Potential Pitfalls in the Diagnostic Criteria for Neurocysticercosis: Are Mimmicks Common?
Received: 10-Jul-2018 / Accepted Date: 06-Aug-2018 / Published Date: 14-Aug-2018 DOI: 10.4172/2314-7326.1000279
Keywords: Diagnostic criteria; Neurocysticercosis; Neuroimaging; T. solium
Introduction
Neurocysticercosis is a worldwide public health problem. It is the most important parasitic infection of the Central Nervous System (CNS) and one of the most common causes of secondary epilepsy in many endemic countries [1-8]. Also, epilepsy has a median lifetime prevalence of 15.8/1,000 in Latin American countries, and a median incidence of 138.2/100,000. Higher NCC estimates are associated with increased prevalence of epilepsy, pointing out to the need to improve our knowledge of the disease as a potential means to decrease epilepsy prevalence [9]. Although the flux of immigrants to developed countries has led to cases being reported in the United States and other firstworld countries [8,10,11], its presentation in these locations is usually due to immigration from endemic countries instead of local transmission [7,8], and its prevalence is 0.2-0.6 per 100 000 inhabitants in some regions of the USA [8]. Diagnostic criteria by Del Brutto and cols have been published in 2001, and a set of revised diagnostic criteria have been released in 2016, aiming to simplify definitions and facilitate its applicability. Greater emphasis has been given to the need of neuroimaging. However, there are several pathologies that can present with similar or even indistinguishable characteristics. Here, we present a set of 5 cases of pathologies that mimicked NCC, described as potential differential diagnosis. All patients were inhabitants of an endemic region and presented with epilepsy.
Diagnosis
Neurocysticercosis is suspected from a patient’s clinical history. Its presentation usually varies from one patient to another, but the most frequent manifestation is seizures; focal signs, headache, and even secondary dementia due to intracranial hypertension are possible, regarding it as a great imitator, and can mimic almost any neurological disorder [7,8,12]. New diagnostic criteria have been published recently [13] emphasizing the importance of both clinical/exposure and neuroimaging criteria, aiming to increase diagnostic accuracy. Neuroimaging criteria, includes head CT, still the best screening procedure due to its lower cost and higher sensitivity for detection of calcified lesions, nevertheless brain MRI has a higher resolution, allows visualization of lesions in the posterior fossa without bony artifacts, and is better to evaluate intraventricular cysticercosis, brainstem cysts, and small cysts over the convexity of cerebral hemispheres [14]. The serologic analysis includes EITB (enzyme-linked immunotransference blotting) with a sensitivity of 98% and higher specificity or detection of anticisticercal antibodies in the CSF by ELISA (Enzyme-Linked Immunosorbent Assay) and is 89% sensitive and 93% specific in patients with viable infections [8].
Classifications of the disease have been described according to imaging studies [15-17]. Recently, Del Brutto and collaborators [13] published diagnostic criteria for neurocysticercosis updating the previous 2001 diagnostic criteria. Main changes in the revisited NCC diagnostic criteria include the division of neuroimaging and clinical/exposure criteria, requiring coexistence of both, as well as the distinction of the major and minor importance of each of them. Neuroimaging criteria have been rearranged as major, minor, and confirmative. Evidence of household contact with T. solium infection has been given greater importance, and epidemiological criteria have been included along with the clinical criteria. Absolute criteria have not changed, as shown in Table 1. According to the 2001 criteria [18] (current at the time that the patients presented to our hospital), the patients described in the following cases should have been diagnosed as definitive cases of neurocysticercosis. However, their clinical course and the lack of response to anticysticercosis treatment led all the way to a biopsy before establishing a true diagnosis. We used the 2016 diagnostic criteria (Table 1) [13], to reevaluate this cases to order to identify its potential usefulness and application, as well as to recognize potential pitfalls.
| Patient | Del Brutto NCC 2001 diagnostic criteria | Diagnostic certainty | Del Brutto NCC 2017 diagnostic criteria | Diagnostic certainty | Carpio 2016 NCC criteria | Diagnostic certainty |
|---|---|---|---|---|---|---|
| Case 1 | 2 Major, 1 Minor, 1 Epidemiological | Definitive NCC | 2 Major Neuroimaging, 2 Minor ( 1 Clinical/1 Exposure) | Definitive NCC | Multiple parenchymal vesicles without scolex associated with seizures, also positive ELISA | Definitive parenchymal NCC |
| Case 2 | 2 Major, 1 Minor, 1 Epidemiological | Definitive NCC | 2 Major Neuroimaging, 2 Minor ( 1 Clinical/1 Exposure) | Definitive NCC | Multiple parenchymal vesicles without scolex associated with seizures, also positive ELISA | Definitive parenchymal NCC |
| Case 3 | 1 Minor, 3 Major, 1 Epidemiological | Definitive NCC | 3 Major Neuroimaging, 2 Minor (1 Clinical/1 Exposure) | Definitive NCC | Any combination of parenchymal cysticercus in different evolutive stages: degenerative nodular, and calcified granulomas | Definitive parenchymal NCC |
| Case 4 | 2 Major, 1 Minor, 1 Epidemiological | Definitive NCC | 2 Major Neuroimaging, 2 Minor ( 1 Clinical/1 Exposure) | Definitive NCC | Any combination of parenchymal cysticercus in different evolutive stages: cystic lesion, and calcified granulomas | Definitive parenchymal NCC |
| Case 5 | 1 Absolute, 1 Major, 1 Minor, 1 Epidemiological | Definitive NCC | 1 Absolute, 1 Major Neuroimaging, 2 (1 Minor/1 Exposure) | Definitive NCC | Single or multiple active parenchymal cysts, with at least one cyst with scolex | Definitive parenchymal NCC |
Table 1: NCC criteria related to the patients presented [NCC criteria from Del Bruto and Carpio proposal related to the patients presented, all of them with the "Definite" criteria of neurocysticercosis].
Case Description
Case 1
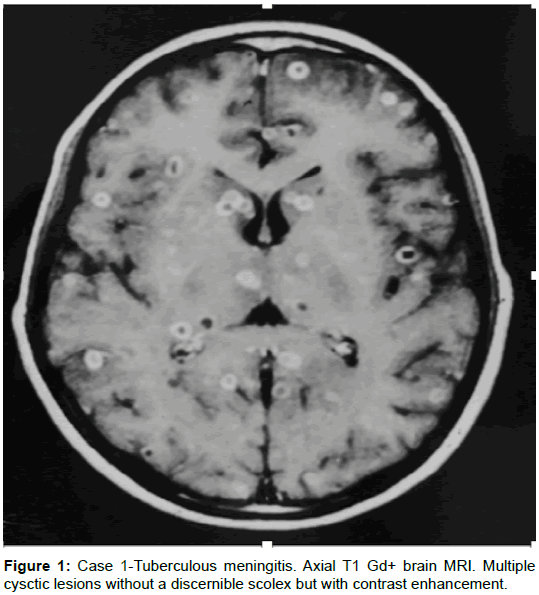
A 14-year-old boy presented with a one-month history of head aches. Three weeks later, generalized, tonic-clonic seizures; preceded by two days of vomiting. The physical examination revealed papilledema. A brain MRI was performed and showed cystic lesions without a discernible scolex, that enhanced with gadolinium, from 2 to 5 mm in diameter, arbitrarily distributed throughout the cerebral parenchyma. The lumbar puncture demonstrated an initial CSF pressure of 380 mmH20; a differential cell count of 120 leucocytes, 60% segmented; glucose 24 mg/dL; and proteins 128 mg/dL. The ELISA testing for cysticercosis was positive, and the equivalent of 1 acid-fast bacillus was found in CSF, for which antimycobacterial treatment was initiated. The patient’s evolution was monitored clinically and by analysis of CSF, which turned out to be satisfactory. The MRI taken after one year of treatment was normal.
Comment: Fifteen to twenty percent of tuberculosis is extrapulmonary [19], but an estimated 10% of immunocompromised patients develop tuberculosis in the CNS. The causal agent is Mycobacterium tuberculosis. Tuberculous meningitis (MTb) is the most frequent manifestation, lasting approximately two weeks in adults (1 day to 9 months). Patients show a normal thorax in 45% of cases when radiological imaging is performed, a positive PPD skin test in 51% of cases, and a mortality rate of 21% [20,21]. Hemiparesis, papilledema, and seizures occur in 10 to 15% of patients. The diagnosis is suspected by the patient’s clinical history, microbiological studies, PCR studies in CSF, as well as neuroimaging. The treatment for pharmaco-sensitive bacilli consists of 2 months of isoniazid, rifampin, pyrazinamide, and streptomycin, followed by 7 to 10 months of isoniazid and rifampin. The treatment varies if resistance is noted. HIV testing is recommended in patients with MTb [22] (Figure 1).
Case 2
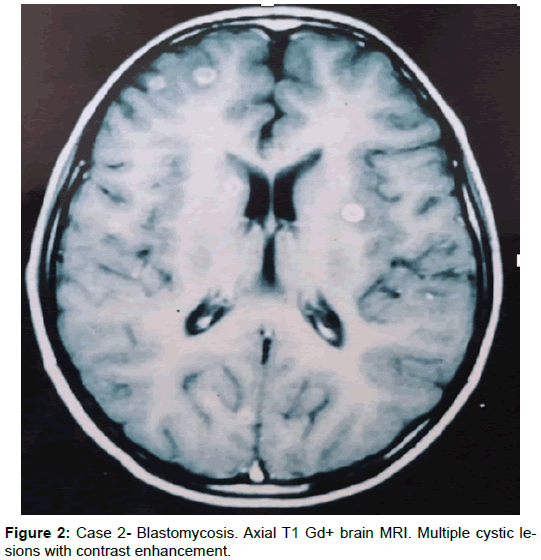
A 12-year-old boy presented with a 6-month history of headaches, vomiting, and generalized seizures. The general medical and neurological examinations were normal. The simple and contrast-enhanced head CT showed multiple hypodense (cystic) lesions with contrast-enhanced annular forms, a finding that was confirmed in subsequent control imaging (CT and MRI), with persistence of these same lesions, in spite of treatment with albendazole for seven months and praziquantel for two weeks. Analysis of the CSF demonstrated an aseptic liquid and positive ELISA testing for cysticercosis. A biopsy was taken from one of the lesions, and North American blastomycosis was diagnosed. The patient started treatment with amphotericin B and continued with fluconazole as an out-patient. After four years of treatment with antimycotic agents, the patient was asymptomatic.
Comment: Blastomycosis is a disease caused by a dimorphic fungus, Blastomyces dermatitidis. Its most common presentation is subacute or chronic pulmonary disease, while disseminated forms are more frequent in the skin, bone, and the genital and urinary tract. Blastomycosis in the CNS is quite rare. However, its presentation has been reported in patients receiving treatment with ketoconazole for the pulmonary form [23-25] (Figure 2).
Case 3
A 45-year-old man presented with a 6-year history of diabetes mellitus, a 2-year history of arterial hypertension, and nine years of progressive intense headaches. In October 1994 the patient presented a simple-partial, left-hemifacial seizure with secondary generalization (1 minor criterion). Head CT showed a lesion highly suggestive of NC (2 major criteria). Anticysticercosis treatment was initiated (inhabitant of an endemic area for cysticercosis: 1 epidemiological criterion). A left frontal craniectomy was performed due to a poor response to pharmaceutic treatment. The macroscopic lesion indicated NC, but the microscopic analysis revealed histoplasmosis. After surgery the patient developed acute intracranial hypertension, aphasia, and right hemiparesis. The patient’s evolution turned out satisfactory after treatment with fluconazole. At present he is asymptomatic.
Comment: The etiological agent of histoplasmosis, Histoplasma capsulatum, is a dimorphic fungus that causes systemic disease. It grows in soil with a high nitrogen content, especially in areas contaminated with the excreta of bats and birds, such as in chicken coops, attics, barns, woodpiles, caves, and roosting areas such as parks. It is highly endemic in the Ohio and Mississippi Valley regions of the United States, the southern fringes of the provinces of Ontario and Quebec in Canada, and scattered areas of Central and South America, as well as in south and southeast Asia. This disease can remain latent in an individual for many years after having abandoned an endemic area. Its principal manifestations are respiratory, and the disseminated form of the disease is rare except in immunosuppressed hosts. Ten to twenty percent of patients with disseminated disease present neurological manifestations, including meningitis, vasculitis, or even mass lesions [26-28].
Case 4
A 17-year-old boy presented with a 7-year history of partial seizures with motor signs in the upper right extremity and with secondary generalization (1 minor criterion). Neuroimaging showed a solitary granulomatous lesion in the left parasagittal, precentral region of the brain (1 major criterion). The CSF analysis demonstrated an elevated concentration of proteins, and the ELISA testing in CSF was positive for neurocysticercosis (1 minor criterion), for which anticysticerci treatment was begun (inhabitant of an endemic area for cysticercosis: 1 epidemiological criterion); however, it was not successful. Therefore, a biopsy was performed, and coccidioidomycosis was diagnosed. Treatment was immediately initiated with amphotericin B and later with fluconazole. At present the patient is receiving phenytoin and is asymptomatic.
Comment: The causal agent of coccidioidomycosis, Coccidioides immitis, is a dimorphic fungus endemic in semiarid climates and include such areas as the central San Joaquin Valley in California, Maricopa and Pima Counties in Arizona, and several western and southwestern counties in Texas. The disease is also endemic in the northern states of Mexico and parts of Venezuela, Paraguay, and Argentina. Cases have been reported in Central America as well. Its most frequent presentation is pulmonary, while isolated presentation in the CNS is quite uncommon. However, when it does occur, its usual manifestation is meningitis. The disseminated form of the disease only occurs in approximately 0.5% of cases, especially in immunocompromised individuals. There have been less than 40 cases of CNS infections of coccidioidomycosis that cause mass lesions reported in the medical literature [29-31].
Case 5
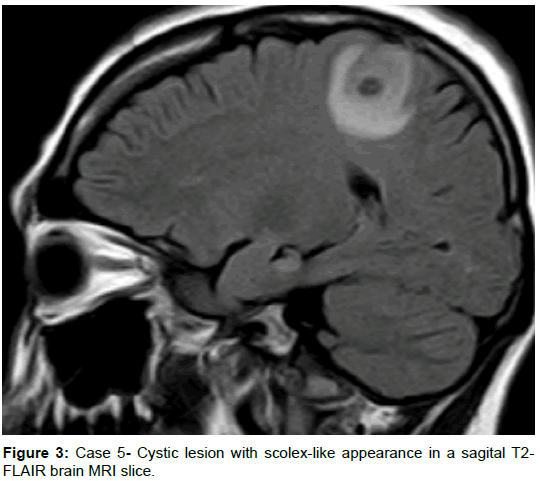
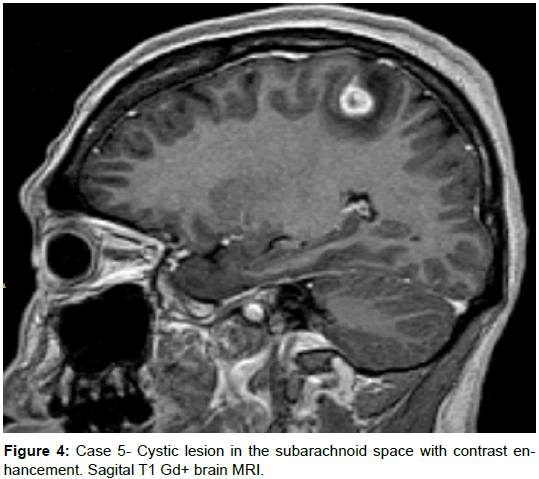
A 35-year-old female from a rural area without a history of comorbidities presented with a 1-month history of a dry cough, and three weeks prior presenting motor focal onset seizures without awareness impairment, initially one crisis per day, but with progression to Epilepsia partial continua in 2 weeks, requiring hospitalization. Head CT scan revealed two hypodense rounded lesions with contrast enhancement, highly suggestive of NCC, receiving treatment with albendazole and antiepileptics. Left hemiparesis was present after the episode of focal onset seizures but had been worse despite the absence of new crisis. Brain MRI was performed and revealed 2 extra-axial lesions with an isointense signal to brain parenchyma on T1, low signal and appearance of a scolex (isointense) on T2-FLAIR, a low signal on T2, without restriction on DWI, with notorious surrounding edema, and clear contrast enhancement. Despite the lack of a history for systemic symptoms, a body CT was performed revealing several hepatic, pulmonary, and one renal mass, a serum LDH of 1100, and HCG beta subunit of 1’505,752 leading to the diagnosis of choriocarcinoma.
Comment: It is essential to keep in mind that some pathologies might have rounded cystic lesions with pseudo scolex that might prompt us to consider a diagnosis of NCC. The characteristics of the two lesions in this patient are virtually indistinguishable from those of a vesicular colloidal phase of NCC. Remnants of neoplastic cells in metastasis, particularly (but not exclusive) in patients with single lesions should prompt the clinician to look for differential diagnosis [13]. Diffusion-weighted images (DWI) and fast imaging employing steady acquisition (FIESTA) can help to visualize these lesions [13], and magnetic resonance spectroscopy (MRS) can be of aid to distinguishing from other pathologies (neoplastic, with increase in choline, and infectious, as described in tuberculomas, with an inversion of the peaks of choline and creatine and a marked peak of lipids) [32] (Figures 3 and 4).
Discussion and Conclusion
NCC is a highly common entity, particularly in developing countries. Diagnostic criteria provide guidance, but knowledge about exceptions and precautions are essential when evaluating a patient with suspected NCC. These findings might apply to countries with a high number of cases of NCC, potentially biasing the evaluation and over-diagnosing the disease, or developed countries with few cases, raising the possibility to overlook the diagnosis of NCC. Neuroimaging techniques do not guarantee that the causal agent of these clinical manifestations is Taenia solium. It is not difficult to confuse other lesions with those caused by T. Solium, and many times these patients meet the diagnostic criteria for NCC previously mentioned and might lead to inappropriate treatment. It is only when anticysticerci treatment fails, and a biopsy is taken that the final diagnosis is reached. In the patients presented in this work, who often required a biopsy to reach a final diagnosis, it is noted that cystic-appearing lesions, that also enhance with contrast administration, are a somewhat common finding in several pathologies, particularly infectious, and that most often this patients might present with seizures, fulfilling 2 major neuroimaging and at least 1 clinical criteria for NCC, providing a “definitive” diagnostic certainty according to the revised diagnostic criteria for NCC (Table 2) [13], which might represent a potential pitfall for such criteria. In the fifth case, the presence of several cystic-appearing lesions, one of them with a dot, highly suggestive of a scolex, must also be carefully evaluated to prevent overlooking a metastatic lesion or other causes for pseudo-scolex, and neuroimaging techniques as DWI, perfusion, ADC, and MRS might be of great aid in this endeavor [17,33].
| Revised diagnostic criteria and degrees of diagnostic certainty for neurocysticercosis. |
| Diagnostic criteria |
| Absolute criteria: |
| • Histological demonstration of the parasite from biopsy of a brain or spinal cord lesion. |
| • Visualization of subretinalcysticercus. |
| • Conclusive demonstration of a scolex within a cystic lesion on neuroimaging studies. |
| Neuroimaging criteria: |
| Major neuroimaging criteria: |
| • Cystic lesions without a discernible scolex. |
| • Enhancing lesions.a |
| • Multilobulated cystic lesions in the subarachnoid space. |
| • Typical parenchymal brain calcifications.a |
| Confirmative neuroimaging criteria: |
| • Resolution of cystic lesions after cysticidal drug therapy. |
| • Spontaneous resolution of single small enhancing lesions.b |
| • Migration of ventricular cysts documented on sequential neuroimaging studies.a |
| Minor neuroimaging criteria: |
| • Obstructive hydrocephalus (symmetric or asymmetric) or abnormal enhancement of basal leptomeninges. |
| Clinical/exposure criteria: |
| Major clinical/exposure: |
| • Detection of specific anticysticercal antibodies or cysticercal antigens by well- |
| standardized immunodiagnostic tests.a |
| • Cysticercosis outside the central nervous system.a |
| • Evidence of a household contact with T. solium infection. |
| Minor clinical/exposure: |
| • Clinical manifestations suggestive of neurocysticercosis.a |
| • Individuals coming from or living in an area where cysticercosis is endemic.a |
| Degrees of diagnostic certainty |
| Definitive diagnosis: |
| • One absolute criterion. |
| • Two major neuroimaging criteria plus any clinical/exposure criteria. |
| • One major and one confirmative neuroimaging criteria plus any clinical/- exposure criteria. |
| • One major neuroimaging criteria plus two clinical/exposure criteria (including at least one major clinical/exposure criterion), together with the exclusion of other pathologies producing similar neuroimaging findings. |
| Probable diagnosis: |
| • One major neuroimaging criteria plus any two clinical/exposure criteria. |
| • One minor neuroimaging criteria plus at least one major clinical/exposure criteria. |
| a. Operational definitions, Cystic lesions: rounded, well defined lesions with liquid contents of signal similar to that of CSF on CT or MRI; enhancing lesions: single or multiple, ring- or nodular-enhancing lesions of 10-20 mm in diameter, with or without surrounding edema, but not displacing midline structures; typical parenchymal brain calcifications: single or multiple, solid, and most usually 10 mm in diameter; migration of ventricular |
| Cyst: demonstration of a different location of ventricular cystic lesions on sequential CTs |
| or MRIs; well-standardized immunodiagnostic tests: so far, antibody detection by enzyme-linked immunoelectrotransfer blot assay using lentil lectin-purified T. solium antigens, and detection of cysticercal antigens by monoclonal antibody-based ELISA; cysticercosis outside the central nervous system: demonstration of cysticerci from biopsy of subcutaneous nodules, X-ray films or CT showing cigar-shape calcifications in soft tissues, or visualization of the parasite in the anterior chamber of the eye; suggestive clinicalmanifestations: mainly seizures (often starting in individuals aged 20-49 years; the diagnosis of seizures in this context is not excluded if patients are outside of the typical age range), but other manifestations include chronic headaches, focal neurologic deficits, intracranial hypertension and cognitive decline; cysticercosis-endemic area: a place where active transmission isdocumented. |
| b. The use of corticosteroids makes this criterion invalid. |
Table 2: Revised diagnostic criteria and degrees of diagnostic certainty for neurocysticercosis [13].
MRS of NCC lesions has shown elevated choline, lactate, succinate, alanine, lipid, and acetate, and decreased creatine and N-acetyl-aspartate. MRS can be of aid to differentiate from a necrotic neoplasm, which demonstrates only lipid and lactate peaks. Since MRS characteristics are similar to other abscesses, DWI can help to differentiate it from pyogenic abscesses [17], which are hyperintense in DWI and Hypointense in ADC, due to the diffusion restriction within the abscess. DWI in NCC can be isointense or slightly hyperintense to the CSF, with ADC similar to CSF (no diffusion restriction) [17,34]. PWI would show an elevation of relative cerebral blood volume within the lesion in neoplasms, unlike NCC (representing an absence of neoangiogenesis and subsequent hyperperfusion) [17].
We believe that the information about potential differential diagnosis according to neuroimaging characteristics should increase, as there have been reports of several pathologies, such as pulmonary neuroendocrine carcinoma [35], cerebral metastasis [36], and even glioblastoma multiform cases [37], as mimmicks of NCC.
Positive ELISA testing in CSF (for IgM against T. solium) has a generally accepted specificity of 95 to 98% [38,39], even though some authors mention a lower specificity [40], and lentil lectin purified glycoprotein enzyme-linked immune-transfer blot (EITB) should be now used but also keeping in mind that its 100% specificity can drop as low as 50% would a single cysticercus be present [41]. Also, EITB does not indicate CNS involvement, and antibodies can persist for long periods or even increase with exposure to the parasite in the absence of infection [41]. As such, caution must be taken when interpreting a positive result, always in the context of compatible clinical data.
Infectious diseases in neurology continue to be not only a diagnostic challenge but also a therapeutic one-one that requires a quicker and more effective approach. Recent revised diagnostic criteria for NCC underlies the importance of neuroimaging in the diagnosis of NCC. However, caution is advised when evaluating cystic lesions with contrast enhancement, and potential mimics of cystic lesions with an apparent scolex, because they most often are accompanied by clinical criteria, fulfilling definitive NCC according to the diagnostic criteria.
Further studies with an emphasis in differential diagnosis will be required to increase our diagnostic certainty when evaluating patients with suspected NCC but atypical clinical and neuroimaging manifestations.
References
- White AC (1997) Neurocysticercosis: A major cause of neurological disease worldwide. Clin Infect Dis 24: 101-105.
- Senanayake N, Roman GC (1993) Epidemiology of epilepsy in developing countries. Bull World Health Organization 71: 247-258.
- Medina MT, Rosas E, Rubio-Donnadieu F (1990) Neurocysticercosis as the main cause of late-onset epilepsy in Mexico. Arch Intern Med 150: 325-327.
- Garcia HH, Gilman R, Martinez M, Alvarado M, Tsang VCW, et al. (1993) Cysticercosis as a major cause of epilepsy in Peru: The Cysticercosis Working Group in Peru (CWG). Lancet 341: 197-200.
- Garcia HH, Del Brutto OH (2000) Taenia solium cysticercosis. Infect Dis Clin North Am 14: 97-119.
- Singh G, Burneo JG, Sander JW (2013) From seizures to epilepsy and its substrates: Neurocysticercosis. Epilepsia 54: 783-792.
- Garcia HH, Del Brutto OH (2005) Neurocysticercosis: Updated concepts about an old disease. Lancet Neurol 4: 653-661.
- Garcia HH, Nash TE, Del Brutto OH (2014) Clinical symptoms, diagnosis, and treatment of neurocysticercosis. Lancet Neurol 13: 1202-1215.
- Bruno E, Bartoloni A, Zammarchi L, Strohmeyer M, Bartalesi F, et al. (2013) Epilepsy and neurocysticercosis in Latin America: A systematic review and meta-analysis. PLoS Negl Trop Dis 20: e2480.
- Scharf D (1988) Neurocysticercosis: Two hundred thirty-eight cases from a California hospital. Arch Neurol 45: 777-780.
- Shandera WX, White AC, Chen JC, Diaz P, Richard A (1994) Neurocysticercosis in Houston, Texas: A report of 112 cases. Medicine (Baltimore) 73: 37-52.
- Estanol B, Kleriga E, Loyo M, Mateos H, Lombardo L, et al. (1983) Mechanisms of hydrocephalus in cerebral cysticercosis: Implications for therapy. Neurosurgery 13: 119-123.
- Del Brutto OH, Nash TE, White AC, Rajshekhar V, Wilkins PP, et al. (2017) Revised diagnostic criteria for neurocysticercosis. J Neurol Sci 372: 202-210.
- Garcia HH, Del Brutto OH (2003) Imaging findings in neurocysticercosis. Acta Trop 87: 71-78.
- Sotelo J, Guerrero V, Rubio F (1985) Neurocysticercosis: A new classification based on active and inactive forms. A study of 753 cases. Arch Intern Med 145: 442-445.
- Salgado P, Rojas R, Sotelo J (1997) Cysticercosis: Clinical classification based on imaging studies. Arch Intern Med 157: 1991-1997.
- Lerner A, Shiroishi MS, Zee CS, Law M, John L Go (2012) Imaging of neurocysticercosis. Neuroimaging Clin N Am 22: 659-676.
- Del Brutto OH, Rajshekhar V, White AC, Tsang VCW, Nash TE, et al. (2001) Proposed diagnostic criteria for neurocysticercosis. Neurology 57: 177-183.
- Kulchavenya E (2014) Extrapulmonary tuberculosis are statistical reports accurate? Therapeutic Advances in Infectious Disease 2: 61-70.
- Saavedra JS, Urrego S, Pérez Ã, Toro ME (2015) Diagnosis of tuberculous meningitis. Acta Neurológica Colombiana 31: 223-230.
- Rodriguez-Leyva I, Hernandez-Gomez JF, Hernandez-Sierra JF (2017) The resurgence of meningeal tuberculosis in Mexico: A social phenomenon. J Neurol Sci 372: 329-330.
- Monge S, Diez M, Pulido F, Iribarren JA, Campins AA, et al. (2014) Tuberculosis in a cohort of HIV-positive patients: Epidemiology, clinical practice, and treatment outcomes. Int J Tuberc Lung Dis 18: 700-708.
- Bariola JR, Perry P, Pappas PG, Proia L, Shealey W, et al. (2010) Blastomycosis of the central nervous system: A multicenter review of diagnosis and treatment in the modern era. Clin Infect Dis 50: 797-804.
- Friedman JA, Wijdicks EF, Fulgham JR (2000) Meningoencephalitis due to Blastomyces dermatitidis: Case report and literature review. In Mayo Clin Proc 75: 403-408.
- Yancey RW Jr, Perlino CA, Kaufman L (1991) Asymptomatic blastomycosis of the central nervous system with progression in patients given ketoconazole therapy: A report of two cases. J Infect Dis 164: 807-810.
- Benedict K, Thompson III GR, Deresinski S (2015) Mycotic infections acquired outside areas of known endemicity, United States. Emerg infect dis 21: 1935-1941.
- Hamada M, Tsuji S (2009) Central nervous system histoplasmosis. Brain Nerve 61: 129-134.
- Klein CJ, Dinapoli RP, Temesgen Z (1999) Central nervous system histoplasmosis mimicking a brain tumor: Difficulties in diagnosis and treatment. In Mayo Clin Proc 74: 803-807.
- Nino Oberto S, Ponce De Leon A, Sierra Madero J (1999) Coccidioides immitis: Primary infection of the central nervous system. Case report and literature review. Rev Invest Clin 51: 43-48.
- Lammering JC, Iv M, Gupta N (2013) Imaging spectrum of CNS coccidioidomycosis: Prevalence and significance of concurrent brain and spinal disease. AJR Am J Roentgenol 200: 1334-1346.
- Saubolle MA, McKellar PP, Sussland D (2007) Epidemiologic, clinical, and diagnostic aspects of coccidioidomycosis. J Clin Microbiol 45: 26-30.
- Pretell EJ, Martinot C, Garcia HH, Alvarado M, Bustos JA, et al. (2005) Differential diagnosis between cerebral tuberculosis and neurocysticercosis by magnetic resonance spectroscopy. J Comput Assist Tomogr 29: 112-114.
- Do Amaral LL, Ferreira RM, Da Rocha AJ (2005) Neurocysticercosis: Evaluation with advanced magnetic resonance techniques and atypical forms. Top Magn Reson Imaging 16: 127-144.
- Gupta RK, Prakash M, Mishra AM (2005) Role of diffusion weighted imaging in differentiation of intracranial tuberculoma and tuberculous abscess from cysticercus granulomas: A report of more than 100 lesions. Eur J Radiol 55: 384-392.
- Lam JC, Robinson SR, Schell A (2016) Pulmonary neuroendocrine carcinoma mimicking neurocysticercosis: A case report. J Med Case Rep 10: 144.
- Coulibaly B, Gautier G, Fuentes S, Ranque S, Bouvier C (2008) Degenerating neurocysticercosis cysts: Differential diagnosis with cerebral metastasis. Rev Neurol (Paris) 164: 948-952.
- Sabel M, Neuen-Jacob E, Vogt C (2001) Intracerebral neurocysticercosis mimicking glioblastoma multiforme: A rare differential diagnosis in Central Europe. Neuroradiology 43: 227-230.
- Wiederholt WC, Grisolia JS (1982) Cysticercosis: An old scourge revisited. Arch Neurol 39: 533.
- Rosas N, Sotelo J, Nieto D (1986) ELISA in the diagnosis of neurocysticercosis. Arch Neurol 43: 353-356.
- Gripper LB, Welburn SC (2017) Neurocysticercosis infection and disease: A review. Acta Trop 166: 218-224.
Citation: Rodriguez-Leyva I, Corés-Enriquez F, Hernández-Gómez JF (2018) Potential Pitfalls in the Diagnostic Criteria for Neurocysticercosis: Are Mimmicks Common? J Neuroinfect Dis 9: 279. DOI: 10.4172/2314-7326.1000279
Copyright: © 2018 Rodriguez-Leyva I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4917
- [From(publication date): 0-2018 - Dec 22, 2025]
- Breakdown by view type
- HTML page views: 3992
- PDF downloads: 925