Propranolol and Structurally Novel Derivatives as Positive Allosteric Modulators of the Alpha-7-nicotinic Acetylcholine Receptor
Received: 10-Feb-2020 / Accepted Date: 25-Feb-2020 / Published Date: 03-Mar-2020 DOI: 10.4172/2167-065X.1000192
Abstract
Purpose: Alpha-7-nicotinic acetylcholine receptor (α7-nAChR) positive allosteric modulators (PAMs) are a potential disease modifying treatment for Alzheimer’s disease, as exemplified by the approved drug galantamine. However, clinical development of this class of compounds has stalled due to poor efficacy and severe adverse reactions, likely related to receptor overstimulation-induced neurotoxicity. In this study, we searched for alternative α7-nAChR PAMs and tested their effect at the receptor.
Methods: A α7-nAChR PAM pharmacophore model was used to screen over 1000 FDA approved drugs and commercially available compounds. Patch clamping was used to assess the six most promising compounds selected from 160 hits. We designed structurally novel derivatives of one of these hits, namely propranolol, and five were synthesized and tested.
Results: Three compounds from the initial virtual screen demonstrated α7-nAChR PAM activity: riboflavin, bromocriptine, and propranolol. Propranolol was noted to possess both α7-nAChR PAM and inhibitory activity at lower and higher concentrations, respectively. Four of propranolol’s analogs also displayed α7-nAChR PAM activity with evidence of functional inhibition at higher concentrations.
Conclusions: Novel compounds with α7-nAChR PAM activity were successfully identified using a computational approach. Several compounds displayed functional inhibition at higher concentrations, which may allow for clinical use without risking overstimulation induced excitotoxicity.
Keywords: Propranolol; Positive allosteric modulator; Alpha-7- nicotinic acetylcholine receptor; Alzheimer’s disease
Introduction
Alzheimer’s disease (AD) is the most common type of dementia and is the 6th leading cause of death in USA affecting over 5.1 million adults over the age of 65 years, while costing the economy over $150 billion per year [1]. Most of the research on AD has focused on the aggregation of tau and -amyloid proteins and on the decrease in acetylcholine, while other studies have investigated the role of the Alpha-7-nicotinic acetylcholine receptor (α7-nAChR) in in vivo AD animal models [2]. Though the mechanisms underlying AD are still not completely clear, the α7-nAChR has been implicated in the pathogenesis of the disease. Positive allosteric modulator (PAM) agonists represent a clinically validated, disease-modifying class of compounds, and when applied in vivo, α7-nAChR PAMs improved cognition and reduced brain tau and -amyloid pathology [3]. Approved therapies for AD, namely four cholinesterase inhibitors (tacrine, rivastigmine, donepezil, and galantamine) and one N-methyl-D-aspartate receptor inhibitor (memantine), are purely symptomatic and do not alter the disease course [1]. Consequently, there is a pressing need to develop new medications with disease modifying effects for AD.
Previous studies have demonstrated that α7-nAChRs are essential in modulating neurotransmission, cognition, anxiety, and sensory gating, and the receptor may have a central role in the pathophysiology of AD [4]. α7-nAChRs are ligand gated ion channels that are comprised of five 7 subunits [4]. By regulating intracellular calcium levels in discrete neuronal locations, these receptors modulate numerous physiological processes in the central nervous system, such as neuronal growth, neuronal differentiation, and pre- and post-synaptic neurotransmitter release [4]. Additionally, the receptor serves as a feedback mechanism for glutamatergic pathways [4]. It is theorized that interactions between neuronal α7-nAChRs and -amyloid, as a result of their high binding affinity, lead to the formation of amyloid plaques [5,6]. When α7-nAChRs are bound by -amyloid, the receptor loses its normal functioning and induces rapid phosphorylation of tau [7]. This process leads to abnormal accumulation and aggregation, followed by formation of the neurofibrillary tangles that are characteristic of AD [7]. Conversely, deletion of α7-nAChR in the mouse model of AD achieves protection of synaptic integrity and relative preservation of learning and memory functions [8]. Therefore, disrupting the interaction between -amyloid and α7-nAChR has been suggested as a validated therapeutic target in treating AD [9].
The α7-nAChR agonists comprise one class of compounds known to block the interaction between -amyloid and α7-nAChR [9]. As these agonists typically lose their effect over time, the development of welltolerated agents that act in the preferred mechanism is an active area of research for AD treatment.
Galantamine, an approved medication for AD, acts primarily as a cholinesterase inhibitor to slow cognitive decline and reduce behavioral symptoms in patients, but it fails to modify the underlying pathophysiology [9]. However, the drug was also discovered to act through a secondary mechanism as a α7-nAChR PAM [10]. α7-nAChR PAM compounds have been demonstrated to affect both -amyloid and tau deposition in AD [5,7]. Though it is unclear if galantamine is able to function this way due to its preferential functioning as an acetylcholinesterase inhibitor, evaluating other α7-nAChR PAMs in animal models of AD may reveal the true effects of these agonists. In studies with galantamine, mice have served as good predictive models, as the animal overexpresses amyloid precursor protein (APP) with three familial AD (FAD) mutations [10]. The 5XFAD mouse model has reproduced memory impairment, plaque formation, reduced anxiety, and neuron loss; administration of galantamine decreased plaque formation and slowed behavioral decline [10,11].
In this study, we have virtually screened FDA-approved drugs using a pharmacophore for a α7-nAChR PAM and tested commercially available compounds for this activity in vitro. Additionally, we have created structurally novel derivatives of one of the resultant hit drugs, propranolol, and screened those new derivatives for α7-nAChR PAM activity, as well. The results of this study suggest that propranolol and the derivatives target α7-nAChR and may potentially be useful in the treatment of AD and other neurodegenerative disorders that involve β-amyloid and/or tau protein deposition in the brain.
Materials and Methods
Pharmacophore modeling and virtual screening
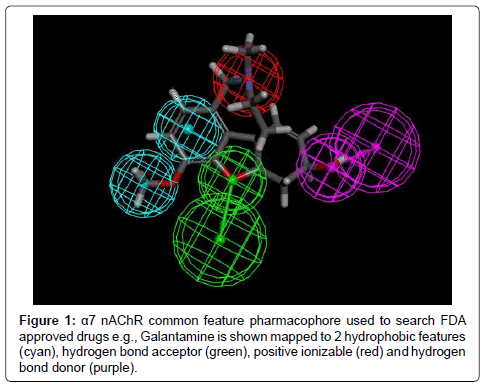
A common feature pharmacophore was developed based on galantamine using commercially available software (Discovery Studio vers 4.1, Biovia, San Diego, CA), as described in detail in our prior publication [12]. Briefly, we started by using galantamine as the template for a common feature pharmacophore model, which was then used to search a 3D database of FDA approved drugs and commercially available compounds [13].
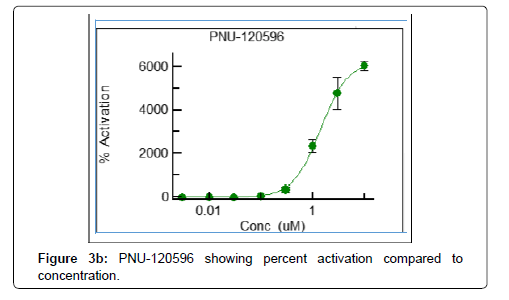
In Vitro patch-clamp assay for PAM activity at the α7 nAChR All patch-clamp testing was performed by ChanTest, Charles River Discovery, Inc. (Cleveland, OH), an independent contract research company, using samples of commercially available compounds procured from Sigma Aldrich, Inc. (St. Louis, MO). During patchclamp testing, all recordings of currents through α7-nAChR were made in a blinded fashion and included measurements of concentrationresponse and rate-dependence of test articles on α7-nAChR activation kinetics [12]. A commercially available positive control, PNU-120596, was used for quality control purposes with all recordings, and test articles were evaluated at eight different concentrations with four replicates per concentration performed to ensure reliability of the data [12]. The compounds’ effects on receptor kinetics were either calculated as percent activation according to previously published formulas, or determined to be present by analysis of variance (ANOVA) using a p-value of less than 0.05 to indicate statistically significant differences between multiple concentrations of a single test article.
Design of structurally novel propranolol derivatives
The 3-D structure for the α7-nAChR has not yet been determined, and consequently we used the acetylcholine binding protein as a substitute for the computer based binding affinity simulations. The 3-D structure of the acetylcholine binding protein was downloaded from the National Protein Data Bank and the 3-D structure for propranolol was downloaded from DrugBank.ca.gov. A set of potential derivatives was then created by systematically replacing single hydrogen atoms in propranolol with either a methyl, hydroxyl, or carbonyl group at all possible locations along the carbon chain of the molecule using the commercially available program Visual Molecular Dynamics. A second commercially available program, PyRx, was then used to determine the predicted binding affinities between individual derivatives and the α7- nAChR.
Synthesis of structurally novel propranolol derivatives
Synthesis of structurally novel propranolol derivatives was performed by a contract research company (BioFocus, Inc., United Kingdom). The structures for all compounds synthesized were confirmed through three methods: high-performance liquid chromatography (HPLC), mass spectroscopy (MS), and nuclear magnetic resonance (NMR) studies.
Results
Virtual screen
The pharmacophore based on galantamine consisted of two hydrophobic features, a hydrogen bond acceptor, a hydrogen bond donor, and a positive ionizabile feature (Figure 1). After screening over 1000 molecules, over 160 remaining molecules that mapped to the pharmacophore was filtered to identify potentially repurposable α7-nAChR PAMs. Molecules that mapped well to the features of either pharmacophore were identified and known PAMs or problematic compounds were removed. The selected compounds were then tested in vitro (Table 1).
| TA # | Test Article ID | EC50 (IC50), M | |
|---|---|---|---|
| Peak | 0.5-AUC | ||
| 1 | Carvedilol | (18.9) | (4.4) |
| 2 | Alfuzosin hydrochloride | (22.9) | (14.2) |
| 3 | Riboflavin | ND | ND |
| 4 | Bromocriptine | ND | (66.1) |
| 5 | Propanolol | (15.9) | (5.3) |
| 6 | Fexofenadine | ND | ND |
| 7 | O-Desmethyl Galantamine | ND | ND |
| 8 | N-Desmethyl Galantamine | ND | ND |
| 9 | Galantamine hydrobromide | ND | ND |
| PC | PNU-120596 | 1.066 | 1.215 |
Table 1: EC50 (IC50) values of Test Articles.
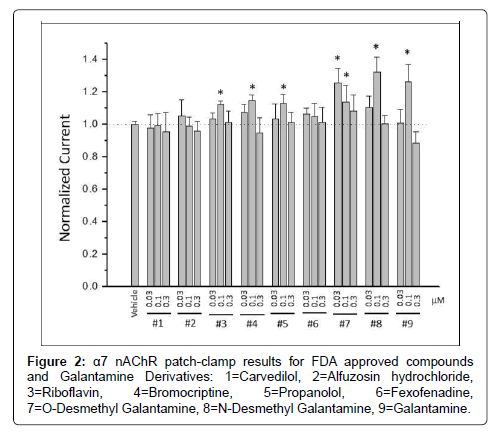
Patch clamp studies of FDA approved compounds and galantamine derivatives
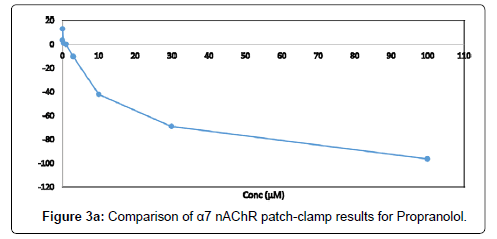
Three compounds: riboflavin, bromocriptine, and propranolol, were identified as displaying PAM effects at the α7-nAChR in addition to galantamine and two galantamine metabolites (O-desmethyl and N-desmethyl) that displayed measurable α7-nAChR PAM activity at lower concentrations (Figure 2). Furthermore, three compounds, carvedilol, alfuzosin hydrochloride, and propranolol, were identified as showing inhibitor effects at the α7-nAChR (Table 1). The IC50 value was greatest for alfuzosin (IC50=22.9), followed by carvedilol (IC50=18.9), and was lowest for propranolol (IC50=15.9). Not only did propranolol yield an order of magnitude of the PAM effect on α7-nAChR that was much lower than that of the commercially available PAM compound used as a positive control PNU-120596, but it also displayed evidence of functional inhibition at higher concentrations (Figure 3). Functional inhibition is not seen with most other commercial α7-nAChR compounds, including PNU-120596 (Figure 3).
Binding affinity studies of propranolol analogs
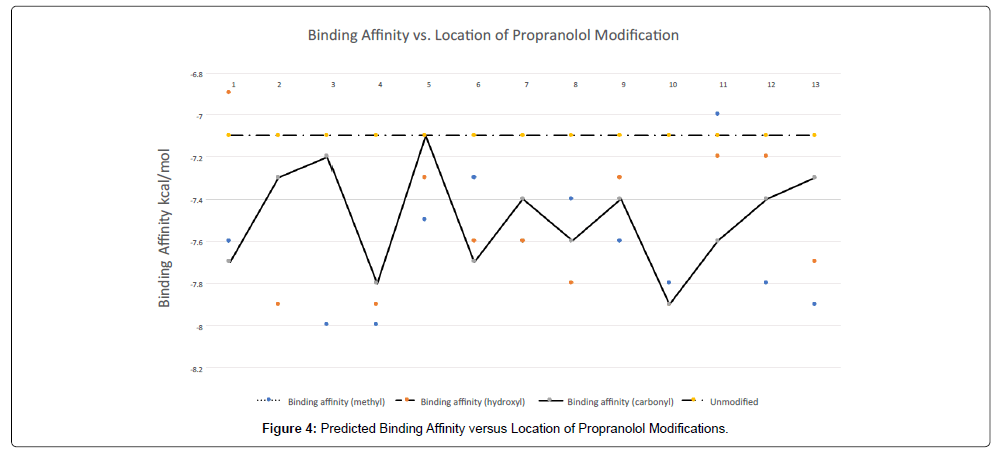
Computational binding affinity calculations for possible propranolol modifications, i.e. additions of either methyl, hydroxyl, or carboxyl groups along the carbon chain of the molecule, were calculated using PyRx and graphed relative to the position of the modification in the molecule (Figure 4). While multiple types and locations of modifications increased the binding affinity with the α7-nAChR, we selected 4 structures to synthesize, including one structure, derivative 6, for which two stereoisomers were possible.
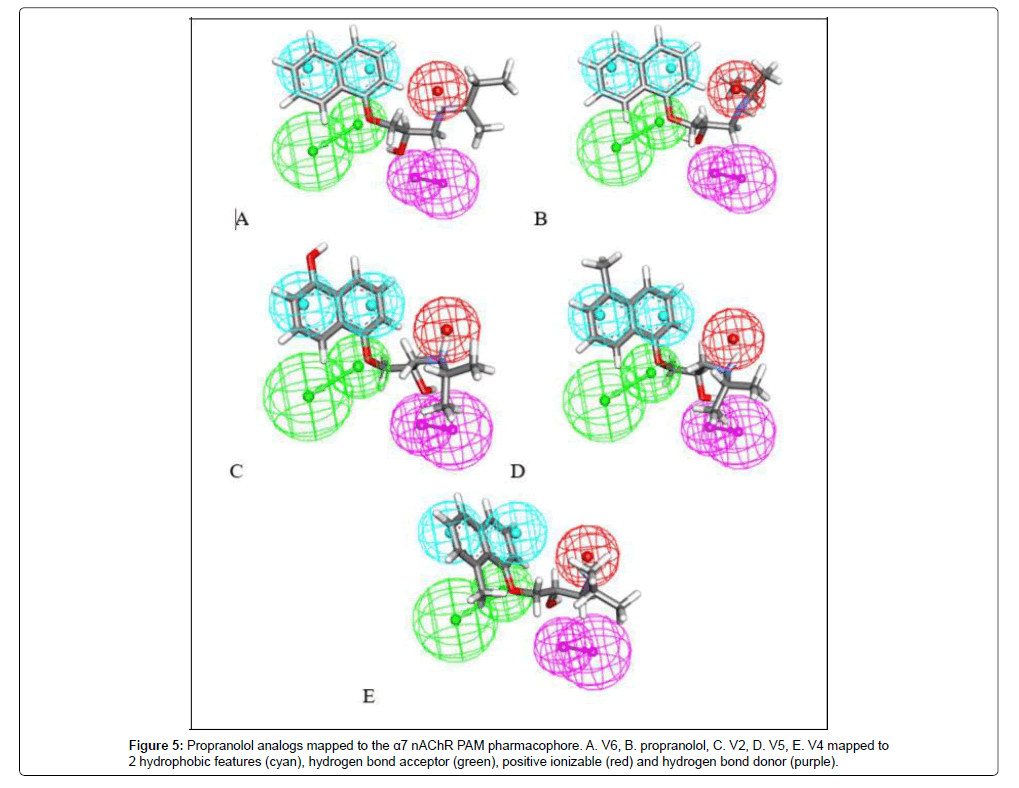
Mapping structurally novel propranolol analogs to the α7- nAChR PAM pharmacophore
The analogs were mapped to the α7-nAChR PAM pharmacophore, allowing up to one feature miss, and all molecules appeared to fit with scores ranging from 0.90-2.81 (Figure 5).
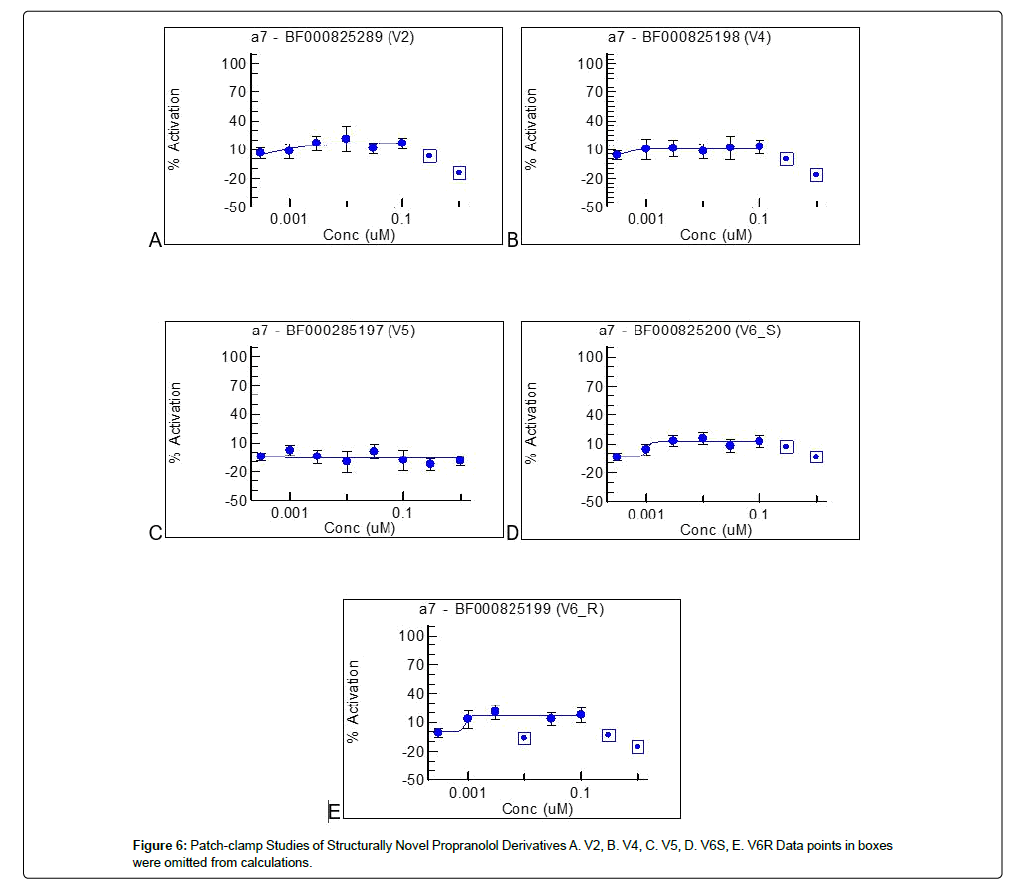
Patch clamp studies of propranolol analogs
Of the five structurally novel propranolol analogs tested, four (derivatives 2, 4, 6S, and 6R) displayed PAM effects at the α7-nAChR (Figure 6). Each of these analogs not only had milder magnitudes of effect than PNU-120596 (similar to the magnitude of effect displayed by propranolol and galantamine), but also displayed functional inhibition at higher concentrations (Figure 6).
Discussion
Current therapies for Alzheimer’s disease are suboptimal as they are purely symptomatic with no disease modifying effect. To date, there are no available medications that target the underlying histopathological changes associated with the disease, namely the formation of β-amyloid and tau protein aggregates. α7-nAChR agonists comprise one class of potentially disease modifying compounds through disruption of the interaction between β-amyloid and α7-nAChR.(9) Consequently, developing novel compounds that act through this mechanism and are well tolerated is an active area of AD research. In this study, we identified commercially available compounds with previously unknown α7- nAChR PAM functionality. For one of these compounds, propranolol, we created four structurally novel derivatives that displayed α7-nAChR PAM functionality, as well as functional inhibition. Further in vivo testing is warranted for possible development of propranolol, as well as all compounds with 7-nAChR PAM functionality, as new therapeutic agents for AD based on these preliminary studies.
Propranolol is particularly well suited for potential repurposing as an α7-nAChR PAM, as it is currently FDA approved in the United States for treatment of a variety of medical disorders, including hypertension, irregular heartbeat, essential tremors, and anxiety, and the drug has been utilized in many countries [14]. Propranolol is considered to be a safe medication that requires no blood-test monitoring, produces minimal side effects, commonly doses well below toxic levels, and is generally well-tolerated [14]. The drug acts as a non-selective -blocker without sympathomimetic activity [14]. Additionally, it demonstrates membrane stabilizing activity and is highly lipid soluble which, when coupled with its low molecular weight, allows for good blood-brainbarrier penetration [14]. Consequently, this medication is ideal for repurposing, and its efficacy in animal models could be assessed prior to treating patients with AD.
We expect that the utility of a well-tolerated and effective α7-nAChR PAM compound would extend beyond treatment of Alzheimer’s disease. For instance, β-amyloid and tau protein deposition have been implicated in the pathogenesis of several other neurodegenerative disorders which may also be amenable to treatment with α7-nAChR agonists [15]. Relatively common and currently untreatable disorders, such as schizophrenia and dementia in Parkinson’s disease in particular, have been suggested as targets for treatment with α7-nAChR PAMs [4].
Several α7-nAChR PAM compounds have been developed by various pharmaceutical companies and have gone on to clinical trials, including: A-582941 (2-methyl-5-(6-phenyl-pyridazin-3-yl)- octahydro-pyrrolo[3,4-c]pyrrole; Abbott Laboratories), ABT-107 (5-(6-[(3R)-1-azabicyclo[2,2,2]oct-3-yloxy]pyridazin-3-yl)-1H-indole; Abbott Laboratories), EVP-6124 (Elan Pharmaceuticals, Dublin, Ireland), SB-206553 (3,5-dihydro-5-methyl-N-3-pyridinylbenzo [1,2-b:4,5-b0] -dipyr-role-1 (2H)-carboxamide), PNU-120596 (1-(5-chloro-2,4- dimethoxy-phenyl)-3-(5-methyl-isoxazol-3-yl)-urea; GSK Pharmaceuticals), and Encenicline (R)-7-chloro-N-quinuclidin- 3-yl)benzo[b]thiophene-2-carboxamide; Forum Pharmaceuticals).
These compounds, which possessed in vitro activity similar in magnitude to PNU-120596 without functional inhibition, have been tested in various Phase 1-3 clinical trials, but none have demonstrated a clear disease modifying effect to date. In September 2015, the FDA placed a clinical hold on three Phase 3 studies of an α7-nAChR PAM in Alzheimer’s disease, which prohibited any further clinical research with these compounds [16]. Additionally, a clinical hold, though later partially lifted, was placed on one Phase 1 study in schizophrenia, due to the compound’s (Encenicline) severe gastrointestinal side effect resulting from overstimulation of nicotinic receptors [16]. Other clinical trials of α7-nAChR PAMs have failed due to lack of efficacy and the compounds are no longer being developed as potential new drugs [17].
The failure of clinical trials involving α7-nAChR PAM compounds, either due to serious adverse events or an inability to demonstrate meaningful clinical benefit, has been a large set back in the field, delaying the introduction of this class of potential therapeutic compounds to the market. These failures have been attributed, in part, to excessive overstimulation of the α7-nAChR causing neuronal excitotoxicity [18]. In general, the α7-nAChR PAMs that have been tested in clinical trials demonstrated a current across the receptor that was augmented by up to 6,000% of normal in in vitro studies [18]. From compounds with similar activity profiles, stimulation of neuronal nAChRs induced neuronal cell death through excitotoxicity [18]. Consequently, a drug with a more modest effect at the receptor, such as propranolol, may offer a therapeutic alternative that delivers the potential benefits of α7-nAChR PAMs in AD, without causing adverse events related to overstimulation of the receptor. However, this hypothesis requires further evaluation in additional controlled studies.
Due to the methods and procedures used throughout this study, there are limitations to our findings and their potential applications. In calculating IC50 for propranolol and the analogs, insufficient concentrations were tested in the lower range, given that could potentially elicit agonist activity. Thus, additional testing with lower concentrations of propranolol and the analogs should be performed to generate their IC50’s. Furthermore, only a select few analogs predicted by the computer as the most likely to have PAM activity were synthesized and tested; many other propranolol analogs with PAM activity likely exist. We did not test propranolol against nicotinic receptors other than the 7-nAChR. Further studies are required to determine whether propranolol acts similarly, i.e. with functional inhibition, with all nicotinic receptors, or if this activity is unique to the 7-nAChR. Furthermore, because the 3-D model for 7-nAChR is not available, an acetylcholine binding protein was used as a substitute. Although this protein is similar to the receptor to a sufficient degree, we cannot be certain that the receptor would act in the same way [19].
There are also limitations that present themselves upon considering future applications of propranolol. For instance, all tests in this study were performed in vitro, thus the same PAM functionality cannot be assumed in vivo due to the possibility of unexpected binding or reaction with other proteins. Future research in mouse AD models is a suitable initial step in understanding the true nature of these molecules. Finally, this preliminary study did not explore the metabolism of the propranolol analogs or their ability to cross the blood-brain-barrier. Further in vitro absorption, distribution, metabolism and excretion studies must be performed to collect this data.
Conclusion
In this study, we identified previously unknown in vitro α7-nAChR PAM activity for several FDA approved medications, including propranolol. Compared to other α7-nAChR PAM compounds that have failed in clinical trials, propranolol possesses a milder and more physiological augmentation of current across the α7-nAChR. More importantly, it displays functional inhibition and may prevent overstimulation of the receptor, which has been a cause of failure in previous studies. We feel that the milder α7-nAChR PAM effect of propranolol may allow for a beneficial disruption of α7-nAChR’s and -amyloid’s interaction, without resulting in adverse events from overstimulation-induced neuronal excitotoxicity. We also designed structurally novel propranolol derivatives that possess α7-nAChR PAM activity. However, these compounds require additional studies to determine whether they may be suitable in treating patients with AD.
Acknowledgement
The authors received support for this project from the Joseph Stahlberg Foundation.
References
- Mielke MM, Vemuri P, Rocca WA (2014) Clinical epidemiology of Alzheimer's disease: assessing sex and gender differences. Clin Epidemiol 6: 37-48.
- Ma KG, Qian YH (2019) Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer's disease. Neuropeptides 73: 96-106.
- West S, Bhugra P (2015) Emerging drug targets for Abeta and tau in Alzheimer's disease: a systematic review. Br J Clin Pharmacol 80: 221-234.
- Toyohara J, Hashimoto K (2010) Alpha 7 Nicotinic Receptor Agonists: Potential Therapeutic Drugs for Treatment of Cognitive Impairments in Schizophrenia and Alzheimer's Disease. Open Med Chem J 4: 37-56.
- Wang HY, Lee DH, D'Andrea MR, Peterson PA, Shank RP, et al. (2000) Beta-Amyloid (1-42) binds to alpha 7 nicotinic acetylcholine receptor with high affinity. Implications for Alzheimer's disease pathology. The Journal of biological chemistry 275: 5626-5632.
- Wang HY, Lee DH, Davis CB, Shank RP (2000) Amyloid peptide A beta (1-42) binds selectively and with picomolar affinity to alpha 7 nicotinic acetylcholine receptors. J Neurochem 75: 1155-1161.
- Wang HY, Li W, Benedetti NJ, Lee DH (2003) Alpha 7 nicotinic acetylcholine receptors mediate beta-amyloid peptide-induced tau protein phosphorylation. The Journal of biological chemistry 278: 31547-31553.
- Dziewczapolski G, Glogowski CM, Masliah E, Heinemann SF (2009) Deletion of the alpha 7 nicotinic acetylcholine receptor gene improves cognitive deficits and synaptic pathology in a mouse model of Alzheimer's disease. The Journal of neuroscience 29: 8805-8815.
- Hashimoto K, Iyo M (2002) Amyloid cascade hypothesis of Alzheimer's disease and alpha 7 nicotinic receptor. Nihon Shinkei Seishin Yakurigaku Zasshi 22: 49-53.
- Bhattacharya S, Haertel C, Maelicke A, Montag D (2014) Galantamine slows down plaque formation and behavioral decline in the 5XFAD mouse model of Alzheimer's disease. PloS one 9: e89454.
- Bhattacharya S, Maelicke A, Montag D (2015) Nasal Application of the Galantamine Pro-drug Memogain Slows Down Plaque Deposition and Ameliorates Behavior in 5X Familial Alzheimer's Disease Mice. Journal of Alzheimer's disease JAD 46: 123-136.
- Ekins S, Mathews P, Saito EK, Diaz N, Naylor D, et al. (2017) Alpha7-Nicotinic acetylcholine receptor inhibition by indinavir: implications for cognitive dysfunction in treated HIV disease. AIDS 31: 1083-1089.
- Motel WC, Coop A, Cunningham CW (2013) Cholinergic modulation by opioid receptor ligands: potential application to Alzheimer's disease. Mini reviews in medicinal chemistry 13: 456-466.
- Wong YC, Krainc D (2017) Alpha-synuclein toxicity in neurodegeneration: mechanism and therapeutic strategies. Nat Med 23: 1-13.
- Yang T, Xiao T, Sun Q, Wang K (2017) The current agonists and positive allosteric modulators of alpha7 nAChR for CNS indications in clinical trials. Acta Pharm Sin B 7: 611-622.
- Guerra-Alvarez M, Moreno-Ortega AJ, Navarro E, Fernandez-Morales JC, Egea J, et al. (2015) Positive allosteric modulation of alpha-7 nicotinic receptors promotes cell death by inducing Ca (2+) release from the endoplasmic reticulum. J Neurochem 133: 309-319.
- Brejc K, van Dijk WJ, Klaassen RV, Schuurmans M, van Der Oost J, et al. (2001) Crystal structure of an ACh-binding protein reveals the ligand-binding domain of nicotinic receptors. Nature 411: 269-276.
Citation: McMurtray A, Saito E, Tsang S, Lee A, Ekins S (2020) Propranolol and Structurally Novel Derivatives as Positive Allosteric Modulators of the Alpha-7- nicotinic Acetylcholine Receptor. Clin Pharmacol Biopharm 9: 192. DOI: 10.4172/2167-065X.1000192
Copyright: © 2020 McMurtray A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4223
- [From(publication date): 0-2020 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 3287
- PDF downloads: 936