gold R, Schmied M, Giegerich G, Breitschopf H, Hartung HP, et al. (1994) Differentiation between cellular apoptosis and necrosis by the combined use of in situ tailing and nick translation techniques. Lab Invest 71: 219-225.
- Sauer H, Stanelle R, Hescheler J, Wartenberg M (2002) The DC electrical-field-induced Ca2+ response and growth stimulation of multicellular tumor spheroids are mediated by ATP release and purinergic receptor stimulation. J Cell Sci 115: 3265-73.
- Valko M, Leibfritz D, Moncol J, Cronin MT, Mazur M et al. (2007) Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 39: 44-84.
- Wartenberg M, Hescheler J, Sauer H (1997) Electrical fields enhance growth of cancer spheroids by reactive oxygen species and intracellular Ca2+. Am J Physiol 272: R1677-1683.
- Sauer H, Bekhite MM, Hescheler J, Wartenberg M (2005) Redox control of angiogenic factors and CD31-positive vessel-like structures in mouse embryonic stem cells after direct current electrical field stimulation. Exp Cell Res 304: 380-390.
- Lantow M, Lupke M, Frahm J, Mattsson MO, Kuster N, et al. (2006) ROS release and Hsp70 expression after exposure to 1,800 MHz radiofrequency electromagnetic fields in primary human monocytes and lymphocytes. Radiat Environ Biophys 45: 55-62.
- Kramer IR, el-Labban NG, Sonkodi S (1974) Further studies on lesions of the oral mucosa using computer-aided analyses of histological features. Br J Cancer 29: 223-231.
- Reibel J (2003) Prognosis of oral pre-malignant lesions: significance of clinical, histopathological, and molecular biological characteristics. Crit Rev Oral Biol Med 14: 47-62.
--
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 15246
- [From(publication date):
February-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 10550
- PDF downloads : 4696