Purification and Characterization of Îñ-Amylase from Bacillus subtilis Isolated from Cassava Processing Sites
Received: 29-Sep-2017 / Accepted Date: 26-Oct-2017 / Published Date: 30-Oct-2017 DOI: 10.4172/2155-6199.1000417
Abstract
This study was designed to purify and characterize of α-amylase from pure strain of Bacillus subtilis. The crude α- amylase was purified by ammonium sulphate precipitation, then loaded on DEAE Sephadex A-50 ion exchange chromatography and gel filtration. The effect of pH, temperature and metal ions were investigated on the purified enzyme. The single protein band on SDS-PAGE suggested that the enzyme was homogenous. Two different activity peaks were observed in ion exchange chromatography designated pool A and pool B with the 8% and 4% yield, 15.93 and 6.44 purification fold and specific activity 2.55 μmol/min/mg and 1.03 μmol/min/mg respectively. The two fractions revealed the same optimum pH 7.0 for the α-amylase activity while the enzyme was relatively stable at pH 4.0 and 7.0 between 20 to 40 minutes and 60 to 80 minutes for pool A and pH 8.0 between 40 and 100 minutes for pool B. At 40°C, optimum temperature was reached, and amylase activity was maintained at 75% and 70% temperature stability between 60 to 80 minutes for pool A and B, less than 20%, the residual activity at 60°C and 70°C was recorded. The incubation of α-amylase with Na+ and Zn2+ ions enhanced/activate the enzyme activity correspondingly, Al3+ and K+ ions exhibited varied degree of inhibition while Ca2+ and Hg2+ ions caused total inhibition on α-amylase activity. The ability of purified α-amylase from Bacillus subtilis under wide range of temperatures and pH suggests its applications in industries and bioremediation of effluent discharge on food processing sites.
Keywords: Bacillus subtilis ; Cassava processing site; α-amylase; Purification; Characterization
Introduction
Globally, indiscriminate dumping or disposal of wastes to the environment affects both macro-and-microorganisms causing ecological imbalance and displacement from their niche. The survival of microorganisms depended on several factors such as biotic, and abiotics. Soil stands as major reservoir of different microorganisms. Biodegradation of wastes on/in soil by the action of microorganisms could prompt a safer environment [1]. Microorganisms isolated from soil stand as potential source of industrial enzymes due to their abundance in nature. However, utilization of these enzymes such as amylases, cellulases, linamarases, mannanase and pectinase etc in our industries are limited due to the poor exploration. Amylases (EC 3.2.1) is a hydrolytic enzyme that catalyze the hydrolysis of amylose and amylopectin from starch, as well as starch derivatives such as dextrin and oligosaccharides liberating reducing groups [2]. Amylase constitute a class of industrial enzymes having approximately 25%-33% of the enzyme in the enzyme market [3,4]. α-amylase can be obtained from several sources, such as plants, animals and microorganisms [5,6]. Commercial application of amylase produced from genus Bacillus spp has been widely used in baking and bread industry, textile and leather, laundry and detergent industry, pulp and paper, pharmaceuticals, feed processing and agriculture [7-9]. Due to technological advancement, α-amylases applications have been extended in many fields such as clinical and analytical chemistry, medicine, brewing and food industry for the starch liquefaction and saccharification, and biogeochemical cycle of carbon [7,10,11]. Enzymes from genus Bacillus spp such as Bacillus subtilis , Bacillus stearothermophilus, Bacillus licheniformis and Bacillus amyloliquefaciens are of special interest in our industrial applications as the sole producer of amylase, its commercial production and ability to withstand high temperatures [7,12]. However, the high activity of exhibited by this enzyme produced from Bacillus subtilis at wide pH and temperatures suggest it applications for various industrial purposes and in bioremediation of soil polluted with effluents discharge on food processing sites.
Materials and Methods
Bacterial isolates
The bacterium (Bacillus subtilis) used in this study was isolated from cassava processing sites. The bacterium was isolated using standard microbiological methods under optimum cultural conditions.
α-amylase assay: α-amylase activity was determined as described by Okolo et al. [13] with slight modification. The reaction mixture comprising of 2.0 ml of 1% corn starch prepared in 0.5 ml of 0.05 M sodium phosphate buffer (pH 7.0) and 0.5 ml of the supernatant of the crude enzyme extract was used to assay for α-amylase.
The blank (control tube) contained 0.5 ml of 0.05 M sodium phosphate buffer (pH 7.0), 0.5 ml of 1% starch solution and 1 ml sterilized distilled water. Both the experimental and control tubes were incubated at 30°C for 10 minutes. At the end of incubation, the tubes were removed from the water bath (Lamfield Medical England Model DK-600), and the reaction was terminated by adding 1 ml of 3,5- dintrosalicylic acid (DNSA) reagent tube. The tubes were incubated for another 5 minutes in a boiling water bath for colour development and cooled rapidly. The activity of the reaction mixture was measured against blank at 540 nm. The amount of liberated reducing sugars (glucose equivalents) were estimated by the dinitrosalicylic acid (DNS) method of Miller. One unit of α-amylase was defined as amount of enzyme producing 1 micromole of glucose equivalent per minute under the experimental conditions.
Protein determination: The protein estimation of the crude extract was determined by Bradford method and Bovine Serum Albumin (BSA) was used as standard Bradford.
Purification and Characterization of α - amylase from Bacillus subtilis
Purification of α-amylase from Bacillus subtilis : The purification of crude extract (enzyme supernatant) obtained after cold centrifugation was done in three stages. In stage one, 400 ml of the supernatant of crude enzyme was precipitated by adding 240 g ammonium sulphate to obtained 60% ammonium sulphate concentration. The concentrates were then cold centrifuged at 6000 rpm for 30 minutes, the precipitate obtained was washed with 100 ml phosphate buffer (pH 6.0) and then dialyzed in the same buffers. In stage two, the partially purified α– amylase obtained was purified on ion exchange column chromatography containing DEAE (diethylamineethyl) Sephadex A-50 (2.0 × 2.5 cm, Pharmacia). The eluates obtained from stage two were washed with 300 ml ion-free water, followed by 200 ml 0.01 M Tris- HCl buffer (pH 8.0). The gel was eluted with NaCl. The absorbance of each of the fraction at time interval was measured. In stage three, 2.5 ml of partially purified α–amylase was loaded onto a column chromatography (2.5 cm in diameter and 30.0 cm high) using Sephadex G-150 (Pharmacia). Phosphate elution buffer at 50 mM pH 6.0 was applied with the flow rate 20 ml/hour. A fraction of 5.0 ml was collected at interval of 30 minutes and the highest α–amylase activity was determined with corn starch as a substrate, at 280 nm (Jenwey 6305). Fractions with α–amylase activity were pooled and concentrated in glycerol solution at 30°C [14].
Characterization of purified α–amylase from Bacillus subtilis
Effect of pH on α–amylase activity and stability: Different buffers (0.05 M): Glycine-HCl (pH 3.0-4.0), sodium acetate buffer (pH 5.0-6.0), sodium phosphate buffer (pH 7.0), Tris-HCl (pH 8.0), glycine-NaOH buffer (pH 9.0) and 1% corn starch as substrate were prepared. The assay was carried out according to the standard assay procedure. The pH stability of the purified enzyme was determined at different pH buffers ranged from 5.0 to 8.0 at room temperature for different time interval up to 2 hours. Thereafter, relative α–amylase activity was assay.
Effect of temperature on α–amylase activity and stability: α–amylase activity was assay by incubating the enzyme reaction mixture at different temperature, 30°C to 90°C for 120 minutes in in 0.05 M sodium phosphate buffer (pH 7.0) and 1% corn starch. The temperature stability was determined by measuring the residual activity at 60°C. After incubation, samples were collected at 20 minutes and thereafter residual activities were evaluated under standard assay conditions.
Effect of metal ions on α–amylase activity: A stock solution of 10 mM for different metal ions was prepared. Two milliliters of the prepared solution was mixed with 2 ml of enzyme solution. The mixture was incubated for 5 minutes at room temperature. Zero point five milliliters (0.5 ml) of the mixture were collected and α–amylase assay was carried out according to the standard assay procedure.
Results and Discussion
Summary of purification
Table 1 shows the α–amylase purification processes. The homogeneity of the purified α–amylase was obtained in three steps with 16% and 7% fold, approximately total protein recovery of 8% and 4% with specific activity 2.55 μmol/min/mg and 1.03 μmol/min/mg respectively of pool A and pool B. The purification fold increased due to desalting of the enzyme. The homogeneity of the crude enzyme could result from the fact that the isolate is from a known source. Purification of amylase from Bacillus subtilis by centrifugation on DEAE-Cellulose and gel filtration Sephadex 75 column with 26 purification fold and 10% yield has been reported [15]. Mukesh et al. [8] reported purifed α–amylase from Bacillus sp. MN123 with 27.41% yield and 2358 U/mg specific activity. The percentage yield 13.4%, 16.3 fold and specific activity 19.8 mM/min/mg of purified xylanase from Bacillus cereus was reported [16].
| Step | Vol. | PC | EA | TP | TA | SA | Yield (%) | Fold |
|---|---|---|---|---|---|---|---|---|
| crude extract | 500 | 7.65 | 1.2 | 3825 | 600 | 0.16 | 100 | 1 |
| ASP (40 - 60%) | 21.5 | 6.11 | 4.65 | 131.37 | 99.98 | 0.76 | 16.66 | 4.75 |
| Dialysis against buffer | 25 | 5.62 | 4.16 | 140.5 | 104 | 0.74 | 13.91 | 4.63 |
| Ion exchange (A 50) | ||||||||
| Pool A | 12.16 | 4.16 | 6.22 | 50.59 | 75.64 | 1.49 | 12.61 | 9.31 |
| Pool B | 8.91 | 3.98 | 4.01 | 35.46 | 35.73 | 1.01 | 5.95 | 6.31 |
| Gel (G-100) | ||||||||
| Pool A | 12.56 | 1.46 | 3.72 | 18.34 | 46.72 | 2.55 | 7.79 | 15.93 |
| Pool B | 10.72 | 1.95 | 2.01 | 20.9 | 21.55 | 1.03 | 3.59 | 6.44 |
Table 1: Purification table of α–amylase produced from Bacillus subtilis. CP=crude protein (mg/ml); TP=total protein (mg); EA=Enzyme activity (micromole/min/mg); SPA=specific enzyme activity (micromole/min/mg); TA=total activity (micromole/min/ml); ASP=Ammonium sulphate precipitation.
The high specific activities of peak A (2.55 micromole/min/mg) and B (1.03 micromole/min/mg) than that of the crude and ammonium sulphate precipitates could be attributed to increase in the level of purity of the enzyme as compared to the crude and the precipitates, starch-hydrolyzing enzymes filtrate isotypes, the effect of synergy on the enzyme activity of the filtered enzymes when compared to the crude [17]. The increase in specific activities for both enzyme after gel filtration could be ascribed to the purification procedure which enhance the specific activity of the desired enzyme after the purification procedure [17].
Elution
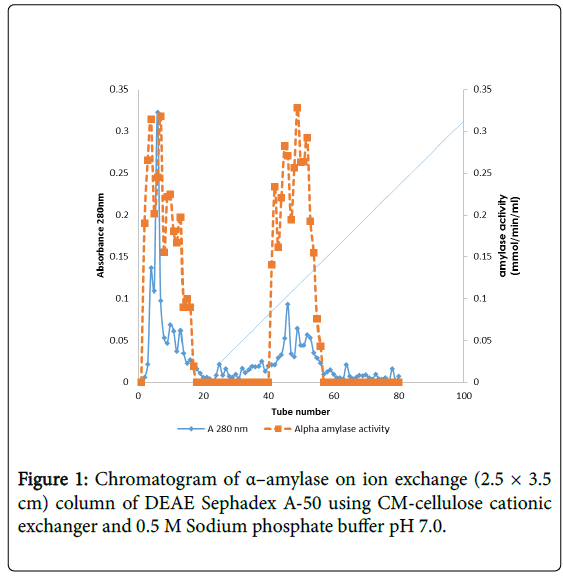
The elution pattern of the α–amylase enzyme on Sephadex A-50 gel filtration of the precipitated enzyme, two enzyme activity designated peaks loop A and loop B were observed showing that this enzyme might have isotypes (Figure 1).
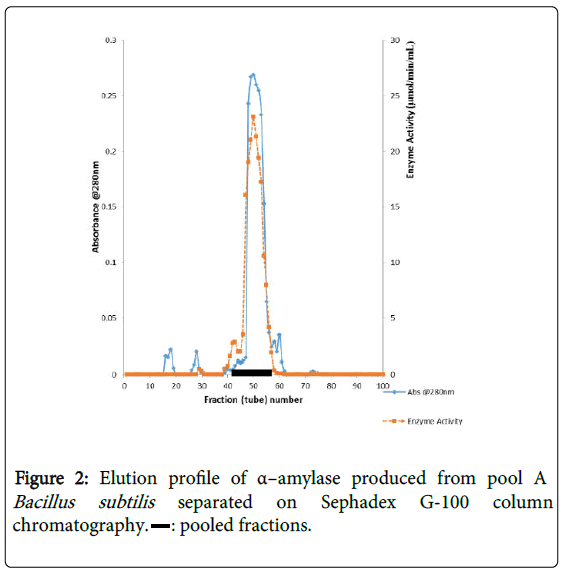
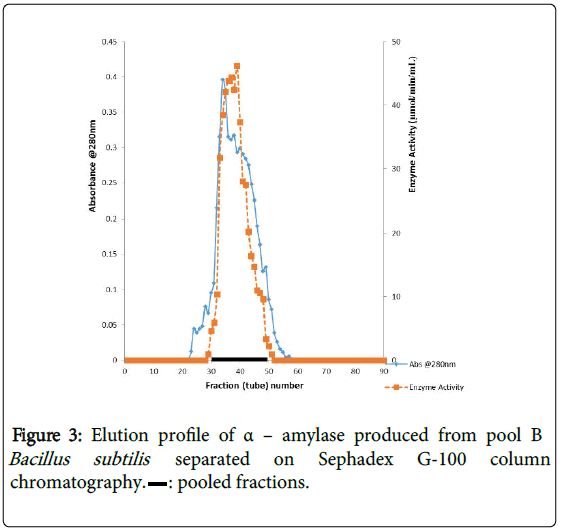
α–amylase activity was detected in fraction tubes 1.00 to 18.00 and 40.00 to 57.00, between fraction tubes 19.00 and 39.00 no enzyme activity was detected. The fractionation tubes (pool A and pool B) with α–amylase activity were separately pooled and further purified separately on Sephadex G-150 (Figures 2 and 3). The highest enzyme activity of the partially purified α–amylase from pool A was attained at fraction tube 50 with specific activity 23.13 μmol/min/mg while the pool B was attained at fraction tube 39 with enzyme activity 42.64 μmol/min/mg respectively. The two forms of amylase activity resulting from the elution process might be due to their molecular weight and catalytic functions [18]. The result is however similar to the findings of Alexander et al. [19] who reported three forms of purified α–amylase, Amyl I, Amyl II and Amyl III from Aspergillus awamori.
Characterization of α–amylase
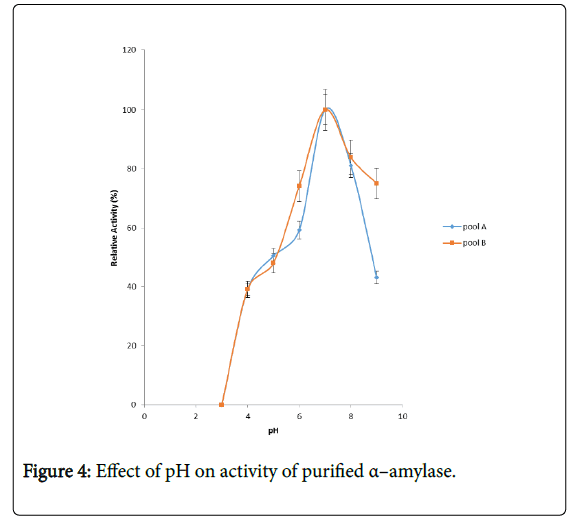
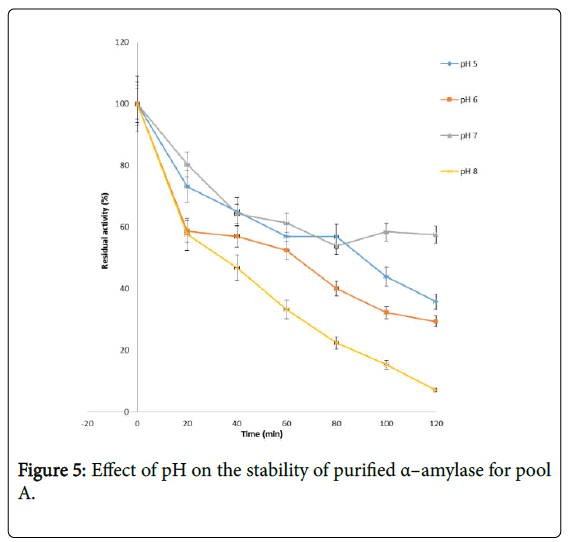
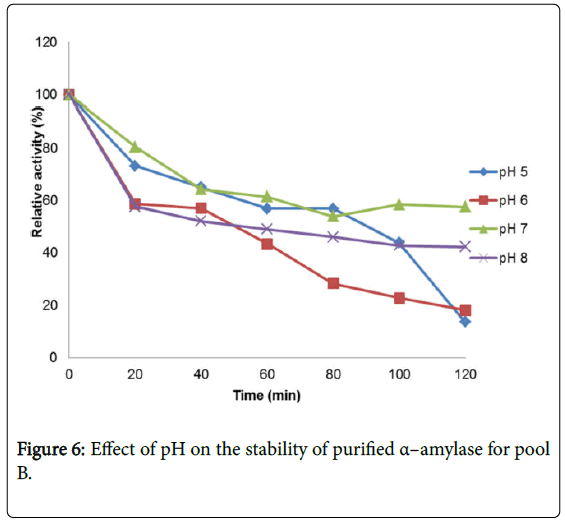
Figure 4 shows the effect of pH on purified α – amylase activity while the pH stability of the purified α–amylase from pool A and pool B are shown (Figures 5 and 6). The α–amylase from pool A and pool B increased progressively up pH 4 with specific activity 39.17 μmol/min/mg. The optimum activity was attained at pH 7.0. The relative activities of pool A and pool B at pH 6.0 was reduced by 34% and 47% and pH 9.0 was reduced by 10% and 48% respectively when compared with the value obtained at pH 7.0. The stability of the enzyme was checked at different pH ranged from 5.0 to 8.0 at room temperature for two hours. The α – amylase activity obtained from pool A was relatively stable between 20 to 40 and 60 to 80 minutes of incubation at pH 4.0 and 7.0 while α–amylase activity obtained from pool B was stable between 40 to 100 minutes of incubation at pH 8.0 respectively. pH of the growth medium plays an important role by inducing morphological change in the nature of microorganism and their enzyme secretion. The decrease in pH beyond optimum might be due to the concomitant alteration in the conformation of the enzyme protein caused by changes in pH of its environment resulting in changes in the three dimensional structure of the enzyme active sites and substrates binding speed to produce maximum product. pH 6.8-7.2 optimum for purified amylase from Bacillus subtilis has been reported [20]. Roy and Rowshanul [16] reported pH 6.0 optimum for purified xylanase from Bacillus cereus . It was reported that the stability of most plant enzymes decreased significantly at pH values below 4.0 and above 7.5, whereas, the majority of the corresponding microbial enzymes are rather stable at pH 7.0 [21]. Mukesh et al. [8] reported α– amylases stability over a wide range of pH 4 to 11. The optimum pH 7.0 and above for α–amylase activity from B. subtilis, B. licheniformis, B. amyloliquefaciens and thermophilic Anoxybacillus flavithermus has been reported [16,22-26] pH 8.0 optimum has been reported for amylase activity from Thermus sp., Bacillus KSM-K38 and Bacillus spp [27,28] Wide range (pH 3.5 to 12) optimum pH for α–amylases has been reported [7,29-32]. The result obtained from this study agrees with the findings of AgüloËglu Fincan and Bukhari and Rehman [32,33] who reported pH 7.0 optimum for purified α–amylase from Bacillus subtilis from local environment.
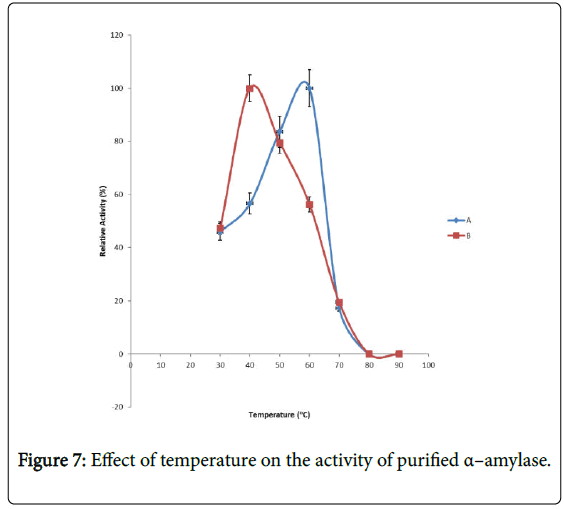
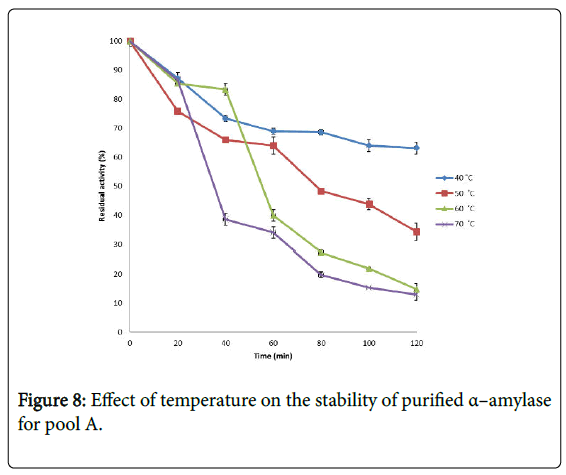
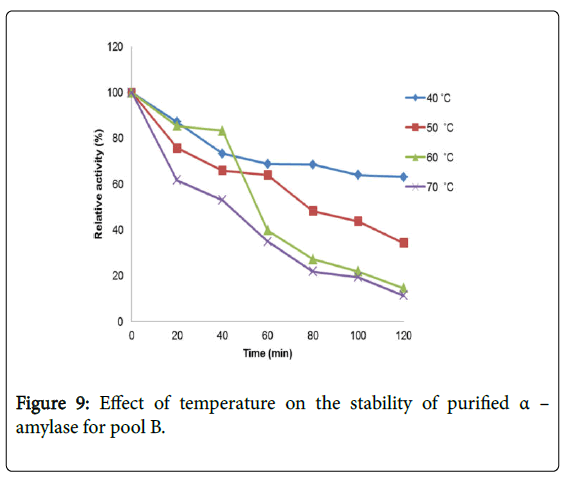
Effect of temperature on α–amylase activity and stability: Figure 7 shows the effect of temperature on purified α–amylase activity while the temperature stability of the purified α–amylase from pool A and pool B are shown in Figures 8 and 9. The α–amylase activity increased with increase progressively with incubation temperature until an optimum percentage relative activity was reached at 40°C and 60°C for pool A and pool B respectively (Figure 7). The purified α–amylase activity from pool A and pool B were relatively thermostable at 40°C and 50°C between 60 and 80 minutes and 40 and 60 minutes before a decline was observed (Figures 8 and 9).
Interest in thermostable amylases has increased tremendously, since resistance to thermal inactivation has become a desirable property in many industrial applications [34]. Enzymes are mostly proteins with a labile nature. Inactivating agents such as heat impair their native conformation so affecting their catalytic functions. It has been reported that most amylases Bacillus spp have an optimum temperature of around 40–70°C [35].
The increase in temperature beyond optimum might cause the denaturation of the enzymes. An optimum temperature 55°C of α– amylase from thermophilic Anoxybacillus flavithermus has been reported [26]. Roy and Rowshanu [16] reported optimum temperature 45°C for purified xylanase from Bacillus cereus . Findings on thermal stability amylase enzyme from bacteria belonging to genus Bacillus has been reported [25]. Averagely, the optimum temperature obtained from this study agree with the findings of Demirkan, Ponnuswamy and Prakash and Mukesh et al. [8,15,31] who reported 50°C optimum for purified α–amylase and cellulase from Bacillus spp from a paddy field.
Effect of metal (ions) on α–amylase activity: Table 2 shows the effect of concentration of metal (ions) on purified α–amylase activity. The metal ions exerted activation and inhibition on the α–amylase activity. The α–amylase activity was stimulated/activated by Na+ and Zn2+ ions correspondingly, Al3+ and K+ ions exhibited varied degree of inhibition while Ca2+ and Hg2+ ions caused total inhibition on α–amylase activity. The salt tolerance test is important in saccharification of starch and in treatment of effluent with high salinity containing starch or cellulose residues in pollution control mechanism [35,36]. Metallic ions can either show the inhibitory or stimulating effect on the activity of enzymes depending on the concentration of the metallic salt solution. Most amylases are known to be metal ion-dependent enzymes, particularly with regard to divalent ions like Ca2+ Mg2+ Mn2+ and Zn2+ [37]. The effect of this metal ions activating the enzyme activity might be due to the presence of chloride in the surrounding soil environment. The inhibition of α–amylase by Hg2+ might indicate the importance of the enzyme function [10]. The inhibition of enzyme activity by metallic ions could be due to competition between the exogenous cations and the protein-associated cations, resulting in decreased metalloenzyme activity [38]. Oboh and Ajele [39] and Mohapatra et al. [40] in their previous studies have reported metallic chlorides as most potent activators of amylase. Demirkan [15] also reported inhibition of purified amylase activity from Bacillus subtilis by metal ions such as Hg2+, Mn+ and Cu2+ while Na+, Zn+ and Mg2+ enhance the enzyme activity. The result obtained from this study contradict Gupta et al. and Mukesh et al. [8,10] who reported enhancement of α-amylase activity from Bacillus sp. MNJ23 by calcium and cobalt due to their high affinity and metalloenzymes nature. Stability of α-amylase activity by Ca2+ ions at high temperatures has been reported [41]. Enhancement of amylase activity by metallic ions might be due to their ability interact with negatively charged amino acid residues such as aspartic and glutamic acid [42], thereby stabilizing and maintaining the enzyme conformation. Researchers have reported inhibition of amylase activity by Ag+, Hg2+, Cd2+, Cu2+, Pb2+, Fe2+, Ni2+, Mn2+ and Zn2+ ions [10,37,43]. The result obtained from this study agrees with the findings of Igarashi et al. [44] who reported strong inhibition 100% (0% relative activity) of Hg2+ ions on alpha amylase from Bacillus specie.
| Metal | Residual activity (%) | |
|---|---|---|
| Pool A | Pool B | |
| Al3+ | 37.61 | 63.54 |
| Na+ | 147.71 | 213.54 |
| Ca2+ | 0 | 0 |
| K+ | 21.10 | 97.92 |
| Hg2+ | 0 | 0 |
| Zn2+ | 156.88 | 277.08 |
| Control | 100 | 100 |
Table 2: Effect of metal ions on purified α–amylase.
Conclusion
The results from this study revealed that Bacillus subtilis isolated from cassava processing sites exhibited the tendency of producing amylase two conspicuous iso-enzymes from the same medium. Thus, this can be employed in agro-processing industries in starch-utilizing industries and food, textile, pharmaceuticals, biofuel, removal of soil pollutant and decontamination of soil debris.
These results suggest that the activity of the enzyme is fairly higher in alkaline pH, making this enzyme attractive for the detergent industry.
References
- Ilangumaran G, Stratton G, Ravichandran S, Shukla PS, Potin P, et al (2017) Microbial degradation of lobster shells to extract chitin derivatives for plant disease management. Front Microbiol 8:781.
- Wolfgang A (2007) Enzyme in Industry: Production and Applications. John Wiley & Sons, New Jersey, USA 11: 8-13.
- Sidhu GS, Sharma P, Chakrabart T (1997) Strain improvement for the production of a thermostable α-amylase. Enzyme and Microb Technol 21: 525-530.
- Rajagopalan G, Krishnan C (2008) Alpha-amylase production from catabolite derepressed Bacillus subtilis KCC103 utilizing sugarcane bagasse hydrolysate. Bioresour Technol 99: 3044-3050.
- Andualem B (2014) Isolation and screening of amylase producing thermophilic spore forming Bacilli from starch rich soil and characterization of their amylase activities using submerged fermentation. Int Food Res J 21: 831-837.
- Fentahun M, Kumari PV (2017) Isolation and screening of amylase producing thermophilic spore forming Bacilli from starch rich soil and characterization of their amylase activity. Afr J Microbiol Res 11: 851-859.
- De Souza PM, Magalhães PO (2010) Application of microbial amylase in industry-a review. Braz J Microbiol 41: 850-861.
- Mukesh DJ, Jayanthi S, Amutha D, Monica D, Bala K (2012) Purification and Characterization of α-Amylase and –Galactosidase from Bacillus Sp. MNJ23 Produced in a Concomitant Medium. American-Eurasian. J Agric Environ Sci 12: 566-573.
- Jaiswal N, Prakash O (2011) Immobilization of soybean α-amylase on gelatin and its application as a detergent additive. Asian Journal of Biochemistry 6: 337-346.
- Gupta R, Gigras P, Mohapatra H, Goswami VK, Chauhan B (2003) Microbial α-amylases: a biotechnological perspective. Process Biochem 38: 1599-1616.
- Luchian M, Csatlos C (2010) Research on the effect of α-amylase used in bakery. Proceeding of the International Conference on New Research in Food and Tourism Bioatlas, University of Brasov, Romania, pp. 111-115
- Shafaat S, Akram M, Rehman A (2011) Isolation and characterization of a thermostable-amylase from Bacillus subtilis. African Journal of Microbiology Research 5: 3334-3338.
- Idriss EE, Makarewicz O, Farouk A, Rosner K, Greiner R, et al. (2002) Extracellular phytase activity of Bacillus amyloliquefaciens FZB45 contributes to its plant-growth-promoting effecta. Microbiology 148: 2097-2109.
- Das P, Kumar P, Kumar M, Solanki R, Kapur MK (2017) Purification and molecular characterization of chitinases from soil actinomycetes. African Journal of Microbiology Research 11: 1086-1102.
- Demirkan ES, Mikami B, Adachi M, Higasa T, Utsumi S (2005) α-Amylase from B. amyloliquefaciens: purification, characterization, raw starch degradation and expression in E. coli. Process Biochemistry 40: 2629-2636.
- Roy N, Habib MR (2009) Isolation and characterization of Xylanase producing strain of Bacillus cereus from soil. Iranian Journal of Microbiology 1: 49-53.
- Lukong B, Awah F, Nwuke C, Fobellah A (2007) Protein Isolation, Purification and Estimation. In: Introduction to Protein Science, Osprey Publication Centre, Owerri, Nigeria pp: 55-56.
- Rezaei PS, Najafpour GD, Shafaghat H, Mahjoub S (2009) Production of amylase from starch using Aspergillus niger NCIM 548. World Applied Sciences Journal 7: 306-311.
- Ciloci A, Bivol C, Stratan M, Reva V, Clapco S, et al. (2012) Production and purification of α-amylase from Aspergillus niger 33-19 CNMN FD 02a mutant form. Analele Universităţii din Oradea, Fascicula Biologie 19: 74-79.
- Tomschy A, Tessier M, Wyss M, Brugger R, Broger C (2000) Optimization of the catalytic properties of Aspergillus fumigatus phytase based on the threeâ€dimensional structure. Protein Science 9: 1304-1311.
- Greiner RC (2009) Toxicology of organophosphate and carbamate compounds. Elsevier Academic Press, Amsterdam, Netherlands, pp: 181-210.
- Tanyildizi MS, Özer D, Elibol M (2005) Optimization of α-amylase production by Bacillus sp. using response surface methodology. Process Biochemistry 40: 2291-2296.
- Haq I, Ashraf H, Qadeer MA, Iqbal J (2005) Pearl millet, a source of -amylase production by Bacillus licheniformis. Bioresour Technol 96: 1201-1204.
- Asoodeh A, Chamani J, Lagzian M (2010) A novel thermostable, acidophilic α-amylase from a new thermophilic “Bacillus sp. Ferdowsicous†isolated from Ferdows hot mineral spring in Iran: Purification and biochemical characterization. International journal of biological macromolecules 46: 289-297.
- Wang SL, Liang YC, Liang TW (2011) Purification and characterization of a novel alkali-stable α-amylase from Chryseobacterium taeanense TKU001, and application in antioxidant and prebiotic. Process Biochemistry 46: 745-750.
- Fincan SA, Enez B, Özdemir S, Bekler FM (2014) Purification and characterization of thermostable α-amylase from thermophilic Anoxybacillus flavithermus. Carbohydrate polymers 102: 144-150.
- Igarashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, et al. (1998) Enzymatic properties of a novel liquefying α-amylase from an alkaliphilic Bacillus isolate and entire nucleotide and amino acid sequences. Applied and Environmental Microbiology 64: 3282-3289.
- Fentahun M, Kumari PV (2017) Isolation and screening of amylase producing thermophilic spore forming Bacilli from starch rich soil and characterization of their amylase activity. African Journal of Microbiology Research 11: 851-859.
- Burhan A, Nisa U, Gokhan C, Omer C, Ashabil A, et al. (2003) Enzymatic properties of a novel thermostable, thermophilic, alkaline and chelator resistant amylase from an alkaliphilic Bacillus sp. isolate ANT-6. Process Biochem 38: 1397-1403.
- Konsula Z, Liakopoulou-Kyriakides M (2004) Hydrolysis of starches by the action of an α- amylase from Bacillus subtilis. Process Biochem 39: 1745-1749.
- Ponnuswamy V, Prakash Vincent SG (2012) Purification and Characterization of Carboxymethyl Cellulase from Bacillus sp. Isolated from a Paddy Field. Polish Journal of Microbiology 61: 51-55.
- Bukhari DA, Rehman A (2015) Purification and Characterization of α-Amylase from Bacillus subtilis Isolated from Local Environment. Pakistan Journal of Zoology 47: 905-911.
- Agülo˘glu Fincan S (2008) Isolation and characterization of Bacillus subtilis α-amylase isolated from C¸ ermik hot water springs. In: 11th-33rd FEBS Congress IUBMB Conference Athens, Greece, p: 275.
- Kumar P, Satyanarayana T (2006) Biotechnological aspects of thermophilic fungal glucoamylases. In: Emerging Trends in Mycology, Plant Pathology and Microbial Biotechnology, BS Publications, Hyderabad, India, Pp: 519-543.
- Cordeiro CAM, Martins MLL, Luciano AB (2002) Production and properties of α -amylase from Thermophilic Bacillus sp. Braz J Microbiol 33: 57-61.
- Al-Qodah Z, Daghstani H, Geopel PH, Lafi W (2007) Determination of kinetic parameters of α-amylase producing thermophile Bacillus sphaericus. African Journal of Biotechnology 6: 699-706.
- Pandey A, Nigam P, Soccol CR, Soccol VT, Singh D, et al. (2000) Advances in microbial amylases. Biotechnol Appl Biochem 31: 135-152.
- Leveque E, Janecek S, Haye B, Belarbi A (2000) Thermophilic archaeal amy-lolytic enzymes. Enzyme and Microbial Technology 26: 3-14.
- Oboh G, Ajele JO (1997) Effect of some Metallic chlorides on the activity of β-amylase. Niger J Biochem Mol Biol 12: 73-75.
- Mohapatra BR, Bapuji M, Sree A (2003) Production of industrial enzymes (Amylase, Carbo xymethyl cellulase and Protease) by bacteria isolated from marine sedentary Organisms. Acta Biotechnol 23: 75-84.
- Chung YC, Kobayashi T, Kanai H, Akiba T, Kudo T (1995) Purification and properties of extracellular amylase from the hyperthermophilic Archaeon Thermococcus profundus DT 5432. Appl Environ Microbiol 61: 1502-1506.
- Linden A, Mayans O, Meyer-Klaucke W, Antranikian G, Wilmanns M (2003) Differential regulation of a hyperthermophilic α-amylase with a novel (Ca, Zn) two-metal center by zinc. Journal of Biological Chemistry 278: 9875-9884.
- Sun H, Zhao P, Ge X, Xia Y, Hao Z, et al. (2010) Recent advances in microbial raw starch degrading enzymes. Appl Biochem Biotechnol 160: 988-1003.
- Igarashi K, Hatada Y, Hagihara H, Saeki K, Takaiwa M, et al (1998) Enzymatic properties of a novel liquefying α-amylase from an alka-liphilic Bacillus isolate and entire nucleotide and amino acid sequences. Applied and Environmental Microbiology 64: 3282-3289.
Citation: Gbenga A, David S, Femi B, Saanu AB (2017) Purification and Characterization of α-Amylase from Bacillus subtilis Isolated from Cassava Processing Sites. J Bioremediat Biodegrad 8:417 DOI: 10.4172/2155-6199.1000417
Copyright: © 2017 Gbenga A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.<
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 8746
- [From(publication date): 0-2017 - Nov 29, 2025]
- Breakdown by view type
- HTML page views: 7546
- PDF downloads: 1200