Renitrogen Fixation Activity in Cyanobacterial Biological Soil Crusts with Domination of the Stigonema Genus Species in Mountain and Plain North- East European Tundra Ecosystems
Received: 08-Sep-2017 / Accepted Date: 07-Oct-2017 / Published Date: 13-Oct-2017 DOI: 10.4172/2573-458X.1000138
Abstract
To model global processes which happen under the climate change, we should know the input of cyanobacterial communities to nitrogen balance in high latitude regions. It is widely known that the Arctic and mountain ecosystems are highly sensitive to changes of the climate. The forecasted increase in temperature in high latitudes, undoubtedly, will cause shifts in scale and rate of biogeochemical N-cycles and result in transformations of plant communities. The reliable forecasting of changes in the functional organization of ecosystems is only possible if vast and broad experimental data is taken into account in the models. Both seasonal and daily values of N2-fixation process has to be evaluated in various plant communities from different zones under variety of conditions. For balanced estimations, the various types of arctic ecosystems have to be included in the studies. The N2-fixation activity is barely studied on large territories of plain and mountain tundra of the NortheastEuropean Arctic. The article sums up results of a research on nitrogenase activity ratesin the soil crusts dominated byStigonema species.The species of the genus arewidespreadinthestudied regions, they have capability to fix nitrogen and they are important contributors to the N-inputin cryogenic disturbed soils. The nitrogen-fixing activity of investigated crusts was studied and the measured rates were 0.59-0.80 mg С2Н4 m-2 h-1under 15°С (frequent midday temperature in July)and 0.97-1.64 mgC2H4m-2 h-1 under 21°С(optimal temperature for N2-fixation in the Arctic). Crusts with the dominance of Stigonema genus from different regions of the European North-East have close rates of N2-fixing activity in latitudinal and altitudinal gradients under similar environmental conditions.The obtained values might be used to calculate the input of crustswith Stigonemato the N-balancein mountain and plain tundras for calculating global balance for high latitudes.
Keywords: Cyanobacteria/cyanoprokaryota; Black Stigonema crusts; N2 fixation; European arctic
Introduction
The spots of the bare ground are one of the most common types of cryogenic micro- and meso-relief in plain and mountain tundras of the Arctic. As environment conditions change and become more extreme, the proportion of area occupied by such spots in tundra communities grows: they can cover up to 50-80% of the ecosystem areas [1]. The spore-forming organisms are the primary colonizers of the bare soils and play a special part in the formation of biological soil crusts (BSC). The most abundant and diverse groups of BSCs are Cyanobacteria and eukaryotic algae which are involved in accumulation of organic matter in the soils and biogeochemical cycles of carbon, nitrogen and other elements [2-5]. Some Cyanobacteria can fix atmospheric nitrogen and, thus, they are a significant source of it in BSCs [6,7]. To assess their input in accumulation of nitrogen in soils disturbed by cryogenic processes, the rate of nitrogen fixation activity should be evaluated using data from daily and seasonal measurements in various types of tundra and mountain communities under environmental conditions. The data could be used in future evaluations of cyanobacterial contribution to biogeochemical nitrogen cycle of tundras in latitudinal and altitudinal gradients [4,8]. Measurements of typical types of BSCs in various tundra and mountain communities are required. The NA (nitrogenase activity) of BSCs in tundra regions have been studied in few areas of Canadian Arctic, Svalbard archipelago, and mountain regions [8-17], in Russian sector of the Arctic, data on nitrogen fixation activity of cryptogamic crusts is limited to regions of the European tundra [18-21]. The studies have shown that nitrogen fixation of BSCs depends on many factors; the most important are light, temperature and moisture of the substrate as well as composition of the dominant species of cyanobacteria in the crust. In the listed above publications a large data and analysis of NA is presented for different BSCs with various types of vegetation and bare ground, as well as rates of NA for Cyanoprokaryota species forming complexes with mosses and lichens. The research of spots with Stigonema remains poorly investigated. To estimate their role in the N cycle it is therefore necessary to conduct field and laboratory measurements of these BSCs since they are the most typical and widespread not only in mountain and plain spotted tundra of the North- East Europe [18-20] but in the other high latitude and mountain regions in the East and West sectors of the Arctic [2,3,17]. The nitrogen fixation rates of the species from Stigonema genus is close to the widespread and well-studied nitrogen fixating species – Nostoc commune Vauch. ex Born. et Flah. which has one of the highest values among cyanobacteria. Yet, the territories occupied by Stigonema in the Arctic and Subarctic are notably larger [17,18].
The article focuses on results of comparative measurements of NA rates in cyanobacterial biological crusts with Stigonema as the major N2 fixing component.
Materials and Methods
The field study sites
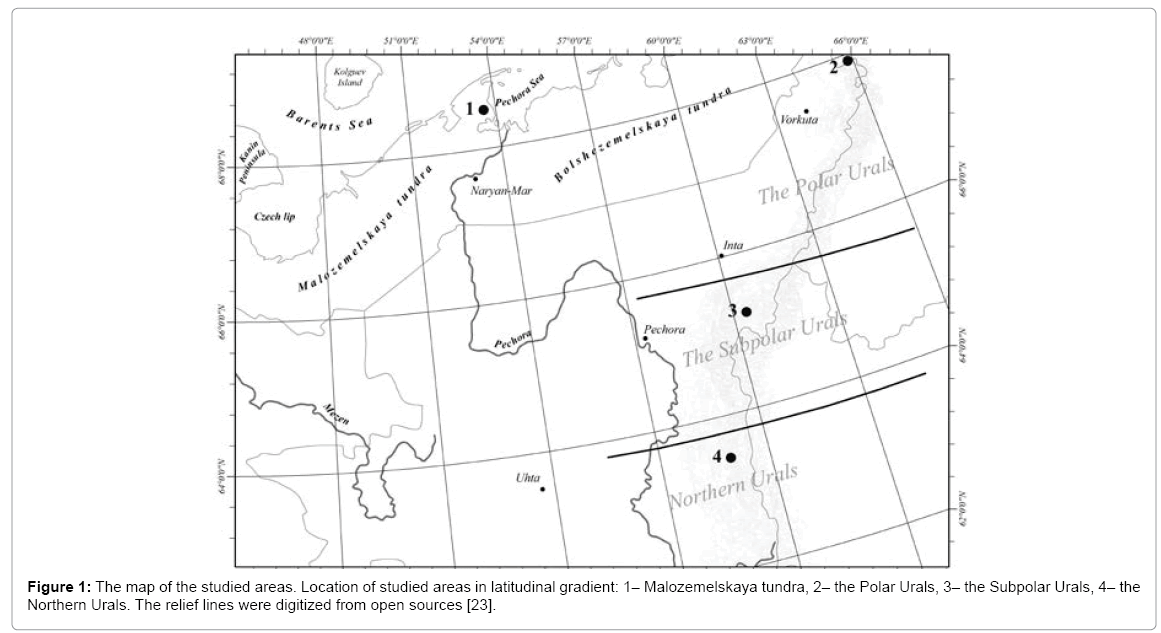
It the field, nitrogenase activity was studied in BSCs of spotted tundra. The measurements in all studied regions were held during the same period of the growing season – between late July and early August. The studies in plain tundra were conducted in 2015 in Malozemelskaya tundra, in the basin of Pechora River, Nenets Reserve (68°25’N, 53°13’E, 28 m above sea level) (Figure 1). The area belongs to the Arctic tundra [22]. In mountain tundra, the research was carried out in the Polar Urals in 2013 (68°28’N, 66°21’E, 200 m asl), in the sub polar Urals during 2013-2016 (65°11’N, 60°18’E, 680-1305 asl) and in the Northern Urals in 2016 (63°23’N, 58°54’E, 750-900 m asl). The Malozemelskaya tundra and Northern regions of the Urals have harsh climate with long extreme winters, cold summers and short vegetation season, with relatively high precipitation, the low values of evaporation and excess moistening. The average temperature of the coldest month (January) is -17 to 21°C; the temperature of the warmest month (July) is 8-14°C. The vegetation period lasts for less than 80 days. Th e precipitation in plain tundras is 350-500 mm in mountain areas is 800-1500 mm per year. Climatic characteristics of the research areas can be found in Atlas of the Arctic [23].
Figure 1: The map of the studied areas. Location of studied areas in latitudinal gradient: 1– Malozemelskaya tundra, 2– the Polar Urals, 3– the Subpolar Urals, 4– the Northern Urals. The relief lines were digitized from open sources [23].
Characteristics of biological soil crusts dominated by Stigonema
For the study, we carefully selected reassembling black BSCs in different studied regions under similar environmental conditions: we took into account a species composition and dominant species abundance in BSCs, their thickness and color, position in microrelief, moisture content in the soil and type of spotted lichen-moss-shrub community they were growing in. To study nitrogen-fixing activity we chose typical biological soil crusts in European North-East tundra, 0.2-0.5 cm thick, with a black surface, dominated by species from Stigonema genus. Such BSCs colonize bare spots formed by cryogenic processes. They occupy microdepressions in the landscape. These habitats are characterized by rapid changes in temperature and moisture content in the soil.
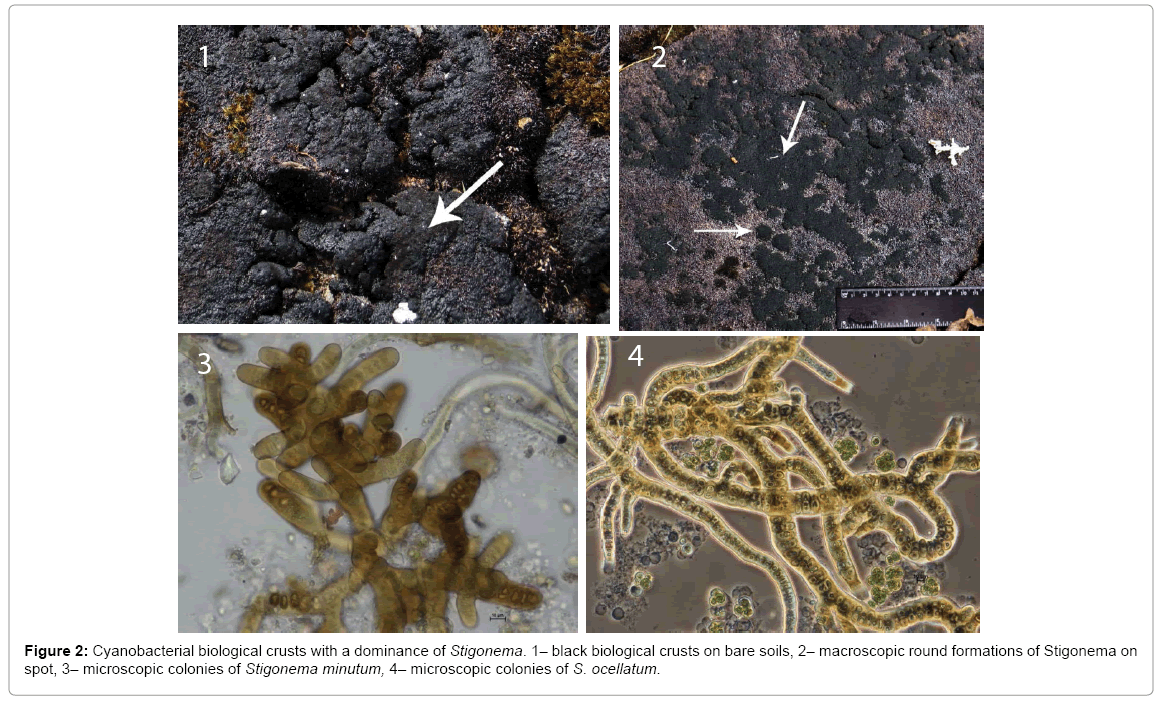
The dominant cyanobacteria species from Stigonema genus formed macroscopic round formations on the surface of BSCs that could be seen without a microscope (Figure 2). The size of the colonies varied from 1 mm to 1-2 cm. The species of macrocolonies were identified using a stereoscopic microscope (Zeiss Axiolab, Germany) and key from Komárek [24]. The BSCs with a dominance of Stigonema (occupied area was no less than 50-80% of the total area of the soil crust) were chosen for the experiments. The microphotography of the samples was done at 200 and 600 magnification with Nikon DS-2M digital camera of Nikon Eclipse 80i microscope in the laboratory.
Measurements of total nitrogen and carbon in black soil crusts
To evaluate the similarity of the BSCs to each other we evaluated the total amount of carbon and nitrogen (mg g-1 dry weight, 2-5 replicates for each studied area). To determine the chemical composition, we took 2-3 mm of cyanobacterial layer (and removed as many mineral particles as possible) and 1 cm thick soil layer under the cyanobacteria layer, both were obtained from the same soil crusts where NA was measured. The analysis was performed on gas chromatography analyzer EA-1100 (CHNS-O) (CE Instruments, Italy) in “Ekoanalit” laboratory, Institute of biology, Komi Science Centre.
Measurement of nitrogenase activity and ecological parameters in the field
The nitrogen fixation activity was measured using the acetylene reduction assay [25]. NA was calculated as the amount of ethylene produced in mg C2H4 m-2; h-1. In all research sites, nitrogen fixing activity was measured when moisture content of the BSCs were constantly high for 2-3 days before the sampling. It is well known that the moisture content of the BSCs has a strong prolonged effect on metabolic activity of BSC. The measurements of NA in the studied regions were carried in wide range of temperatures but in the article we present the comparisons of NA in different regions under 15 ± 0.5°C (the frequent midday temperature in July in the studied regions) and 21 ± 0.5°C which is close to the optimal temperature of NA in cyanobacterial crusts in High Arctic regions [9,10,13]. For the measurements we used soil crusts with the surface area of 3 × 4 cm2 and a height of 1 cm (5-12 replicates for each study area). The BSCs were weighted before and after the NA measurement for further calculations of the moisture content of the BSCs. The samples were placed in 130 ml conical flasks, their initial structure was intact. The flasks were sealed with a rubber stopper which was adapted to allow sampling of gas mixtures. Before sampling, 10 ml of air was extracted from the flask. After 13 ml of 100% acetylene was added to create a 10% acetylene mixture. The temperature of BSCs was kept close to the outside air temperature with a difference less than 1.5°C. For each NA measurement 3 ml sample was taken and injected into 12 ml Labco Exetainer flasks (Labco Limited, United Kingdom). The first sample was taken after 30 min after acetylene was added. The second sample was taken after 90 min (1 h after the first sample was taken). During measurements in the flasks, the light intensity and crust temperature were monitored in control flask. The Photosynthetically Active Radiation (PAR) was registered by PAR-sensor of HOBO H-21 logger (Onset Computer Corporation, USA). The temperature was measured by thermistor 2 mm in diameter HOBO H01-001-01 logger (Onset Computer Corporation, USA), inserted inside the surface of soil crust by 3 mm. The PAR during field measurements were in the range between 200-250 μmol m-2 s-1 under these conditions the difference between temperatures in the flasks and outside was less than 1.5°C.
The ethylene was measured in the flasks in the laboratory using gas chromatograph Zvet-800 (Russia) with Porapak N 80/100 separator in 2 m stainless steel column with internal diameter 2 mm. The 0.8 ml of the gas mixture was injected into the column. Standard Linde Gas mixtures were used for calibration of Zvet-800.
Statistical analysis
Statistical analysis was performed using STATISTICA 6.0 program (StatSoft, USA). Significant differences between the means (p<0.05) have been tested by One-way ANOVA followed by post- hoc Tukey test.
Results
Characteristics of studied black Stigonema crusts
In the studied BSCs two species from Stigonema genus dominated: Stigonema minutum [Ag.] Hassall ex Born. et Flah. and S. ocellatum [Dillw.] Thuret ex Born. et Flah. (Figure 2). These species are typical for bare soils, or sometimes the surface of the moss. They form massive colonies which look like round black or black-green spots. Both species have a wide distribution in the Arctic and mountain regions [24]. The species have intercalary and terminal heterocytes involved in active fixation of nitrogen. The mentioned species can form crusts together or separately of each other. In drier habitats the crusts are dominated by Stigonema minutum, in wetter – by St. ocellatum. The abundance of other species was low in soil crusts dominated by Stigonema species. The diazotrophic subdominants of the studied soil crusts are Scytonema ocellatum [Dillw.] Lyngb. ex Born. et Flah., S. hofmannii C. Ag. ex Born. et Flah., species from Nostoc ssp. genus, Calothrix parietina (Näg.) Thuret ex Born. et Flah., Tolypothrix tenuis Kütz., Dichothrix gypsophila (Kütz.) Born. et Flah. Other species with low abundance are Petalonema crustaceum C. Ag. ex Kirchn., Tolypothrix distorta Kütz. ex Born. et Flah., Trichormus variabilis (Kütz. ex Born. et Flah.) Komárek et Anagnostidis, from Calothrix ssp. genus, etc.
Concentrations of total nitrogen and carbon in Stigonema crusts and in the soil layer under are presented in Table 1. The low content of these elements in the soil crusts is connected with large portion of inorganic matter tightly attached to the organic layer of BSCs. The nitrogen concentration in cyanobacterial layer was about 2-8 times higher and carbon 3-24 times higher than in the underlying soil layers. The mean values of total nitrogen and carbon in the crusts showed no statistical difference (Tukey test, p>0.05) (Table 1).
| Malozemelskaja (2015) | Рolar Ural (2013) |
Subpolar Ural (2015,2016) | Northern Ural (2016) | |
|---|---|---|---|---|
| Ncb | 4.4 ± 0.9 (n=4) | 4.7 ± 0.8 (n=2) | 4.2 ± 0.8 (n=5) | 4.0 ± 0.8 (n=4) |
| Ccb | 92 ± 9 (n=4) | 55 ± 10 (n=2) | 71 ± 7.2 (n=5) | 77 ± 7.5 (n=4) |
| Ccb/Ncb ratio | 21 | 12 | 17 | 19 |
| N1cm | 0.7 ± 0.15 (n=4) | 0.6 ± 0.16 (n=2) | 0.7 ± 0.12 (n=5) | 1.9 ± 0.2 (n=4) |
| C1cm | 11 ± 2.6 (n=4) | 5.5 ± 1.3 (n=2) | 7.8 ± 1.2 (n=5) | 31 ± 5 (n=4) |
| C1 cm/N1 cm ratio | 16 | 9 | 12 | 16 |
Note: SD: Standard Deviations
Table 1: The total amount of nitrogen and carbon in cyanobacterial layer of the crusts (cb) and soil layer (1 cm) under it.
N: Total Nitrogen, mg g-1 dry weight; C: Total Carbon, mg g-1 dry weight
Mean value of n samples ± SD
Nitrogenase activity of black Stigonema crusts in the studied areas
The comparative results of NA rates under optimal combination of the conditions (moisture content 160-180% of the dry weight and PAR 200-250 μmol m-2 s-1) are shown in Table 2. As expected, the NA rates under 21°C are 1.5-2 times higher than at 15°C. There is no big difference in values of NA in latitudinal and altitudinal gradients. Between all the studied BSCs the highest rates were recorded for the crusts of the Polar Urals, the lowest – for the Subpolar Urals.
| Nitrogenase activity | Malozemelskaja tundra (2015) | Рolar Ural (2013) |
Subpolar Ural (2015, 2016) | Northern Ural (2016) |
|---|---|---|---|---|
| NA 15 | 0.63 ± 0.11(n=12) | 0.80 ± 0.31(n=8) | 0.59 ± 0.08(n=12) | 0.61 ± 0.14(n=10) |
| NA 21 | 1.02 ± 0.14(n=4) | 1.64 ± 0.86(n=4) | 0.97 ± 0.17 (n=5) | 1.27 ± 0.45(n=10) |
Note: SD: Standard Deviations
Table 2: Mean values of nitrogenase activity rates (mg С2Н4 m-2 h-1) of Stigonema crusts at 15 ± 0.5°С (NA15) and 21 ± 0.5°С (NA 21).
Mean value of n samples ± SD
Discussion
It is well known that, free-living diazotrophic Cyanobacteria have the biggest input in N2 fixing of the biological soil crusts since their biomass and rates of nitrogen activity are the highest among other microbial components in high latitudes [4,20,17]. The contribution of N2 fixation by Cyanobacteria depends on many factors; the most important are the species composition and abundance, type of ecosystem and seasonal microclimate conditions [17]. In the studied plain and mountain tundra, the BSCs with Cyanobacteria are important and constant component of the landscapes. In Malozmelskaya tundra the coverage of BSCs could reach few m2 in diameter and might occupy from 50 to 80% of the area of spotted tundra phytocoenoses [22]. In mountain tundra of Northern Urals, the BSCs don’t exceed 1-2 m2 and might occupy from 5 to 30% of area of spotted tundra [22]. As our research showed, the species from Stigonema genus dominate in BSCs. The species of this genus are widespread in the studied regions, they are able to fix nitrogen as well and, thus, they are important contributors to the N input these ecosystems.
The total nitrogen in the crusts of all investigated was quite similar and averaged from 4.0-4.7 mg g-1 in dry weight (Table 1). The total carbon had more variable values from 55 to 92 mg g-1 in dry weight. The crusts of the plain and mountain tundras had close values of total nitrogen, carbon and C/N ratio which may indirectly indicate similarity in composition of their nitrogen-fixing components. We want to note that the obtained values of total nitrogen and carbon are close to those for organic part of black soil crusts of Svalbard archipelago: the content of total nitrogen was 6.6 mg g-1 dry weight, and the ratio of C/N - 16 [8], but unfortunately, the mentioned article does not contain data on nitrogen-fixing activity of the crusts. While comparing the data with hot desert regions it could be noted that in the BSCs from the south (the measurements were carried on soil crusts with domination of species from Nostoc and Scytonema genus), the total amount of nitrogen was 1.8 mg g-1 in dry weight, of carbon – 49.2 mg g-1 in dry weight [26]. Those values are significantly lower than observed in BSCs of the crusts in the northern regions.
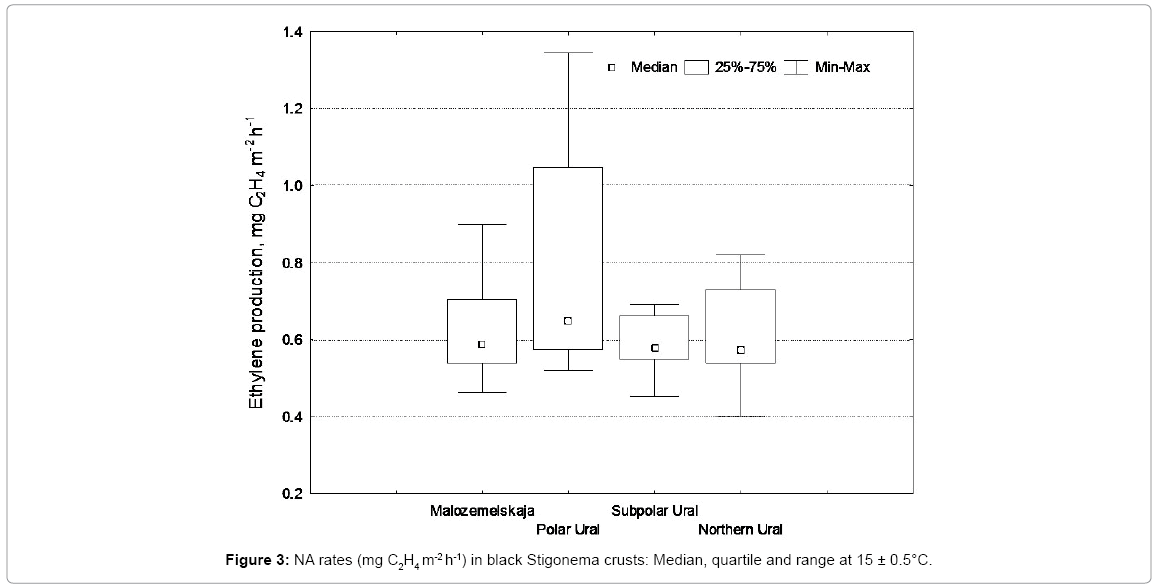
The highest rates of NA in tundra regions were recorded in July, the month with the high temperatures and the best combination of optimal climatic parameters. For example, in Canadian Arctic the maximal rates of NA in similar to ours BSCs with domination of Stigonema species (Stigonema turfaceum (Berk.) Cooke and S. minutum) were observed in July in the range of 0.87-1.73 mg C2H4 m-2 h-1 under 20°C [17]. These rates are closer to our July NA rates of 0.97-1.64 mg C2H4 m-2 h-1 under 21°C. In the studied region, the usual midday temperature in July doesn’t exceed 15°C as long-term observations show and the most of our field measurements were conducted under that temperature. Figure 3 shows the range of values, median and quartile values of NA rates in Stigonema crusts under 15°C. NA rates of black crusts from the Polar Urals stand out among the others with a wide range of values. This could be, attributed to a large variation in coverage of soil crusts by Stigonema colonies in comparison to other areas, as well as greater participation of active N2 fixing Cyanobacteria Nostoc commune in the formation of some of the studied soil crusts in the region. There are no statistically significant differences between the mean values of NA rates of the compared soil crusts (Tukey-test p>0.05) under 15°C as well as under 21°C. The absence of significant differences is also proved by the median values which are close to each other for the all studied BSCs (Figure 3).
Conclusion
No significant differences were found in species composition, dominant complex, or rates of nitrogen fixation, the content of total nitrogen in cyanobacterial crusts from similar habitats, on spots of bare ground in depressions of microrelief in spotted lichen-mossshrub communities of plain and mountain tundra. Black crust with a dominance of species from Stigonema genus from different regions of the European North-East tundra in latitudinal and altitudinal gradients living in similar environmental conditions showed close values of nitrogen-fixing activity rates. Our values for lowland and mountain tundras of the European part of Russia support the assumption by Liengen and Olsen [10] that the same rate of N2 fixation of cyanobacteria is observed in communities of similar tundra vegetation ecosystems in different areas High Arctic. The obtained values might be used to calculate the input of BSCs with Stigonema to the N balance in mountain and plain tundra of North-Eastern European ecosystems and their transformation under climatic change аnd also while calculating global balance of high altitude regions.
Acknowledgement
The research was supported by a grant from the Russian Foundation for Basic Research No. 15-04-06346.
References
- Bliss LC, Heal OW, Moore JJ (1981) Tundra ecosystems: A comparative analysis.Cambridge University Press.
- Belnap J (2003) Factors influencing nitrogen fixation and nitrogen release in biological soil crusts. Biological Soil Crusts: Structure, Function and Management, pp: 241-261.
- Büdel B (2005) Microorganisms of biological crusts on soil surfaces soil biology. Microorganisms in Soils: Roles in Genesis and Functions. Berlin Heidelberg, Springer-Verlag, pp: 307-323.
- Elbert W, Weber B, Büdel B, Andreae MO and Pöschl U (2009) Microbiotic crusts on soil, rock and plants: Neglected major players in the global cycles of carbon and nitrogen? Biogeosciences Discuss 6: 6983-7015.
- Weber B, Büdel B, Belnap J (2016) Soil crusts: An organizing principle in drylands. Berlin, Springer.
- Evans RD, Johansen JR (1999) Microbiotic crusts and ecosystem processes. Crit Rev Plant Sci 18: 183-225.
- Vincent WF (2000) Cyanobacterial dominance in the polar regions. The ecology of cyanobacteria: Their diversity in time and space, Dordrecht, London, Boston, Kluwer Academics Publishers, pp: 321-340.
- Yoshitake S, Uchida M, Koizumi H, Kanda H, Nakatsubo T (2010) Production of biological soil crusts in the early stage of primary succession on a High Arctic glacier forelan. New Phytol 186: 451-460.
- Lennihan R, Chapin DM, Dickson LG (1994) Nitrogen fixation and photosynthesis in high arctic forms of Nostoc commune. Can J Botan 72: 940-945.
- Liengen T, Olsen RA (1997) Nitrogen fixation by free-living cyanobacteria from different coastal sites in a high arctic tundra, Spitsbergen. Arct Antarct Alp Res 29: 470-477.
- Liengen T (1999) Conversion factor between acetylene reduction and nitrogen fixation in free-living cyanobacteria from high arctic habitats. Can J Microbiol 45: 223-229.
- Liengen T (1999) Environmental factors influencing the nitrogen fixation activity of free-living terrestrial cyanobacteria from a high arctic area, Spitsbergen. Can J Microbiol 45: 573-581.
- Dickson LG (2000) Constraints to nitrogen fixation by cryptogamic crusts in a polar desert ecosystem, Devon Island, N. W. T., Canada. Arct Antarct Alp Res 32: 40-45.
- Zielke M, Ekker AS, Olsen RA, Spjelkavik S, Solheim B (2002) The influence of abiotic factors on biological nitrogen fixation in different types of vegetation in the high arctic, Svalbard. Arct Antarct Alp Res 34: 293-299.
- Zielke M, Solheim B, Spjelkavik S, Olsen RA (2005) Nitrogen fixation in the high arctic: Role of vegetation and environmental conditions. Arct Antarct Alp Res 37: 372-378.
- Hobara S, McCalley C, Koba K, Giblin AE, Weiss MS, et al. (2006) Nitrogen fixation in surface soils and vegetation in an Arctic tundra watershed: A key source of atmospheric nitrogen. Arct Antarct Alp Res 38: 363-372.
- Stewart KJ, Coxson D, Grogan P (2011) Nitrogen inputs by associative cyanobacteria across a low Arctic tundra landscape. Arct Antarct Alp Res 43: 267-278.
- Getzen MV, Kostajev VJ, Patova EN (1997) Role of nitrogen-fixing cryptogamic plants in tundra. Disturbers and Recovery in Arctic Lands an Ecological Perspective. Netherlands, Kluwer Academic Publishers, pp: 135-150.
- Patova E, Sivkov M (2002) Diversity of soil cyanophyta, CO2-gas exchange and acetylene reduction of the soil crust in the cryogenic soils (East-European tundra). Nova Hedwigia 123: 387-395.
- Davydov D (2010) Cyanoprokaryota and their role in the process of nitrogen fixation in terrestrial ecosystems of the Murmansk region. Ðœoscow, GEOS.
- Patova E, Sivkov M, Patova A (2016) Nitrogen fixation activity in biological soil crusts dominated by cyanobacteria in the subpolar urals (European North-East Russia). FEMS Microbiol Ecol 92.
- Aleksandrova VD, Komarov VL (1980) The Arctic and Antarctic: Their division into geobotanical areas. Cambridge University Press.
- Atlas of the Arctic (1985) Moscow, Main Department of Geodesy and Cartography.
- Komárek J (2013) Cyanoprokaryota 3. Part 3: Heterocytous Genera. Süβwasserflora von Mitteleuropa. Bd. Berlin, Springer Spektrum.
- Stewart WD, Fitzgerald GP, Burris RH (1967) In situ studies on N2 fixation using the acetylene reduction technique. Proc Natl Acad Sci U S A 58: 2071-2078.
- Zhou X, Smith H, Giraldo Silva A, Belnap J, Garcia-Pichel F (2016) Differential responses of dinitrogen fixation, diazotrophic cyanobacteria and ammonia oxidation reveal a potential warming-induced imbalance of the N-cycle in biological soil crusts. PLoS ONE 11: e0164932.
Citation: Patova E, Sivkov M, Patova A (2017) Renitrogen Fixation Activity in Cyanobacterial Biological Soil Crusts with Domination of the Stigonema Genus Species in Mountain and Plain North-East European Tundra Ecosystems. Environ Pollut Climate Change 1:138. DOI: 10.4172/2573-458X.1000138
Copyright: © 2017 Patova E, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5358
- [From(publication date): 0-2017 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 4418
- PDF downloads: 940