Research Article Open Access
Removal of Heavy Metals Pb, Zn and Cu from Sludge Waste of Paper Industries Using Biosurfactant
| Nurul Hidayati*, Tini Surtiningsih and Ni’matuzahroh | |
| Biology Department, Faculty of Science and Technology, Airlangga University, Indonesia | |
| Corresponding Author : | Nurul Hidayati Biology Department Faculty of Science and Technology Airlangga University, Indonesia Tel: +62315914042 E-mail: ukhti.abel@gmail.com |
| Received August 25, 2014; Accepted October 17, 2014; Published October 20, 2014 | |
| Citation: Hidayati N, Surtiningsih T, Ni’matuzahroh (2014) Removal of Heavy Metals Pb, Zn and Cu from Sludge Waste of Paper Industries Using Biosurfactant. J Bioremed Biodeg 5:255. doi:10.4172/2155-6199.1000255 | |
| Copyright: © 2014 Hidayati N, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Increasing public awareness of environmental pollution influences the search and development of technologies that help in cleanup of organic and inorganic contaminants such as metals. Sludge waste of paper industries as toxic and hazardous material from specific source containing Pb, Zn, and Cu metal from waste soluble ink. An alternative and eco-friendly method of remediation technology is the use of biosurfactants and biosurfactant-producing microorganisms. Soil washing is among the methods available to remove heavy metal from sediments. The purpose of this research is to study effectiveness of biosurfactant with concentration=CMC for the removal of heavy metals, lead, zinc and copper in batch washing test under four different biosurfactant production by microbial origin; Pseudomonas putida T1(8), Bacillus subtilis 3K, Acinetobacter sp, and Actinobacillus sp was grown on mineral salt medium that had been already added with 2% concentration of molasses that it is a low cost application. The samples were kept in a shaker 120 rpm at room temperature for 3 days. Supernatants and sediments of sludge were separated by using a centifuge and samples from supernatants were measured by Atomic Absorption Spectrophotometer. The highest removal of Pb was up to 14.04% by Acinetobacter sp. Biosurfactant of Pseudomonas putida T1(8) have the highest removal for Zn and Cu was up to 6.5% and 2.01% respectively. Biosurfactant have a role for removal process of the metals, including wetting, contact of biosurfactant to the surface of the sediments and detachment of the metals from the sediment. Biosurfactant has proven its ability as a washing agent in heavy metals removal from sediments, but more research is needed to optimize the process of removal heavy metals.
| Keywords |
| Biosurfactant; Molasses; Removal heavy metal; Sludge waste paper industries |
| Introduction |
| Among the different contaminants, heavy metals have received special attention due to their strength and persistence in accumulating in ecosystems, where they cause damage by moving up the food chain to finally accrue in human beings, who are at the top of the chain food. The toxic potential of heavy metals with regards to the human body is diverse and, because of their toxicity and persistence in nature, the levels of heavy metals in the environment need to be controlled by mandating waste treatment at the sources of pollution. The development of new treatment technologies is required at these sources; however, even though there is awareness of this problem, sustainable solutions are not easily accessible. In general, the conventional treatment methods used to remove metals from wastewater are inefficient and cost-prohibitive [1]. Sludge waste of paper industries as toxic and hazardous material from specific source containing Pb, Zn, and Cu metal from waste soluble ink. According to regulation of Ministry of Environmental no.33/2009 that all the industries are mandatory to clean up contaminated soil from hazardous waste [2]. |
| The use of microbial metabolic ability or their enzymes for degradation/removal of environmental pollutants provides an economic and safe alternative compared to other physicochemical methodologies. Biosurfactants are a structurally diverse group of surface-active substances produced by microorganisms. All biosurfactants are amphiphiles, they consist of two parts-a polar (hydrophilic) moiety and non polar (hydrophobic) group. A hydrophilic group consists of mono- , oligo- or polysaccharides, peptides or proteins and a hydrophobic moiety usually contains saturated, unsaturated and hydroxylated fatty acids or fatty alcohols [3]. |
| Biosurfactant activities depend on the concentration of the surfaceactive compounds until the critical micelle concentration (CMC) is obtained. At concentrations above the CMC, biosurfactant molecules associate to form micelles, bilayers and vesicles [4]. |
| Biosurfactants produced by microorganisms show promise for enhancing organic compound biodegradation in the presence of metals. Application of biosurfactants or microorganism produced biosurfactants in in situ co-contaminated sites bioremediation seems to be more environmentally compatible and more economical than using modified clay complexes or metal chelators [5]. |
| Materials and Methods |
| Bacterial isolates |
| A Biosurfactant-producing bacterium used in this study was isolated from oil contaminated soil in PERTAMINA storage-Tanjung Perak Surabaya. The isolated bacteria were storaged in Microbiologi Laboratory, Biology Departemen Airlangga University Surabaya. The bacterium was maintained on nutrient agar (NA) and was incubated at 28°C for 24 hours. |
| Bacterial culture |
| Synthetic mineral salt medium with adding molasses 2% (v/v) was used as growth substrate. Mineral salts medium developed by Pruthi and Comeotra [6] used in this study contained per liter distilled water, KH2PO4, 5.0g; K2HPO4, 2.0g; FeSO4.7H2O, 0.0006g; (NH4)2SO4, 3.0g; NaCl, 10g; MgSO4. 7H2O, 0.2g; CaCl2, 0.01g;MnSO4. H2O, 0.001g; H3BO3, 0.001g, ZnSO4. 7H2O, 0.001g; CuSO4. 5H2O, 0.001g; CoCl2.6H2O, 0.005g; and Na2M0O4. 2H2O, 0.001 g, pH was adjusted to 7.0. In each flask containing various microbial producing biosurfactant; Pseudomonas putida T1(8), Bacillus subtilis 3K, Acinetobacter sp, and Actinobacillus sp were inoculated at 4% (v/v), OD=0.5, λ=650 nm. Cultivations were performed in 250 mL flasks containing 50 mL broth medium. The mixture was placed on a reciprocal shaker at 120rpm, at 28°C, for 4 days incubation to produce a well-dispersed suspension and to isolate biosurfactant producing bacteria. |
| Test of biosurfactant production |
| Bacterial culture centrifuged at 9000 rpm for 15 min for separating cells from supernatant. Biosurfactant production from obtained supernatant was determined by measuring emulsification activity, surface tension reduction and crude biosurfactant yield. |
| Measurement of emulsification test (E24) |
| E24 of samples were determined by adding 1 mL of kerosene to the same amount of cell free cultures, mixing with a vortex for 2 minutes, and leaving to stand for 24 hours. The E24 index is given as percentage of height of emulsified layer (cm) divided by total height of the liquid column (cm) [7]. |
| Measurement of surface tension value |
| The sample is inserted into the petri dish, which is previously washed with 5% HCl solution, rinsed with distilled water, and dried. Surface tension measurement of the liquid sample was conducted using tensiometer Du Nouy. The value of surface tension is the value seen on the tensiometer when the tensiometer ring was untied from the sample surface. The value of surface tension was expressed in mN / m and reported as a result by using the formula [8]. |
| r=r0x θ/θ0 |
| r=surface tension of liquid samples |
| r0=surface tension of water at t°C |
| θ=surface tension of the sample. |
| θ0=surface tension of water |
| Measurement of crude biosurfactant yield |
| Biosurfactant was extracted from supernatant by 20% saturation (NH4)2SO4 precipitation. The remained product was lyophilized using freeze dryer to obtain crude biosurfactant, and then weighed. The value was assessed as crude biosurfactant yield. |
| Batch Washing Test |
| The biosurfactant saolution was diluted with buffer phosphate pH 7: KH2PO4 (mr 136.08) 7.11 g/L and K2HPO4 (mr 174.1) 11.59 g/L. Measuring pH of buffer using pH meter. NaOH and HCl used for adjusted pH until get pH 7 [9]. A quantity of 12 g from sludge was placed in each 500 mL vial. 120 mL of biosurfactant solution was added to each vial. The samples were kept at room temperature on a shaker 120 rpm for 3 days. Supernatants and sediment particles were separated by using a centrifuge (3000 rpm, 30 min) and samples from supernatants were taken. All test were performed in triplicates. The sample were digested in order to release the heavy metals trapped in the biosurfactant micelles and then the concentrations of copper and other metals in each sample were measured by the Anatomic Absorption Spectrophotometer [10]. |
| Results and Discussion |
| Biosurfactant production |
| Molasses used as substrate is important in biosurfactant production. It is a viscous by-product of the processing of sugar cane, grapes or sugar beets into sugar and usually constitutes an industrial waste. Molasses are an important carbon source for microbial growth. Dessai and Banat [11] used molasses and cornsteep liquor as the primary carbon and nitrogen source to produce biosurfactant. |
| Evaluation of biosurfactant activity was done by surface tension determination and emulsification activity. This process also to detect biosurfactant existence. |
| Surface tension determination |
| Surface tension determination is an important point in biosurfactant production by Acinetobacter sp. A best level of biosurfactant production is characterized by it capacity to reduce surface tension [12]. |
| From the Table 1, it can be explained that Pseudomonas putida T1(8) can reduce the surface tension from 61.67 dyne/cm to 32.14 dyne/cm (29.53 dyne/cm of decrease). A potential bacterium in biosurfactant production can reduce the surface tension ≥ 10 dyne/cm. It means that in this research, Pseudomonas putida T1(8) biosurfactant had the highest potentiality to produce biosurfactant than 3 others. |
| Emulsification activity test of supernatant |
| In this research, kerosene was used to determine the emulsification activity of supernatant. The result is shown on the Table 2. The stability value (%) indicate the power of the biosurfactant produced by microbe on hydrocarbon emulsification test [9]. |
| The data in Table 2 explain that the highest emulsification activity id Pseudomonas putida T1(8) supernatant that can emulsify kerosene at a range of 35.18% (1 hour). After 24 hours the emulsification percentages drop to 31.69% which can explain emulsification instability. Pseudomonas putida T1(8) has a high potentiality in emulsification activity, comparing with control (water: 0%). Being highly potential in kerosene emulsification, Pseudomonas putida T1(8) has a high capacity in the production of bioemulsifier substance. |
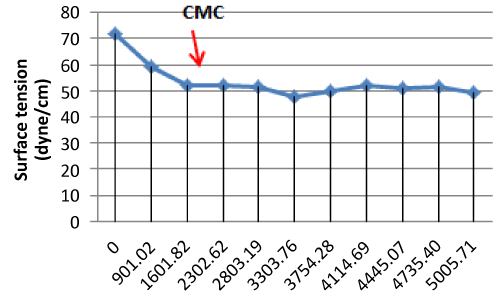
| Critical Micelle Concentration (CMC) Value |
| The CMC is commonly used to measure the efficiency of surfactant. Efficient biosurfactants have a low CMC, which means that less biosurfactant is required to decrease the surface tension [11]. |
| The highest CMC value is from Actinobacillus sp, it have CMC value 3303.76 mg/L or 3.30376 g/L (dry weight) with surface tension value 57.17 dyne/cm. Then the lowest CMC value in this research is from Pseudomonas putida T1(8). CMC value of Pseudomonas putida T1(8) is 1601.82 mg/L or 1.60182 g/L (dry weight) with surface tension value 51.91 dyne/cm. It showed on the Figure 1. |
| Surface tensions, CMC, emulsification stability value constitute the biosurfactant characteristics which depend on substrate type [10]. A biosurfactant is efficient if the CMC is about 1- 2000 mg/L [13]. |
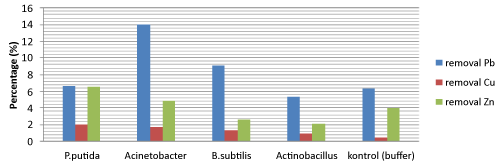
| Removal heavy metal Pb, Zn, and Cu using biosurfactant |
| The diagram showed that the highest removal of Pb was up to 14.04% by Acinetobacter sp. Biosurfactant of Pseudomonas putida T1(8) have the highest removal for Zn and Cu was up to 6.5% and 2.01% respectively. Compare with Dahrazma [10], removal Cu and Zn using 5% Rhamnolipid are 12.9% and 10.7%. It caused by different kind of biosurfactant and substrate (Figure 2). |
| The value removal heavy metal using buffer phosphate as control have a high enough, it maybe because buffer phosphate support absorption process for removal heavy metal. According to Roane and Pepper [14], they explain that most heavy metals are cations and this determines their sorption to negatively charged functional groups that are present in: cell surfaces, which are generally anionic at a pH of between 4 and 8; surfaces with residual hydroxides (OH-) or thiol (SH-) and anionic salts, such as PO4- and SO4-, humic acid, and clay minerals. |
| The usefulness of biosurfactants for bioremediation of heavy metal contaminated soil is mainly based on their ability to form complexes with metals. The anionic biosurfactants create complexes with metals in a nonionic form by ionic bonds. These bonds are stronger than the metal’s bonds with the soil and metal-biosurfactant complexes are desorbed from the soil matrix to the soil solution due to the lowering of the interfacial tension. The cationic biosurfactants can replace the same charged metal ions by competition for some but not all negatively charged surfaces (ion exchange). Metal ions can be removed from soil surfaces also by the biosurfactant micelles. The polar head groups of micelles can bind metals which mobilize the metals in water [15]. |
| Conclusion |
| In conclusion, the highest removal of Pb was up to 14.04% by Acinetobacter sp. Then biosurfactant of Pseudomonas putida T1(8) have the highest removal for Zn and Cu was up to 6.5% and 2.01% respectively. These molasses could be of great alternative substrate for biosurfactant production for using continously. Biosurfactant has proven its ability as a washing agent in heavy metals removal from sediments, but more research is needed to optimize the process of removal heavy metals. |
| Acknowledgements |
| This work was supported by Biology Department, Faculty of Science and Technology, Airlangga University. The authors are very grateful to microbiology laboratory staffs and other students for their help and support. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
| Figure 1 | Figure 2 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14987
- [From(publication date):
December-2014 - Sep 01, 2025] - Breakdown by view type
- HTML page views : 10213
- PDF downloads : 4774
