Pre-harvest Microbial Contamination of Tomato and Pepper Plants: Understanding the Pre-harvest Contamination Pathways of Mature Tomato and Bell Pepper Plants Using Bacterial Pathogen Surrogates
Received: 20-Oct-2015 / Accepted Date: 21-Dec-2015 / Published Date: 28-Dec-2015 DOI: 10.4172/2329-8863.1000204
Abstract
Tomatoes and bell peppers have been previously incriminated in outbreaks of foodborne illnesses due to contamination by human pathogens such as E. coli O157:H7 and Listeria monocytogenes in the field. The objectives of the present study were to investigate (i) the potential entry of E. coli (EC) and L. innocua (LI) from soil to various non-edible and edible parts of the tomato and pepper plants, and (ii) the ability of EC and LI to survive in the plant environment (soil, rhizosphere and phyllosphere). Mature tomato and bell pepper plants cultivated in a greenhouse were soil-inoculated with a bacterial suspension (ca. 108 cfu/ml) of EC or LI. Tomatoes and peppers were also artificially contaminated on the surface with 1 ml of an overnight culture of EC and LI (ca. 109 cfu/ml). Samples of vegetables as well as non-edible parts (soil, roots, stem, foliage) were subjected to microbiological analyses by plating on Eosin Methylene Blue Agar and Listeria Identification Agar to recover EC and LI respectively. Although these bacteria were recovered at population densities of 3.0-3.6, 1.8-2.2 and <0.7 log cfu/g in the bulk soil, roots and foliage respectively, we were unable to recover these bacteria from the edible tomato and pepper fruits. When tomatoes and peppers were spot-inoculated on the surface with EC or LI, the vegetables analyzed were shown to harbor viable bacterial cells for up to 48 h after inoculation. Overall, the potential for systemic uptake and translocation of human pathogens from soil to the edible plant parts was found to be negligible in tomato and pepper plants. However, overhead (spray or sprinkler) irrigation with contaminated water could create opportunities for the deposition and subsequent persistence of human pathogens on the edible surface of vegetables even after harvest. These findings therefore underscore the need for adoption of Good Agricultural Practices (GAPs) by growers and Good Manufacturing Practices (GMPs) by post-harvest handlers of fresh produce.
Keywords: Contamination; Escherichia coli; Listeria innocua;Tomato; Pepper; Ralstonia solanacearum; Pseudomonas fluorescens
404020Introduction
Fresh vegetables contain rich sources of many nutrients and provide numerous health benefits, so nutritionists and health professionals highly recommend increasing consumption of these important foods [1]. Tomatoes and peppers represent some of the vegetables that are most commonly consumed in the raw state. However, these vegetables have also been the source of recent outbreaks of foodborne illnesses in developed countries, which have caused sickness, hospitalizations, and deaths of consumers, as well as serious adverse economic impact on growers and processors [2]. Since 1990, up to 15 outbreaks of salmonellosis have been linked to the consumption of fresh tomato fruits in developed countries such as the United States [3]. Trace-back investigations of outbreaks linked to tomatoes have concluded that the fruits were generally contaminated in the field [4]. Suggested sources ranged from animals in nearby pastures or wetlands to water used for irrigation or pesticide applications [4]. Orozco et al. [5] detected Salmonella in 1.8% of tomatoes grown hydroponically in a greenhouse prior to an extreme weather event during which time floodwaters entered several of the houses. Immediately after the floodwaters had disappeared, the contamination rate increased to 9.4% [5]. Bell peppers also represent a major world commodity by virtue of their high content in vitamin A and C as well as the presence of the compound responsible for the irritation (“hotness”) called capsaicin [6]. The production of hot and sweet peppers for vegetable uses has increased by more than 21% since 1994 [6]. Peppers are commonly used fresh in condiments, sauces, salads, meats and vegetable dishes [6]. Unfortunately, peppers form increasingly recognized vehicles for transmission of foodborne pathogens [7]. A study conducted on the prevalence of Salmonellain peppers showed that 10 out of a total of 27 samples from a pepper production system tested positive for Salmonella and were identified as either Salmonella enterica serovar Typhimurium (91% of 54 cases) or Salmonella enterica serovar Enteritidis (9% of cases) [6].
Given the high frequency of microbial contamination of raw tomatoes and peppers, there has been a concern regarding the potential for human pathogens to become internalized within plant tissue [8]. In the current study, tomato and pepper plants, belonging to the family of Solanaceae, were used as model host systems to study their susceptibility to uptake and persistence of bacterial human pathogens. E. coli O157:H7 is one of the most common zoonotic enteric pathogens associated with vegetables given its widespread presence in animal manure used in produce cultivation [9]. Listeria monocytogenes on the other hand, is a common geophilic (soil-borne) bacterium and is ubiquitous in vegetation [10]. In addition, the role of plant commensal bacteria such as plant pathogen Ralstonia solanacearum and plant beneficial bacteria Pseudomonas fluorescens in enhancing or hindering internalization of human pathogens in vegetables is of equal interest. Indeed, previous research has suggested that bacterial plant pathogens can enhance infiltration or internalization of human pathogens in the roots, leaves and fruits of food crops. Moreover, P. fluorescens represents one of the most abundant soil resident species that usually confer several benefits to the plants. It is thus hypothesized that the presence of phyto-pathogenic species such as R. solanacearum might enhance the uptake of human pathogens in plants due to ability of R. solanacearum to produce plant lesions and wounds which may act as sites of coinfection by human pathogens. Plant pathogens may also have the ability to depress the defense mechanisms of plants, thus enhancing colonization and persistence of human pathogens. On the other hand, it is hypothesized that non-pathogenic P. fluorescens will discourage uptake or internalization of human pathogens since literature has shown that it acts as an excellent plant competitor against non-resident human pathogenic bacteria.
The objectives of the present study were therefore to: (i) investigate the potential uptake, infiltration or internalization of bacterial human pathogens from soil into the edible parts of tomato and bell pepper plants, (ii) investigate the influence of plant pathogen and plant beneficial bacteria on the uptake or internalization of human pathogens and (iii) investigate the survivability of human pathogens in the soil, rhizosphere and phyllosphere of tomato plants.
Materials And Methods
Assessing the potential for systemic uptake of E. coli and L. innocua in tomato and pepper plants
Soil sterilization: The oven was preheated to 82-88°C (180°- 190°F). Ten kg of soil was spread evenly in a large pan to a maximum depth of 10 cm. The pan was sprayed with water to moisten slightly and then covered tightly with aluminum foil. At the center of the covered baking pan, a thermometer probe was inserted into the soil and the pan placed into the oven. Once the soil temperature reached 82-88°C, the temperature was maintained for 60 minutes following which the pan was removed from the oven and allowed to completely cool. Once cooled, soil was transferred to clean gunny bags. Given the limited capacity of the oven, multiple cycles were run to sterilize several batches of soil.
Plant preparation: Tomato (Solanum lycopersicum var. St Pierre) and bell pepper (Capsicum annum var. Nikita) seeds were used. Briefly, seeds were disinfected with 70% ethyl alcohol (EtOH) for 3 min, rinsed in sterile water, and soaked in Javel commercial bleach (0.525% sodium hypochlorite) for 15 min. Seeds were then rinsed in sterile water three times (5 min each rinse). Subsequently, they were sowed in steamsterilized soil contained in Styrofoam plug trays and grown in a Biosafety Level 1 (BSL-1) greenhouse located at the Mauritius Sugar Industry and Research Institute, Reduit. Plants were watered on a daily basis with sterile water. Seedlings were transplanted at 2 weeks of age to potting bags containing steam-sterilized soil (∼1 kg) placed in plastic saucers to serve as a water reservoir for indirect irrigation. The pH and water activity of the soil were regularly monitored with a pH meter (Mettler Toledo) and a water activity meter (Novasina) respectively. Over the period of October 2013 to December 2014 chamber temperatures ranged from 21 to 32°C (daytime) and 12 to 23°C (nighttime) and the relative humidity varied between 65 to 81%. The saucer was refilled with ca. 50 ml sterile water daily. Additionally, the soil was supplemented with ‘Terreau’ or peat (Stender) as per the manufacturer’s instructions to maintain plant growth, to speed up harvest time and increase yields.
Experiment Design: Two plant types (tomato and pepper) were investigated in this part of the study. The plants were given one of 7 treatments (Sterile water, EC, EC + R, EC + P, LI, LI +R, LI + P) where EC, LI, P and R stand for Escherichia coli, Listeria innocua, Pseudomonas fluorescens and Ralstonia solanacearum respectively. Each inoculation treatment was carried out in duplicates. The plants were grown in two separate batches. A total of 56 plants (7 treatments × 2 plant types × 2 plants per treatment × 2 batches) were considered. The different treatments given to the plants are summarized in the (Table 1).
| Treatments | Details of inoculation of potted vegetable plants |
|---|---|
| Water | Addition of 100 ml of sterile water to the potted vegetable |
| E | Inoculation of each potted vegetable type with 200 ml of diluted suspension of overnight culture of E. coli with a cell density of ca. 108 cfu/ml; twice a week |
| EC+P | Inoculation of each potted vegetable type with 200 ml of diluted suspension of overnight culture of E. coli with cell density of ca. 08 cfu/ml& 200 ml of diluted suspension of overnight culture of P. fluorescens with cell density of ca. 107 cfu/ml on alternate days; twice a week |
| EC+R | Inoculation of each potted vegetable type with 200 ml of diluted suspension of overnight culture of E. coli with cell density of ca. 108 cfu/ml& 200 ml of overnight culture of R. solanacearum with cell density of ca. 107 cfu/ml on alternate days; twice a week |
| LI | Inoculation of each potted vegetable type with 200 ml of diluted suspension of overnight culture of L. innocua with cell density of ca. 108 cfu/ml;twice a week |
| LI + P | Inoculation of each potted vegetable type with 200 ml of diluted suspension of overnight culture of L. innocua with cell density of ca. 108 cfu/ml& 200 ml of diluted suspension of overnight culture of P. fluorescens with cell density of ca. 107 cfu/ml on alternate days; twice a week |
| LI + R | Inoculation of each potted vegetable type with 200 ml of diluted suspension of overnight culture of L. innocua with cell density of ca. 108 cfu/ml& 200 ml of diluted suspension of overnight culture of R. solanacearum with cell density of ca. 107 cfu/ml on alternate days; twice a week |
Table 1: Inoculation treatments of plants.
Soil Inoculation:
Bacterial cultures: E. coli ATCC 25922 strain was provided by the Food Technolog Laboratory of the Ministry of Agro-Industry and Food Security of Mauritius. The strain was plated onto Eosin Methylene Blue medium (HiMedia) and incubated for 24 h at 37°C for confirmatory identification of E. coli. Pseudomonas fluorescens ATCC 13525 (Microbiologics Ltd) and was revived on Pseudomonas CFC medium. Colonies that were straw coloured with a greenish tinge were presumed to be P. fluorescens and confirmed by oxidase and catalase tests. Listeria innocua ATCC 33090 (Microbiologics Ltd) and was revived on Polymyxin Acriflavin Lithium-Chloride Ceftazidime Aesculin Mannitol (PALCAM) medium (HiMedia). Olive green colonies with dark sunken centers and black haloes were confirmed to be L. innocua. L. innocua hydrolyzes aesculin to form aesculetin and dextrose. Aesculetin reacts with ammonium ferric citrate and forms a brown-black complex seen as a black halo around colonies. An environmental isolate of Ralstonia solanacearum was generously provided by Dr S. Ganeshan, from the Mauritius Sugar Industry Research Institute. The isolate was obtained from the ooze of a tomato plant suffering from bacterial wilt disease. The isolate was plated onto triphenyl tetrazolium chloride (TTC) medium (Sigma) and incubated overnight at 27°C. Strains were stored at -80°C in glycerol stocks.
Inoculum preparation: The cells of the four cultures were adapted to grow on Plate Count Agar supplemented with 100 μg/ml of nalidixic acid (Sigma) (PCA-N) to select for Nalidixic-acid (NA) resistant strains of E. coli, L. innocua, P. fluorescens and R. solanacearum. NA-resistant mutant strains were subsequently transferred on fresh Plate Count Agar supplemented with 100 μg/ml of NA and plates incubated overnight at 35°C to yield solid cultures. Stock cultures of NA resistant strains of E. coli, L. innocua, R. solanacearum and P. fluorescens were also stored in TSB-N broth containing 25% glycerol (Sigma) at −18°C. To prepare liquid cultures, a single colony of each NA-resistant strain was transferred to 200 ml of tryptic soy broth (TSB-N) and placed on an orbital shaker at 35°C for 18 h.
Soil inoculation of plants: On the day of inoculation of the plants, 100 ml of each culture was mixed with 900 ml of sterile water (10-fold dilution of an overnight culture) to serve as the inoculum for the plants. The concentration of each culture was determined by serial dilution and plating on PCA-N. In addition, the population density of E. coli and L. innocua recovered from the soil immediately after inoculation was also determined. Various treatments were given to the tomato and pepper plants upon fruit set (Table 1). Plants serving as negative controls were treated with sterile water. Tomato plants were staked and strung to bamboo sticks to ensure upright growth. All plants were watered once or twice daily as needed.
Microbiological analysis of vegetables at harvest: Vegetables reaching commercial maturity were harvested by plucking tomato and pepper fruits. Tomatoes and peppers were blended with 0.1% Buffered Peptone Water at a 1:4 ratio. Vegetable samples were blended with 0.1% Buffered Peptone Water at a 1:4 ratio. Vegetables were macerated for 10 minutes into a slurry. The slurry and its serial dilutions were then plated onto Eosin Methylene Blue agar or PALCAM agar supplemented with 100 μg/ml of Nalidixic acid and plates incubated at 44 or 35°C respectively for 48 h. In addition, primary samples suspected to be contaminated with E. coli or L. innocua were subjected to primary enrichment in Lauryl Tryptose broth (LTB) and Half-Fraser broth respectively and incubated at 44 and 35°C for 24 h. Broths were supplemented with NA to a final concentration of 100 ug/ml. Aliquots of LTB and Half- Fraser Broth were then transferred for secondary enrichment into EC and Fraser broths supplemented with NA, and incubated at 44°C and 35°C for 24 h respectively. A loopful of secondary enrichment broth was then streaked onto EMB-N or PALCAM-N and plates incubated at 44 or 35°C respectively for 24 h. Colonies with characteristic green metallic sheen on EMB-N or olive green colonies with a surrounding black halo on PALCAM-N were presumed to be Nalidixic-acid resistant E. coli or L. innocua respectively.
Assessing the translocation potential of E. coli and L. innocua into different sections of the tomato plant
This experiment was conducted to investigate the translocation potential of soil-inoculated E. coli and L. innocua into different parts of the tomato plants (S. lycopersicum cv. St Pierre). Mature tomato plants (past fruit set) were soil-inoculated with 200 ml of a 10-fold dilution of a late-log phase culture of NA-resistant E. coli or L. innocua. The population density of the suspension was ca. 8 log cfu/ml. After 24 h, the plants were cut into 3 sections: the roots, stems and foliage.
Assessing the persistence of E. coli and L. innocua in rhizosphere soil
Soil microcosms were set up consisting of a polypropylene tray containing 2 kg (dry wt) of soil mixed with live roots of an uninoculated tomato plant. Initial water activity of the soil-roots mix was ca. 0.3. The microcosm was inoculated with 200 ml of a suspension of NA-resistant E. coli or L. innocua having a cell density of ca. 108 cfu/ ml resulting in a theoretical final population density of ca. 107 cfu/g of soil. The inoculum was homogeneously stirred into the soil-roots mix and the microcosm covered with aluminum foil. Microcosms were incubated in the dark at 25°C for 7 days with daily addition of 100 ml of sterile water. Soil was collected daily and subjected to microbiological, water activity and pH analyses. In order to determine the population density of bacteria present in the microcosms at daily intervals, about 25 g of soil was taken and mixed with 225 ml of 0.1% buffered peptone water in a sterile stomacher bag. This soil suspension was ten-fold serially diluted in 0.1% buffered peptone water and plated on EMB-N and PALCAM-N agar. Plates were subsequently incubated for up to 48 h at 44°C and 35°C respectively. Soil water activity and pH were determined using a dew point water-activity meter (Novasina) and a pH meter (Mettler-Toledo) respectively.
Assessing the survivability of E. coli and L. innocua on the surface of tomato and pepper fruits
Tomato and pepper plants were cultivated as described previously. At fruit set, a spot inoculation method was used to artificially contaminate the tomatoes and peppers since it allows the deposition of a known amount of cells onto the surfaces, regardless of weight/size. A total of 54 tomatoes and 30 peppers were used for the spot-inoculation study. Mature red ripe tomato and pepper fruits were spot-inoculated with 1000 ul of late-log phase cultures of Nalidixic-acid resistant L. innocua or E. coli on the pericarp and calyx using an appropriate micropipettor. In addition, tomatoes and peppers were also spotted with sterile water as a negative control. Tomatoes and peppers were aseptically harvested after 24 h and 48 h by plucking the fruits together with the stem or peduncle. After aseptically removing the peduncle and calyx, each fruit was then placed in an individual sterile Whirl-Pak filter bag containing 40 ml of 0.1% BPW. To recover bacteria from the surface of fruits, each tomato or pepper fruit was gently hand-massaged for 2 min, and then the rinsate was diluted 10-fold in 0.1% Buffered Peptone Water, and 0.1-ml aliquots of the appropriate dilutions were spread-plated onto EMB-N or PALCAM-N. Plates were incubated and enumerated after 24 h as described previously.
Results And Discussion
Translocation of E. coli and L. innocua in tomato and pepper plants
In this part of the study, E. coli ATCC 25922 and L. innocua ATCC 33090, non-pathogenic surrogate microorganisms were used in lieu of the enteric pathogens Salmonella or E. coli O157:H7 and the ubiquitous soil-borne pathogen L. monocytogenes respectively, to avoid introduction of pathogenic agents in the BSL-1 greenhouse. Other authors including Ingham et al. [11] and Wood et al. [12] have also resorted to non-pathogenic surrogates to circumvent this limitation. Examples of surrogates that have been used in planta studies include E. coli Shiga toxin-negative E. coli O157:H7 [9,13], Listeria innocua [14], and avirulent Salmonella [9]. In using these surrogates, the assumption has been made that they would respond similarly as the pathogenic agent.
(Table 2) summarizes the results obtained for the soil-inoculation experiment of tomato plants. The population density of E. coli and L. innocua recovered from all tomato fruits was below the limit of detection of the plating methodology (E. coli isolates originating from three tomato samples yielded negative results upon biochemical identification, thus confirming their absence. (Table 3) indicates that similar to tomato fruits, E. coli and L. innocua were also undetectable (E. coli ATCC 25922 and L. innocua ATCC 33090 were used as non-pathogenic surrogates to mimic Salmonella spp. or E. coli O157:H7 and L. monocytogenes respectively. Similar to our findings, other authors have also reported the inability to detect Salmonella in tomatoes that have been artificially contaminated with the microorganisms via soil [15,16]. Contrary to our findings however, Zheng et al. [17] has shown that Salmonella was capable of internalizing in tomato plants through the roots provided there are favorable conditions for this to occur. Zheng et al. [17] also indicated that uptake of Salmonella through the roots of S. lycopersicum Micro- Tom grown in sandy loam soil led to the contamination of developing tomato fruits. The authors further noted that fruit contamination rate was much higher with Salmonella introduction through flowers (70.4%) than through the rhizosphere (5.5%). Hence, the phenomenon of Salmonella enterica internalizing tomato plants through the root system remains a largely controversial issue.
| Bacterial Human Pathogen Surrogates (BHPS) | Inoculum Level of BHPS (log cfu/ml) | Plant Commensal Bacteria (PCB) | Inoculum Level of PCB (log cfu/ml) | BHPS Population in fruits (log cfu/g) | # Presumptive Positive Samples/Total Samples |
|---|---|---|---|---|---|
| ------ | 0 | ------ | 0 | < 1.7 | 0/22 |
| EC | 8 | ------ | 0 | < 1.7 | 2/34 |
| EC | 8 | RS | 7 | < 1.7 | 0/24 |
| EC | 8 | PF | 7 | < 1.7 | 1/35 |
| LI | 8 | ------ | 7 | < 1.7 | 0/18 |
| LI | 8 | RS | 7 | < 1.7 | 0/15 |
| LI | 8 | PF | 7 | < 1.7 | 0/17 |
Table 2: Internalization rate of E. coli (EC) and L. innocua (LI) in tomato fruits via soil.
| Bacterial Human Pathogen Surrogates (BHPS) | Inoculum Level of BHPS (log cfu/ml) | Plant Commensal Bacteria (PCB) | Inoculum Level of PCB (log cfu/ml) | BHPS Population in fruits (log cfu/g) | # Presumptive Positive Samples/ Total Samples |
|---|---|---|---|---|---|
| ------ | 0 | ------ | 0 | < 2.2 | 0/9 |
| EC | 8 | ------ | 0 | < 2.2 | 0/17 |
| EC | 8 | RS | 7 | < 2.2 | 0/12 |
| EC | 8 | PF | 7 | < 2.2 | 1/18 |
| LI | 8 | ------ | 7 | < 2.2 | 0/11 |
| LI | 8 | RS | 7 | < 2.2 | 0/12 |
| LI | 8 | PF | 7 | < 2.2 | 0/20 |
Table 3: Internalization rate of E. coli (EC) and L. innocua (LI) in pepper fruits via soil.
Tables 2 and 3 also compared the translocation potential of E. coli and L. innocua in the presence of plant pathogen Ralstonia solanacearum and plant beneficial bacterium Pseudomonas fluorescens. R. solanacearum is a soil-borne pathogen that infects the roots of plants including tomatoes and peppers leading to bacterial wilt disease. Good Agricultural Practices (GAP) guidelines urge growers not to harvest fruits from diseased plants infected by plant pathogens in fear that the plant’s compromised immune system would make them more susceptible to human pathogens such as S. enterica, E. coli O157:H7 or even L. monocytogenes [18]. Indeed, R. solanacearum when added to soil has the ability to infect the plant through natural openings or through wounds in the roots [18], thus potentially increasing the chances for ingress of human pathogens. In this study, the influence of R. solanacearum, a plant pathogen, on the uptake of pathogen surrogates in food crop plants was thus of interest. (Tables 3 and 4) indicate that systemic uptake of E. coli and L. innocua from roots to fruits did not occur in the presence of either plant pathogen R. solanacearum or plant beneficial bacterium P. fluorescens. Contrary to our findings, Pollard et al. [19] demonstrated that R. solanacearum could enhance S. enterica survival and its transportation throughout the internal tissues of tomato plants, causing an increase in S. enterica populations on plants [19]. This is because phytopathogenic bacteria, such as the wilt pathogen R. solanacearum, have the ability to digest pit membranes, having pores of about 0.3 um [20], allowing water to move freely from the stem into a petiole [21-30]. Indeed, certain laboratory models have demonstrated internalization of wilt pathogen Ralstonia solanacearum by tomato roots and then movement up the xylem of the plant [4]. Overall, findings of the current work indicate that the presence of a prototypic plant pathogen exemplified by R. solanacearum and a typical beneficial plant bacterium such as P. fluorescens did not have any effect on the susceptibility of tomato and pepper plants to uptake of bacterial human pathogens. According to Van der Schoot [22], certain cultivars of Solanaceae may possess a type of resistance against wilt pathogens rendering their pit membranes resistant to digestion. Resistance to infection by the plant pathogen or resistance to colonization by the plant beneficial bacteria could have explained the inability to detect any of the plant commensal bacteria or pathogenic surrogates.
| Population density (log cfu/g) of E. coli on the surface of tomatoes | |||
|---|---|---|---|
| Sample ID | Day 0 | Day 1 | Day 2 |
| Sample 1 | 7.8 | 3.6 | < 0.7 (-) |
| Sample 2 | 7.7 | 3.1 | < 0.7 (-) |
| Sample 3 | 8.4 | 3.7 | < 0.7 (-) |
| Sample 4 | 8.6 | 4.3 | < 0.7 (+) |
| Sample 5 | 7.2 | 4.4 | < 0.7 (+) |
| Sample 6 | 8.8 | 3.7 | < 0.7 (-) |
| Sample 7 | 8.2 | 3.0 | < 0.7 (-) |
| Sample 8 | 7.7 | 3.0 | < 0.7 (-) |
| Sample 9 | 8.3 | 4.1 | < 0.7 (-) |
| Mean | 8.1 ± 0.48 | 3.6 ± 0.51 | < 0.7 (2/9) |
Table 4a: Survival of E. coli spot-inoculated on tomatoes.
| Population density (log cfu/g) of L. innocua on the surface of tomatoes | |||
|---|---|---|---|
| Sample ID | Day 0 | Day 1 | Day 2 |
| Sample 1 | 7.4 | < 0.7 | < 0.7 (-) |
| Sample 2 | 7.7 | < 0.7 | < 0.7 (-) |
| Sample 3 | 8.3 | < 0.7 | < 0.7 (-) |
| Sample 4 | 7.6 | < 0.7 | < 0.7 (-) |
| Sample 5 | 7.2 | < 0.7 | < 0.7 (-) |
| Sample 6 | 8.1 | 4.96 | < 0.7 (-) |
| Sample 7 | 7.4 | < 0.7 | < 0.7 (+) |
| Sample 8 | 8.8 | < 0.7 | < 0.7 (-) |
| Sample 9 | 8.2 | < 0.7 | < 0.7 (-) |
| Mean | 8.2 ± 0.49 | 1.2 ± 0.00 | < 0.7 (1/9) |
Table 4b: Survival of L. innocua spot-inoculated on tomatoes.
Translocation of E. coli and L. innocua to different sections of the tomato plant
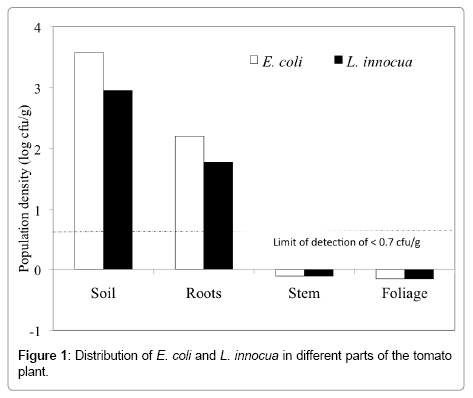
The localization and population density of E. coli and L. innocua in different parts of the tomato plant is depicted in (Figure 1). Our study indicated that E. coli and L. innocua were recovered from bulk soil and roots at population densities of 3.0-3.6 log cfu/g and 1.8-2.2 log cfu/g respectively 24 hrs post-inoculation. However they were undetectable (23] similarly applied water contaminated with Salmonella directly onto the soil of pots containing tomato plants (S. lycopersicum cv. Cherry Gold) and also could not recover Salmonella from the stems or fruits of the tomato plant although populations in the soil ranged from 2.3 to 3.7 log cfu/g. In addition, another study found no evidence of Salmonella enterica serovar Montevideo on the stems, leaves, or fruit of tomato plants (S. lycopersicum L. cv. Trust) when soil-inoculated with contaminated water [4]. This is very similar to our data where plants artificially soilcontaminated with E. coli and L. innocua did not show evidence of translocation of the bacteria to the aerial parts of the plant. However, presence of E. coli and L. innocua in the bulk soil as well as in the roots was observed as indicated in Figure 1. Contrary to our findings where we observed a relatively lower population of these bacteria on roots (1.8-2.2. log cfu/g) than in the bulk soil (3.0-3.6 log cfu/g), Semenov et al. [24] found the densities of S. typhimurium and E. coli O157:H7 in bulk soil and rhizosphere (roots) to be similar following addition of manure to soil. Similarly, Habteselassie et al. [25] found comparable numbers of E. coli cells in bulk and rhizosphere soil when manure was added to pots in which lettuce was being grown. Overall, presence of human pathogens in the bulk soil and on the rhizoplane may not necessarily guarantee entry of the bacteria through the roots to the aerial parts of the plant such as the leaves, flowers or fruits. Contrary to our observation, Hintz et al. [16] reported that repeated application of Salmonella enterica serovar Newport to the root zone via irrigation water has the potential to contaminate various tissues of the tomato plant Solanum lycopersicum cv. Solar Fire. Likewise, Zheng et al. [17] demonstrated that of 22 tomato plants grown with Salmonella-infested soil, 22% (4 out of 18) contained endophytically colonized Salmonella based on direct plating or enrichment procedures, including two stem samples (11.1%), one leaf sample (5.5%), and one fruit sample (5.5%). S. enterica serovar Saintpaul was also isolated from a single positive leaf sample and S. Newport was found on the surface and within the single positive tomato sample (5.5%).
Survival of E. coli and L. innocua in soil mixed with live roots
Plant roots are known to modify their immediate habitat by changing the soil porosity and clustering properties [26] and such physical alterations are likely to impact the microbial community near those roots (i.e., the rhizosphere community). Live roots release root exudates that have the potential to significantly affect the microbial population including the fate of pathogens in the rhizosphere of food crops [27]. These exudates serve as nutrient sources for the bacteria in the vicinity of the roots and could therefore promote the extended survival of pathogens in soil. Taking this into consideration, we thus designed a microcosm consisting of a mix of autoclaved soil and live roots, since previous research has shown that E. coli O157:H7 survived longer in rhizosphere soil compared to free soil [28].
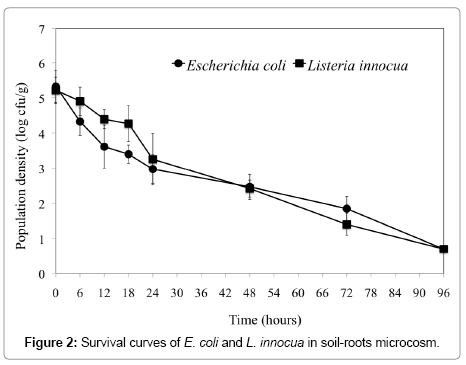
The survival curves of E. coli and L. innocua in the soil-root mix is shown in Figure 2. Both bacterial species exhibited a slow decline from an initial population of 5.2-5.3 to E. coli had a characteristic concave curvature with slightly higher death rate in the first 12 hrs. Islam et al. [29] indicated that survival curves generally exhibit a concave curvature with initial decreases that are log-linear. It is to be noted that conditions of the microcosm were particularly optimized to promote survival of the inocula in the soil-roots mix by protecting against dessication. This was achieved by daily watering with sterile water (soil aw ~ 0.97-0.99) and shielding against UV radiation by covering with foil. Indeed, a critical factor influencing bacterial persistence in the soil is the moisture availability.
Literature has shown the variable persistence of different microorganisms in different agricultural niches [30]. Bell et al. [31] and Micallef et al. [32] indicated that Salmonella can also persist in the tomato-growing environment including the soil. Bernstein et al. [33] reported that S. Newport is capable of persisting in potting medium for 4.7 to 10 weeks. Even among Samonella serovars, there were considerable differences in their persistence; S. Newport and S. Javiana appeared to persist in sandy loam soil more efficiently than other serovars, including S. montevideo, S. saintpaul, and S. typhimurium. In addition to Salmonella, enteric bacteria such as E. coli O157:H7 as well as other fecal microorganisms have been demonstrated to survive for extended periods in soils. Reported survival times of E. coli O157:H7, E. coli O26, Salmonella, Listeria, Campylobacter and Cryptosporidium in soil are up to 6 months, 3 years, 2 years, 20 days and 3 months respectively [34]. Indeed, there is considerable evidence to support the fact that pathogens can survive for widely varying periods of time in the soil and even on produce [33]. The relatively short survival times of E. coli and L. innocua noted in our study (≤ 4 days) could be due the high air temperatures (27-31°C) of the greenhouse during the experiment. Indeed lower survival rates were noted by Fremaux et al. [35] at higher air and soil temperatures. Semenov et al. [24] also reported that the survival of S. typhimurium and E. coli O157:H7 declined with increasing mean soil temperature.
Survival of E. coli and L. innocua on the surface of tomato and pepper fruits
(Tables 4a and 4b) show the population density of E. coli and L. innocua recovered after 24 and 48 h from tomatoes that have been spot-inoculated with the bacteria. Our results show that tomatoes surface-contaminated with E. coli still harbored the bacteria after 24 h at varying density of 3.0-4.4 log cfu/g. However, after 48 h, E. coli was below the limit of detection of the plating methodology (E. coli was still detected on the samples after enrichment and streaking in 2 out of 9 samples. L. innocua lost their viability quicker, dropping from an initial of 8.2 log cfu/g to a mean density of 1.2 log cfu/g after 24 h. After 48 h, L. innocua was detected in only 1 out of 9 samples.
Peppers were surface-inoculated with E. coli or L. innocua at a mean population density of 7.3 to 7.8 log cfu/g respectively. The population declined to 3.5-4.2 log cfu/g after 24 h; after 48 h the bacteria were undetectable by plating although E. coli was detected after enrichment in 6 out 14 samples (Table 5a). L. innocua on the other hand was undetectable in all samples tested after 48h (Table 5b). Taken together, our findings highlight the differential survival of E. coli, a zoonotic bacterium of an intestinal origin, and L. innocua, an environmental bacterium that predominantly resides in soil, on the surface of fruits. The relatively poor colonizing abilities of these bacteria as epiphytes could partly be attributed to the waxy cuticle and regular topography (smoothness) of the tomato and pepper exocarp. Guo et al. [4] also mentioned that bacteria can more readily colonize and penetrate fruit tissue in the early stages of fruit development prior to deposition of the waxy materials. Erickson et al. [13] noted that E. coli O157:H7 cells had a greater propensity to attach to coarse, porous, or injured surfaces than uninjured smooth surfaces of green peppers. The smooth and topographically uniform surface of peppers is thought to be devoid of any microenvironments that can afford protection to the deposited inoculum. Hence, it is not surprising to observe a rapid decline in the bacterial population from an initial 7.8 log cfu/g to 4.2 and <0.7 log cfu/g after 24 h and 48 h respectively.
| Population density (log cfu/g) of E. coli on the surface of peppers | |||
|---|---|---|---|
| Day 0 | Day 1 | Day 2 | |
| Sample 1 | 8.5 | 4.8 | < 0.7 (-) |
| Sample 2 | 8.2 | 4.7 | < 0.7 (+) |
| Sample 3 | 7.9 | 4.6 | < 0.7 (+) |
| Sample 4 | 8.0 | 4.6 | < 0.7 (+) |
| Sample 5 | 7.4 | 3.9 | < 0.7 (-) |
| Sample 6 | 7.6 | 4.2 | < 0.7 (-) |
| Sample 7 | 8.4 | 4.0 | < 0.7 (-) |
| Sample 8 | 7.2 | 4.1 | < 0.7 (+) |
| Sample 9 | 8.1 | 4.5 | < 0.7 (-) |
| Sample 10 | 7.4 | 4.4 | < 0.7 (+) |
| Sample 11 | 7.6 | < 0.7 | < 0.7 (-) |
| Sample 12 | 7.8 | 2.8 | < 0.7 (-) |
| Sample 13 | 8.0 | 4.2 | < 0.7 (+) |
| Sample 14 | 7.5 | 3.5 | < 0.7 (-) |
| Overall | 7.8 ± 0.40 | 4.2 ± 0.54 | < 0.7 (6/14) |
Table 5a: Survival of E. coli spot-inoculated on peppers.
| Population density (log cfu/g) of L. innocua on the surface of peppers | |||
|---|---|---|---|
| Day 0 | Day 1 | Day 2 | |
| Sample 1 | 7.9 | 4.1 | < 0.7 (-) |
| Sample 2 | 6.8 | 3.0 | < 0.7 (-) |
| Sample 3 | 7.4 | 3.0 | < 0.7 (-) |
| Sample 4 | 7.2 | 4.9 | < 0.7 (-) |
| Sample 5 | 6.9 | 3.6 | < 0.7 (-) |
| Sample 6 | 6.7 | 5.2 | < 0.7 (-) |
| Sample 7 | 7.6 | 2.0 | < 0.7 (-) |
| Sample 8 | 7.5 | 3.0 | < 0.7 (-) |
| Sample 9 | 6.9 | 2.0 | < 0.7 (-) |
| Sample 10 | 7.9 | < 0.7 | < 0.7 (-) |
| Sample 11 | 7.6 | < 0.7 | < 0.7 (-) |
| Sample 12 | 6.8 | < 0.7 | < 0.7 (-) |
| Sample 13 | 7.9 | 3.2 | < 0.7 (-) |
| Sample 14 | 7.2 | 2.8 | < 0.7 (-) |
| Sample 15 | 6.9 | 4.2 | < 0.7 (-) |
| Sample 16 | 7.1 | 4.8 | < 0.7 (-) |
| Overall | 7.3 ± 0.42 | 3.5 ± 1.10 | < 0.7 (0/16) |
Table 5b: Survival of L. innocua spot-inoculated on peppers.
Vegetables can be indirectly contaminated in the field when the soil in which they are cultivated becomes contaminated for e.g., during drip-irrigation with contaminated water. In addition to drip-irrigation, vegetables can also be directly contaminated during overhead irrigation with contaminated water through splash dispersal of the bacteria onto the fruit surface [36]. Wei et al. [37] previously demonstrated the survival and growth of Salmonella deposited as an aqueous cell suspension on natural openings of the tomato fruit such as the stem scar. Contrary to Wei et al. [37], we noted that the inoculum deposited on the surface did not grow; instead the population declined rapidly to below detectable levels after 48 h post-inoculation. Wei et al. [37] mentioned that survival of the bacteria was most likely dependent on the inoculum size; when small populations of S. montevideo of 2.8-3.9 log cfu/ml were placed on the smooth periderm of tomato fruits, none could be detected after overnight storage. However, when the concentration of inoculum was increased to 9.5 log cfu/ml, the bacterium could be detected up to three days later. In our experiment, a volume of 1 ml of the overnight culture having a cell density of ca. 9 log cfu/ml was aliquoted on the fruit resulting in the deposition of ca. 109 cells on the fruit. In spite of the high inoculum, the population rapidly declined to 3-4 log cfu/g and to undetectable levels after 24 and 48 h respectively. It has also been mentioned elsewhere that better survival of the inocula was observed when the bacterial cells were suspended in a buffer as compared with distilled water. In our experiment, a 10-fold dilution of the culture was effected in distilled water rather than buffer. The use of plain water over buffer could have contributed to the poor viability of the culture. Finally, the disparity between Wei et al. [37] and our results could be due to the different bacterial species used in our inoculum.
Within the plant production systems, two very different environments are encountered, the rhizosphere (below-ground bacterial habitat) and phyllosphere (above-ground surfaces of a plant as a habitat for microorganisms). This pioneering study examined how introduction of bacterial human pathogens in the rhizosphere and phyllosphere of commercially important food crops affected their microbial safety. Our findings revealed that artificial introduction of E. coli and L. innocua in the rhizosphere of tomato and bell pepper plants did not result in translocation of the bacteria into the fruits 24 h post-inoculation although a relatively high surviving population was noted in the bulk soil and in the roots. Moreover, the presence of plant pathogen Ralstonia solanacearum and plant beneficial bacteria Pseudomonas fluorescens did not influence the systemic uptake of human pathogenic bacteria from the soil to the aerial parts of the plants. However, when E. coli and L. innocua were deposited onto the surface of tomato and pepper fruits, they remained viable for up to 48 h. Hence, a preventative approach to minimizing the risks of pre-harvest contamination of tomatoes and peppers is through avoiding contact between mature fruits and environmental sources of human pathogens such as overhead or sprinkler irrigation water.
References
- Garrow JS, Ralph A, James WPT(2000) Human Nutrition and Dietetics, Elsevier, Amsterdam.
- Beuchat LR1 (2002) Ecological factors influencing survival and growth of human pathogens on raw fruits and vegetables.Microbes Infect 4: 413-423.
- Erickson MC, Webb CC, Diaz-Perez JC, Phatak SC, Silvoy JJ, et al.(2010) Surface and internalized Escherichia coli O157:H7 into field-grown spinach and lettuce treated with spray-contaminated irrigation water. J Food Prot73:1023–1029.
- Guo X1, Chen J, Brackett RE, Beuchat LR (2001) Survival of salmonellae on and in tomato plants from the time of inoculation at flowering and early stages of fruit development through fruit ripening.Appl Environ Microbiol 67: 4760-4764.
- Orozco L1, Rico-Romero L, EscartÃn EF (2008) Microbiological profile of greenhouses in a farm producing hydroponic tomatoes.J Food Prot 71: 60-65.
- Stine SW1, Song I, Choi CY, Gerba CP (2005) Effect of relative humidity on preharvest survival of bacterial and viral pathogens on the surface of cantaloupe, lettuce, and bell peppers.J Food Prot 68: 1352-1358.
- Burke G(2008) Mexican peppers posed problem long before outbreak. Assoc. Press. Yahoo News.
- Beuchat LR(1996) Pathogenic microorganisms associated with fresh produce. Journal of Food Protection 59: 204-216.
- Cooley MB1, Miller WG, Mandrell RE (2003) Colonization of Arabidopsis thaliana with Salmonella enterica and enterohemorrhagic Escherichia coli O157:H7 and competition by Enterobacterasburiae.Appl Environ Microbiol 69: 4915-4926.
- Critzer FJ1, Doyle MP (2010) Microbial ecology of foodborne pathogens associated with produce.CurrOpinBiotechnol 21: 125-130.
- Ingham SC, Losinski JA, Andrews MP, Breuer JE, Breuer JR, et al.(2004) Escherichia coli contamination of vegetables grown in soils fertilized with non-composted bovine manure: Garden-scale studies. Applied and Environmental Microbiology 70: 6420-6427.
- Wood JD, Bezanson GS, Gordon RJ, Jamieson R(2010) Population dynamics of E. coli inoculated by irrigation into the phyllosphere of spinach grown under commercial production conditions. International Journal of Food Microbiology 143: 198-204.
- Erickson MC1, Webb CC, Diaz-Perez JC, Phatak SC, Silvoy JJ, et al. (2010) Infrequent internalization of Escherichia coli O157:H7 into field-grown leafy greens.J Food Prot 73: 500-506.
- Girardin H, Morris CE, Albagnac C, Dreux N, Glaux C, et al.(2005)Behaviour of the pathogen surrogates Listeria innocua and Clostridium sporogenes during production of parsley in fields fertilized with contaminated amendments. FEMS Microbiology and Ecology 54: 287-295.
- Guo X, van Iersel MW, Chen J(2002) Evidence of association of salmonellae with tomato plants grown hydroponically in inoculated nutrient solution. Applied and Environmental Microbiology 68: 3639-3643.
- Hintz LD, Boyer RR, Ponder MA, Williams RC, Rideout SL(2010) Recovery of Salmonella enterica Newport introduced through irrigation water from tomato (Lycopersicumesculentum) fruit, roots, stems, and leaves. Hort Science 45:675-67.
- Zheng J1, Allard S, Reynolds S, Millner P, Arce G, et al. (2013) Colonization and internalization of Salmonella enterica in tomato plants.Appl Environ Microbiol 79: 2494-2502.
- Upreti R, Thomas P(2015) Root-associated bacterial endophytes from Ralstoniasolanacearum resistant and susceptible tomato cultivars and their pathogen antagonistic effects. FrontMicrobiol 6:255.
- Pollard S, Barak J, Boyer R, Reiter M, Gu G (2014) Potential interactions between Salmonella enterica and Ralstoniasolanacearum in tomato plants. Journal of Food Protection 77: 320-324
- Goodman RN, Kira´ly Z, Zaitlin M(1967)the biochemistry and physiology of infectious plant disease. Van Nostrand, Princeton, NJ.
- Prior PH, Beramis M, Chillet M, Schmit J (1990) Preliminary studies for tomato bacterial wilt (Pseudomonas solanacearumE.F.Sm.) resistance mechanisms. Symbiosis 9: 393-400.
- Van der Schoot C, van Bel AJE(1989) Architecture of the intermodal xylem of tomato (Solanumlycopersicum) with reference to longitudinal and lateral transfer. Amer J Bot 76: 487-503.
- Jablasone J, Brovko LY, Griffiths MW(2004)a research note: the potential for transfer of Salmonella from irrigation water to tomatoes. J Sci Food Agric 84:287-289.
- Semenov AV1, van Bruggen AH, van Overbeek L, Termorshuizen AJ, Semenov AM (2007) Influence of temperature fluctuations on Escherichia coli O157:H7 and Salmonella entericaserovarTyphimurium in cow manure.FEMS MicrobiolEcol 60: 419-428.
- Habteselassie MY1, Bischoff M, Applegate B, Reuhs B, Turco RF (2010) Understanding the role of agricultural practices in the potential colonization and contamination by Escherichia coli in the rhizospheres of fresh produce.J Food Prot 73: 2001-2009.
- Feeney DS1, Crawford JW, Daniell T, Hallett PD, Nunan N, et al. (2006) Three-dimensional microorganization of the soil-root-microbe system.MicrobEcol 52: 151-158.
- Haichar FZ1, Marol C, Berge O, Rangel-Castro JI, Prosser JI, et al. (2008) Plant host habitat and root exudates shape soil bacterial community structure.ISME J 2: 1221-1230.
- Ibekwe AM1, Watt PM, Shouse PJ, Grieve CM (2004) Fate of Escherichia coli O157:H7 in irrigation water on soils and plants as validated by culture method and real-time PCR.Can J Microbiol 50: 1007-1014.
- Islam M1, Morgan J, Doyle MP, Phatak SC, Millner P, et al. (2004) Fate of Salmonella entericaserovarTyphimurium on carrots and radishes grown in fields treated with contaminated manure composts or irrigation water.Appl Environ Microbiol 70: 2497-2502.
- Barak JD1, Liang AS (2008) Role of soil, crop debris, and a plant pathogen in Salmonella enterica contamination of tomato plants.PLoS One 3: e1657.
- Bell RL, Cao G, Meng J, Allard MW, Keys C(2012) Salmonella Newport contamination of produce: ecological, genetic, and epidemiological aspects. In: AS Monte, PED Santos (eds), Salmonella: classification, genetics and disease outbreaks. Nova Science Publishers, Inc., Hauppauge, NY.
- Micallef SA, Rosenberg RE, Goldstein A, George L, Kleinfelter MS, et al(2012) Occurrence and antibiotic resistance of multiple Salmonella serotypes recovered from water, sediment and soil on mid-Atlantic tomato farms. Environ Res 114: 31-39.
- Bernstein N, Sela S, Neder-Lavon S(2007) Effect of irrigation regimes on persistence of Salmonella entericaserovar Newport in small experimental pots designed for plant cultivation. IrrigSci 26: 1-8.
- Nicholson FA1, Groves SJ, Chambers BJ (2005) Pathogen survival during livestock manure storage and following land application.BioresourTechnol 96: 135-143.
- Fremaux B1, Delignette-Muller ML, Prigent-Combaret C, Gleizal A, Vernozy-Rozand C (2007) Growth and survival of non-O157:H7 Shiga-toxin-producing Escherichia coli in cow manure.J ApplMicrobiol 102: 89-99.
- Jablasone J, Warriner K, Griffiths M(2005) Interactions of Escherichia coli O157:H7, Salmonella Typhimurium, and Listeria monocytogenes plants cultivated in a gnotobiotic system. International Journal Food Microbiology 99: 7-18.
- Wei CI, Huang JM, Lin WF, Tamplin ML, Bartz JA(1995) Growth and survival of Salmonella Montevideo on tomatoes and disinfection with chlorinated water. J Food Prot 8: 829- 836.
Citation: Ganeshan S, Neetoo H (2015) Pre-harvest Microbial Contamination of Tomato and Pepper Plants: Understanding the Pre-harvest Contamination Pathways of Mature Tomato and Bell Pepper Plants Using Bacterial Pathogen Surrogates. Adv Crop Sci Tech 4:204. DOI: 10.4172/2329-8863.1000204
Copyright: © 2015 Ganeshan S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15532
- [From(publication date): 12-2016 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 13801
- PDF downloads: 1731