Research Article Open Access
Rapid Response by the Ectomycorrhizal (ECM) Community of a Lodgepole Pine Forest to Diesel Application as Indicated by Laccase Gene Fragment Diversity
| Ken Cullings* and Julia DeSimone | |
| NASA-Ames Research Center, MS 239-11, Moffett Field, CA 94035-1000, USA | |
| Corresponding Author : | Ken Cullings NASA-Ames Research Center, MS 239-11 Moffett Field, CA 94035-1000, USA Tel: 805-698-1610 Fax: 650-604-3954 E-mail: cullings1@earthlink.net |
| Received May 14, 2014; Accepted July 11, 2014 Published July 15, 2014 | |
| Citation: Cullings and DeSimone (2014) Rapid Response by the Ectomycorrhizal (ECM) Community of a Lodgepole Pine Forest to Diesel Application as Indicated by Laccase Gene Fragment Diversity. J Bioremed Biodeg 5:1000235. doi:10.4172/2155-6199.1000235 | |
| Copyright: © 2014 Cullings K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
A combination of field baiting and molecular methods was used to identify basidiomycete fungi that are able to withstand conditions within diesel contamination in a pine forest soil. Diesel was applied to 3, 1 meter x 1 meter blocks, which penetrated to a depth of 4 cm. After two weeks fungi that exhibited positive growth responses were identified by amplifying laccase (phenol oxidase) gene fragments using primers specific to basidiomycete laccase genes. The internal transcribed spacer (ITS) region of the nuclear ribosomal DNA (nrDNA) gene repeat was amplified as well to provide a broad community screen, to indicate the pool of basidiomycete fungi that could respond to diesel application. Results indicated that ectomycorrhizal (ECM) fungi in the genera Russula, Piloderma and the ECM species Tricholoma saponaceum (rather than wood-rotting basidiomycetes) responded rapidly to diesel application. These results support recent hypotheses regarding the ecological role of ECM fungi in carbon cycling via exploitation of short chain phenolic carbon compounds. Further the ECM fungi that responded correspond roughly to classification of ECM exploration type, a criterion that describes ECM fungi on the basis of their hyphal growth patterns and potential for enzymatic activity. Finally, because it is apparent that these ECM fungi can survive and express laccase genes under conditions created by diesel contamination, these data provide new models for phytoremediation and site restoration strategies that utilize ECM fungi and pines.
Laccases are easily inducible, are involved in lignin beak-down, utilize a broad range of substrates are often extracellular and reduce toxicity of these compounds via immobilization to humic substances, thus lowering their bioavailability [1]. Laccases are easily inducible, utilize a broad range of substrates, and are often extracellular. Phenolic-based substrates that are utilized by laccases include polycyclic aromatic hydrocarbons (PAH) and polychlorinated by phenols (PCB’s), both of which are widespread soil and water contaminants of anthropogenic origin. Because of these utilities, the range of industrial uses is wide and includes enzymatic remediation of soil and water contaminants, polymer synthesis, pulp bleaching, lignolytic-cellulosic biofuels production, and textile dye bleaching [1]. Hence, fungal laccases are considered excellent candidates for use in enzyme-based remediation strategies [1,8-10].
Petroleum hydrocarbon contamination of boreal and forest soils is a persistent problem with several sources including leaking transfer pipes and storage tanks, and surface spills [2]. While there are indications that microbial processes can aid in the remediation of spills in these ecosystems, our understanding of the microbial communities and how they function under spill conditions are not completely understood [2]. In this study, we “baited” soils in a lodgepole pine (Pinus contorta) forest with diesel, and used molecular-genetic methods to assess the effects of contamination on basidiomycete laccase gene diversity. We performed this work in a field setting rather than in the lab so that all processes governing and impacting fungal function and diversity would be intact. Laccases catalyze the reduction of oxygen to water, and in so doing oxidize phenolic-based substrates [1].
The goal of this endeavor was to determine 1) whether fungal communities would respond rapidly to the addition of this substrate in terms of detection of laccase genes by PCR and also in terms of measurable changes in laccase diversity following 2 weeks of exposure, 2) whether wood rotting or ECM fungi would dominate the taxa that do respond and 3) to provide new candidate fungal species that could be explored as potential enzyme sources and also as specific plant/fungal combinations for phytoremediation and/or habitat restoration strategies.
Two weeks following application, 9 soil samples (3 samples from within each of 3 plots within each block) 1 centimeter (cm) diameter X 4 cm in depth) were taken from each paired treatment and control plot, for a total of 27 from both treatment and control, and a grand total of 54 soil samples.
Polymerase Chain Reaction products were cloned using the Qiagen PCR Cloning plus Kit, plating with Kanamycin selection and blue/white screening, and modified by the protocol by adding 5 ul of sample to the ligation as described by Cullings and Hanely [12]. Thirty sequences from each plot were considered for species identification, providing a target total of 270 sequences for sequence analysis. Sequences were subjected to BLAST analysis to determine closest taxonomic affinities.
Sequencing of 150 clones from diesel-contaminated blocks (30 from each of the 5 of 9 plots that amplified) revealed 12 unique laccase fragments (Table 1) belonging to ECM fungi in the Russulaceae, Atheliaceae, Tricholomataceae and also one individual taxon in the Agaricaceae. None of the 6 sequences sharing homology with those in the Tricholomataceae or Agaricaceae shared sufficient homology with a species in any genus to allow identification beyond the family level. The 4 sequences with homology to the Russulacae and Athelaceae shared homology with the genera Russula and Piloderma respectively, but none in Genbank matched with sufficient homology to identify below the genus-level.
Sequencing of ITS clones provided additional resolution. In keeping with the laccase fragments, 2 species of the genus Russula were detected, as were 4 Piloderma taxa. One definitive identification by greater than 95% BLAST search match was obtained in the Tricholomataceae, Tricholoma saponaceum (Table 2). In addition, the ITS screen indicated the presence of ECM genera in several prominent EM fungal groups (Suilloid group of fungi, the Cortinariaceae, Cantharellaceae, Hydnaceae, Mycenaceae and Thelephoraceae) (Table 3) that were absent from the laccase gene pool.
These results are in keeping with recent data that indicate that some ECM fungi (Gomphidius viscidus and Laccaria bicolor) can grow with diesel as their sole nutrient source [13]. This is perhaps not surprising. ECM fungi are recently evolved from wood-rotting basidiomycetes [14], and are obligate mutualists that form intimate associations with the roots of host trees. However, despite being dependent upon a mutualistic interaction with a host tree for carbon uptake and survival, many ECM fungi demonstrate the ability to utilize lignin as a nutrient source [15,16]. Further, many produce laccases and other enzymes involved in carbon cycling in natural ecosystems [1,2,7,9,10,12,17,18]. Indeed, recent evidence suggests that many EM fungi possess the ability to adopt at least a partial saprophytic habit in times of low host carbon availability [17,19-22], or when carbon substrate is added [21]. Because of these findings, it has been hypothesized that they may derive at least part of their carbon nutrition by enzymatic means [16,19,20,23], though their capacity to break down wood is the topic of considerable debate [1]. However, while they may not possess the capacity to fully break down the complex lignin polymers in wood, it has been hypothesized that ECM may play a significant role in carbon cycling via metabolism of the shorter chain phenolic breakdown products created by the metabolism of lignin by other microbes [20]. These smaller compounds are analogous to components of many anthropogenic contaminants, represented here by diesel.
Our data revealed genera in ECM fungal 3 families that exhibited positive responses to diesel application; the Russulaceae (2 Russula species), Atheliaceae (3 Piloderma species), and the Trichoomataceae. Russuloid fungi are known to have laccase activity [23], and species within the Russulaceae can exhibit significant oxidative enzyme activities [24]. However, culture-based experiments involving measurement of potential remediation function of specific Russula species obtained from non-contaminated soils indicate that these fungi are often poor performers [23]. Despite this, it was noted that fungi are likely to perform differently in a natural setting and in symbiosis with their host trees than they do in pure culture, particularly if these fungi are obtained from contaminated soils which would presumably screen for more active isolates.
ECM fungi can often be classified by hyphal “exploration type”, which is based on their foraging distance in the soil, which can often be correlated with potential to produce oxidizing enzymes [25]. Russula species are classified by this system as a type that possesses high phenol oxidizing potential, and hence potentially have a high ability to utilize substrate via lignolysis. In keeping with this classification, the results of our study indicate that some Russula species exhibit rapid, positive growth responses when exposed to diesel, supporting the hypothesis that at least some ECM fungi could fulfill a role in carbon cycling in forest ecosystems through the utilization of less recalcitrant phenolic compounds, if not from complete breakdown of woody substrate [20].
The data also indicated positive responses by fungi in the family Tricholomataceae. However, genetic matches to members of the Tricholomataeae were low, 70% or less, indicating gaps in our knowledge regarding the diversity of these taxa. Indeed, molecular evidence indicates that this family is paraphyletic with subgroups that are comprised of several other families [26]. Hence, conclusions regarding these taxa are less certain. Despite this, our studies of effects of decreased host photosynthetic potential on ECM communities indicate that both groups can be prevalent members of the soil hyphal community in pine forests [12]. Further, Tricholoma species possess laccase and proteinase activity [27,28] and significant lignin-degrading potential has been demonstrated in several Tricholoma species [16,24]. Indeed, T. aurantium may utilize low molecular weight lignin-derived substrates more efficiently than some traditional wood-rotting fungi [16]. Though the laccase data indicate more species in this family than our ITS screen(most likely due to the paraphyletic nature of the Tricholomataceae) our data support the notion that members of this ECM genus could take part in carbon cycling in forest soils and could provide useful models for ectomycorrhiza-mediated restoration strategies in diesel contaminated soils.
Multiple laccase-like genes have been detected in Piloderma (Atheliaceae) species [29]. Because of this, and because they are often detected in organic soil layers, it has been hypothesized that they may have some role in lignin degradation processes [29]. Our data indicate that Piloderma taxa exhibit positive growth responses to diesel application, suggesting that they could be utilizing short chain phenolic compounds as nutrient sources. Unfortunately, over 3 fruiting seasons we found no Piloderma fruiting bodies. Hence, identifications cannot be made to the species level at this time.
The majority of fungi in the soil pool did not exhibit a positive PCR response to diesel application. Most notable in this group of fungi are those in the Cortinariaceae and the Suilloid group (e.g., Suillus and Rhizopogon species). Suilloid fungi tend to be long distance exploration types that in general lack lignin-degrading abilities [25]. However, some Suillus species can exhibit significant phenol-oxidation activity in field settings in which all factors that influence function are present and intact [12,19] and in some remediation settings [23]. Rhizopogon sp, however, exhibit little if any remediation potential [23], a finding that would seem to be supported by the lack of growth response we measured in our study. Cortinarioid fungi tend to be medium distance types, also generally lacking in phenol-oxidizing activity. Thus, in addition to being an indicator of physiological potential, Agerer’s exploration type may provide a rough indicator of fungi for potential screening for ectomycorrhizal-mediated phytoremediation of phenolic-based contaminants and as enzyme sources for industrial use.
In conclusion, our data indicate that the ECM community can respond rapidly to application of diesel as a potential nutrient source. Further, our results provide new information as to the range of environmental conditions that can be tolerated by some Russuloid and Athelioid fungi, and fungi in the Tricholomataceae. Finally, even if the ECM fungi in this system are playing no role in diesel degradation, ECM fungi can protect the host from damage when growing in harsh environments [30] thereby enhancing their survivability in extreme habitats. Thus, our data provide new ECM models for further studies into phytoremediation or habitat restoration strategies that utilize specific host/fungal combinations.
References
- Baldrian P (2006) Fungal laccases - occurrence and properties. FEMS Microbiol Rev 30: 215-242.
- Robertson SJ1, McGill WB, Massicotte HB, Rutherford PM (2007) Petroleum hydrocarbon contamination in boreal forest soils: a mycorrhizal ecosystems perspective. Biol Rev CambPhilosSoc 82: 213-240.
- Saparrat MC1, Guillén F, Arambarri AM, Martínez AT, Martínez MJ (2002) Induction, isolation, and characterization of two laccases from the white rot basidiomyceteCoriolopsisrigida. Appl Environ Microbiol 68: 1534-1540.
- Taprab Y1, Johjima T, Maeda Y, Moriya S, Trakulnaleamsai S, et al. (2005) Symbiotic fungi produce laccases potentially involved in phenol degradation in fungus combs of fungus-growing termites in Thailand. Appl Environ Microbiol 71: 7696-7704.
- Kerem Z1, Friesem D, Hadar Y (1992) Lignocellulose Degradation during Solid-State Fermentation: Pleurotusostreatus versus Phanerochaetechrysosporium. Appl Environ Microbiol 58: 1121-1127.
- Eggen T, Majcherczyk A (1998) Removal of polycyclic aromatic hydrocarbons(PAH) in contaminated soil by white rot fungus Pleurotusostreatus. International Biodet and Biodeg 41: 111-117.
- Bamforth S, Singleton I (2004) Review: Bioremediation of polycyclic aromatic hydrocarbons: current knowledge and future directions. J Chem Tech Biotech 80: 723-736.
- Ang E, Zhao H, Obbard JP(2005) Recent advances in the bioremdiation of persistent organic pollutants via biomolecular engineering. Enz Micro Tech 37: 487-496.
- Camarero S1, Ibarra D, Martínez MJ, Martínez AT (2005) Lignin-derived compounds as efficient laccase mediators for decolorization of different types of recalcitrant dyes. Appl Environ Microbiol 71: 1775-1784.
- Soden DM1, Dobson AD (2001) Differential regulation of laccase gene expression in Pleurotussajor-caju. Microbiology 147: 1755-1763.
- Luis P, Walther G, Kellner H, Martin F, Buscot F (2004) Diversity of laccase genes from basidiomycetes in a forest soil. Soil Bio Biochem 36: 1025-1036.
- Cullings K, Hanely J (2010) Dwarf Mistletoe Effects on Soil Basidiomycete Community Structure, Soil Fungal Functional Diversity, and Soil Enzyme Function: Implications for Climate Change. Soil Bio Biochem 42:1176-1178.
- Li X1, Huang Y, Wei ZC (2008) [Degradation effect of two pure cultured ectomycorrhizal fungi on diesel]. Ying Yong Sheng Tai XueBao 19: 1579-1584.
- Hibbett DS1, Gilbert LB, Donoghue MJ (2000) Evolutionary instability of ectomycorrhizal symbioses in basidiomycetes. Nature 407: 506-508.
- Haselwandter K, Bobleter O, Read DJ (1990) Degradation of 14C-labelled lignin and dehydropolymer of coniferyl alcohol by ericoid and ectomycorrhizal fungi. Arch Micro 153: 52-354.
- Trojanowski J, Haider K, Hu¨tterman A (1984) Decomposition of 14C-labelled lignin holocellulose and lignocellulose by mycorrhizal fungi. Arch Micro 139: 202-206.
- Courty PE, Bréda N, Garbaye J (2007 Relation between oak tree phenology and the secretion of 130. organic matter degrading enzymes by Lactarius quietus ectomycorrhizas before and during bud break. Soil Bio Biochem 39: 1655-1663
- Pritsch KS, Raidl E, Markensteiner H, Blashcke, Agerer R, et al. (2004) A rapid and highly sensitive method for measuring enzyme activities in single mycorrhizal tips using 4-methylumbelliferone-labeled fluorogenic substrates in a mciroplate system. J Micro Met 58: 233-241.
- Cullings K1, Ishkhanova G, Henson J (2008) Defoliation effects on enzyme activities of the ectomycorrhizal fungus Suillusgranulatus in a Pinuscontorta (lodgepole pine) stand in Yellowstone National Park. Oecologia 158: 77-83.
- Cullings K1, Courty PE (2009) Saprotrophic capabilities as functional traits to study functional diversity and resilience of ectomycorrhizal community. Oecologia 161: 661-664.
- Cullings K, Ishkhanova G, Ishkhanov G (2010) Induction of saphophytic behavior in the ectomycorrhizal fungus Suillusgranulatus by litter addition in a Pinuscontrota (Lodgepole pine) stand in Yellowstone. Soil Bio Biochem 42: 1976-1981.
- Tedersoo L, Kõljalg U, Hallenberg N, Larsson KH (2003) Fine scale distribution of ectomycorrhizal fungi and roots across substrate layers including coarse woody debris in a mixed forest. New Phytol 159:153-165.
- Mehard A, Cairney JWG (2000) Ectomycorrhizas-Extending the capabilities of rhizosphere remediation? Soil Bio Biochem 32: 1475-1484.
- Gramss G, Gunther TH, Fritsche W (1998) Spot tests for oxidative enzymes in ectomycorrhizal, wood and litter decaying fungi. Myco Res 102: 67-72.
- Agerer R (2001) Exploration types of ectomycorrhizae: A proposal to classify ectomycorrhizalmycelial systems according to their patterns of differentiation and putative ecological importance. Mycorrhiza 11: 107-114.
- Moncalvo JM1, Lutzoni FM, Rehner SA, Johnson J, Vilgalys R (2000) Phylogenetic relationships of agaric fungi based on nuclear large subunit ribosomal DNA sequences. SystBiol 49: 278-305.
- Kim JH1, Kim YS (2001) Characterization of a metalloenzyme from a wild mushroom, Tricholomasaponaceum. BiosciBiotechnolBiochem 65: 356-362.
- Wang HX1, Ng TB (2004) Purification of a novel low-molecular-mass laccase with HIV-1 reverse transcriptase inhibitory activity from the mushroom Tricholomagiganteum. BiochemBiophys Res Commun 315: 450-454.
- Chen DM, Bastias BA, Taylor AFS,Cairney JWG (2003) Identification of laccase-like genes in ectomycorrhizalbasidiomycetes and transcriptional regulation by nitrogen in Pisolithusbyssium. New Phyto 157: 547-554.
- Cullings K1, Makhija S (2001) Ectomycorrhizal fungal associates of Pinuscontorta in soils associated with a hot spring in Norris Geyser Basin, Yellowstone National Park, Wyoming. Appl Environ Microbiol 67: 5538-5543.
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 |
Figures at a glance
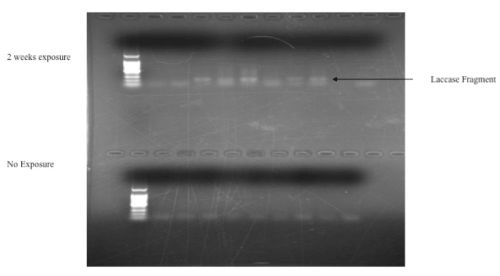 |
| Figure 1 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 13675
- [From(publication date):
September-2014 - Sep 04, 2025] - Breakdown by view type
- HTML page views : 9150
- PDF downloads : 4525
