Response to Combination Intranasal Corticosteroid and Intranasal Antihistamine in Allergic Rhinitis, A Questionnaire Based Study in ENT Outpatients
Received: 02-Feb-2017 / Accepted Date: 27-Feb-2017 / Published Date: 28-Feb-2017 DOI: 10.4172/2161-119X.1000294
Abstract
Aim: To analyse the effect of combination intranasal corticosteroid and antihistamine spray in patients suffering from proven Allergic Rhinitis who have failed primary care therapy. Background: Allergic Rhinitis is prevalent in the community and worldwide, with a rising trend, it also impacts on health related quality of life, sleep and (in appropriate age groups) school attendance. Method: The MSNOT-20 is a validated and reliable questionnaire for use in Rhinitis. It was used in two 4 month periods (2014 and 2016) in patients with Allergic Rhinitis who have failed primary care treatment. They were then commenced on a combination nasal spray of azelastine hydrochloride (antihistamine) and fluticasone propionate (corticosteroid). Results: There was improvement in overall symptom severity score (MSNOT-20 score) for each patient, with improvement in all five subgroups; nasal, paranasal, sleep, social and emotional. The MSNOT-20 score is closely correlated with sleep, social and paranasal subgroups. The top 5 most severe symptoms identified by MSNOT-20 questionnaire objectively were also identified by patients as their most troublesome symptoms and include (in descending order); blocked nose, need to blow nose, sneezing, runny nose, post nasal discharge. Conclusion: Allergic rhinitis has widespread impact on different domains of the patients’ life. This combination treatment is an effective treatment regime for patients who have failed primary care and has been proven to be of benefit in seasonal and perennial Allergic Rhinitis by improving all symptom subgroups
Keywords: Allergic rhinitis; Nasal spray; Corticosteroid; Anti-allergy drugs
254652Introduction
Allergic rhinitis
Rhinitis means inflammation of the nasal mucous membrane; when this is caused by an abnormal IgE-mediated response to an otherwise innocuous stimulant, i.e., an allergen, this is known as allergic rhinitis and is the most common form of non-infectious rhinitis [1-4].
Affecting 1 in 4 people seasonal allergic rhinitis, also known as hay fever [5], presents with nasal congestion, rhinorrhea, itching and/or sneezing. Allergic rhinoconjucntivitis is the associated watery eyes, itching, burning/irritability, redness and injection of the conjunctiva which has been documented in 71% of European patients who concurrently had nasal symptoms [6].
Importantly, Allergic Rhinitis has consequences beyond its prevailing symptoms by having significant impact on quality of life and social life, these include mood changes, anxiety, depression and impairment of cognitive function [7].
Skin Prick Testing (SPT) is used to identify allergens patients are allergic to and has been shown to be superior to patient-reported allergen identification or allergens as identified on allergy history take [8]. One meta-analysis reported that on SPT the top three allergens identified in 15 developed countries (covering Europe including the UK, USA and Australia) were house dust mite, grass pollen and cat (median prevalence across all centres 21.7%, 16.9% and 8.8%, respectively) [9].
Epidemiological data suggests that worldwide 400 million people are currently suffering from allergic rhinitis and that its prevalence is on the rise globally [1]. Though the cause for this rising trend is unknown, contributing factors include a higher concentration of air borne allergens, poor air quality due to pollution, poor indoor ventilation, smoking and a more sedentary lifestyle among others [10].
Treatment of allergic rhinitis
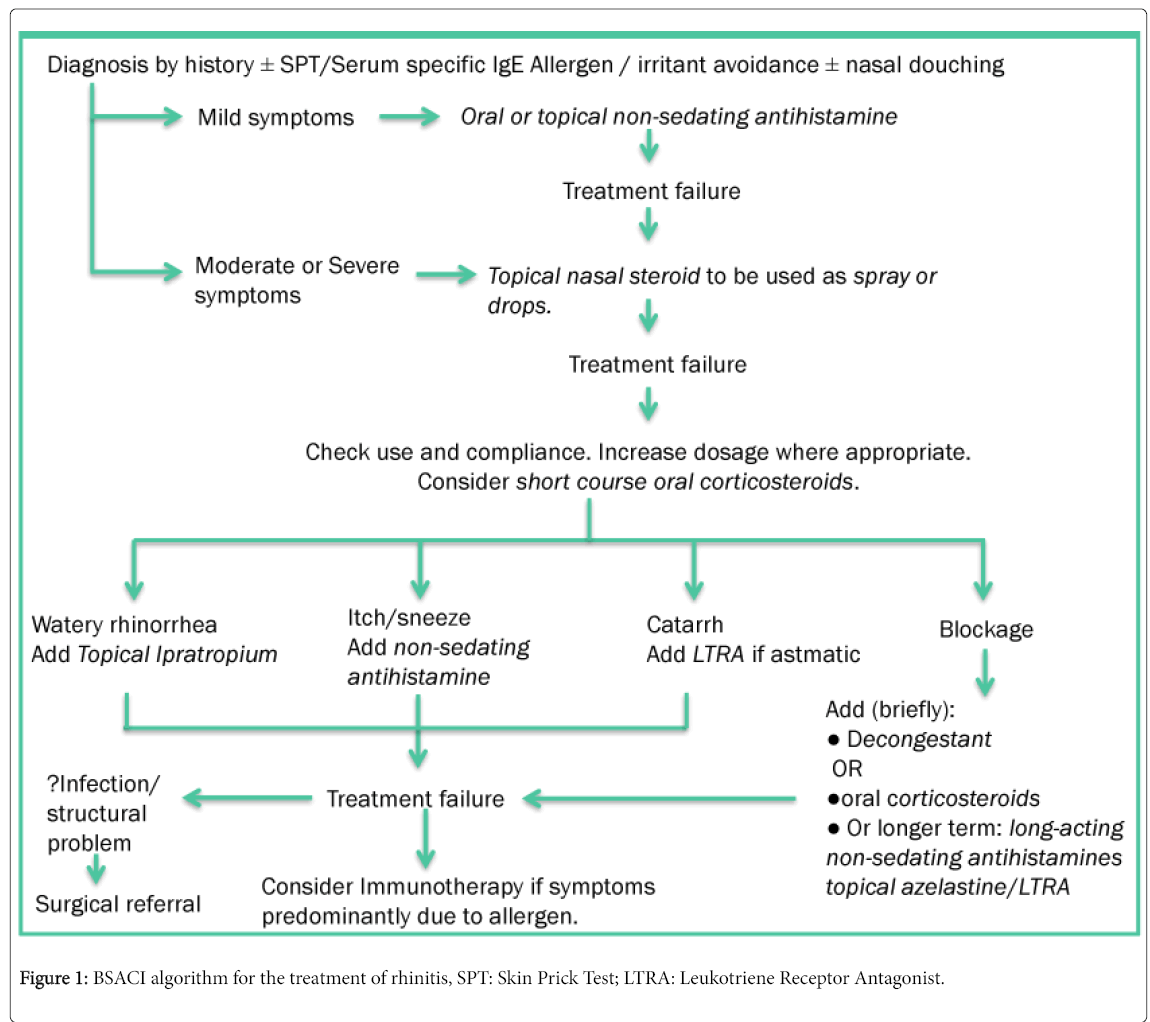
The British Society of Allergy and Clinical Immunology (BSACI) have developed a pathway for the treatment of Rhinitis, adapted in Figure 1 [2].
Primary care has an increasingly heavy burden of Allergic Rhinitis, in England primary care prescriptions for Allergic Rhinitis have gone up by 41.7% from 2001-2005, which includes antihistamines and topical nasal corticosteroids (also includes topical nasal antihistamine and topical nasal cromoglicate) [11].
Specialist centres can provide Immunotherapy for patients who have had optimal medical treatment yet symptom control has been either minimal or non-existent, there is impact on quality of life and no contra-indications to immunotherapy. Immunotherapy treatment itself has lasting benefits for the first few years after treatment though this will diminish over time [12].
Study aim
The primary aims of this study is to analyse the effect of combination nasal spray (azelastine hydrochloride and fluticasone propionate) in patients suffering from proven allergic rhinitis who have failed primary therapy in the GP setting.
Secondary aims included subgroup analysis as defined by the MSNOT-20 questionnaire and exploring symptom responsiveness based upon skin prick test results.
Methods
The MSNOT-20 questionnaire is a validated, disease specific questionnaire (Appendix 1) which can identify rhinitis, its associated impact on quality of life and disease response to treatment in the adult population, its modified version has similar proven qualities in the paediatric population of 11-16 year olds [3,13]. It consists of three sections; section one comprises of demographic details, section two is the disease specific section and section three is the quality of life section. In the disease specific section, patients would describe the severity of their symptoms based on a Likert 1 to 5 scale with 5 being quantified “as bad as it can be”. The related questions are grouped together into subgroups, each of which is composed of the following questions from this section:
Nasal: questions 1, 2, 3, 19
Paranasal: 5, 6, 7, 8, 9, 10-can be further split into the below for further analysis
• Sinus: 5, 6, 10
• Ear: 7, 8, 9
Sleep: 11, 12, 13,14
Social: 15, 16, 17
Emotional: 18, 20
Inclusion criteria: Patients being referred from their GP to the ENT and Allergy department at Royal National Throat, Nose and Ear hospital, London due to nasal and sinus symptoms. These patients showed no response to optimal primary care treatment along the Figure 1 algorithm (which begins with monotherapy agents) or practice-specific guidelines. Participants were enrolled in two fourmonth periods, February-May 2014 and February-May 2016.
Exclusion criteria: Patients with nasal polyposis.
At first clinical contact patients had a full clinical assessment, completed the MSNOT-20 questionnaire and, if relevant, had skin prick test.
Subsequent management plan including pharmaceutical option was discussed; if eligible and clinically appropriate (using treatment guidelines and clinical experience), treatment was advised with the aforementioned combination nasal spray and, following informed consent, initiated as per manufacturers guidelines; 1 spray in each nostril twice a day in those over 12 years (at the time of the study). Where needed, a patient information leaflet and clinical demonstration of the correct technique when using a nasal spray was carried out.
As this particular brand combination was initially not available at the index hospital a GP prescription was provided. After four weeks, patients were asked to repeat section two of the MSNOT-20 questionnaire again, results were sent back to the researchers. Data was then collated and statistical analysis was carried out.
Results
In total, 48 candidates (24 males, 24 females) were eligible for inclusion into the study, age range 20-69 with just under 2/3rd (65%) being in the 20-44 age group. There was no statistically significant difference in symptom severity between males and females and between those with a positive family history compared to those without.
Before and after treatment comparisons
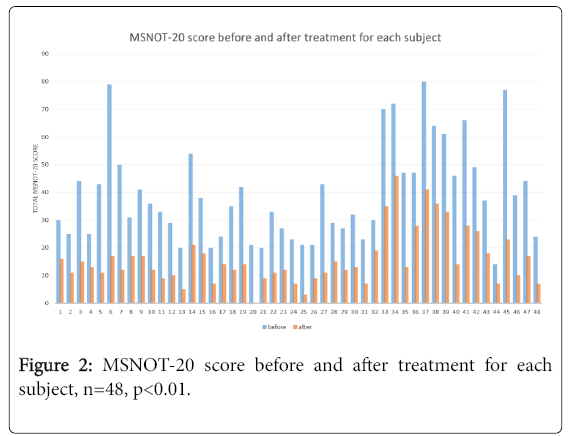
The sum of the symptom severity patients reported for each of the twenty questions were calculated to produce the MSNOT-20 score and is represented in Figure 2 for each subject. All patients had significant improvement in their overall symptom severity.
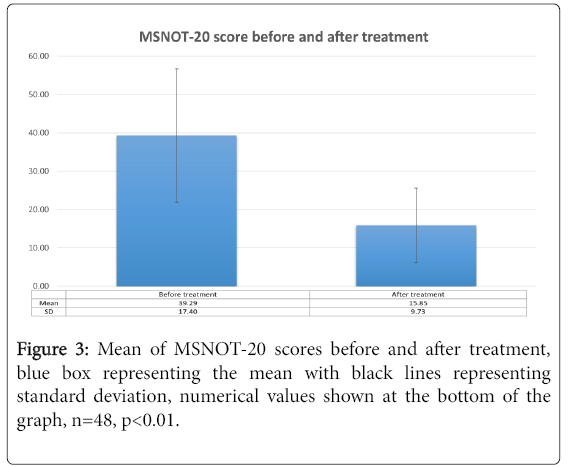
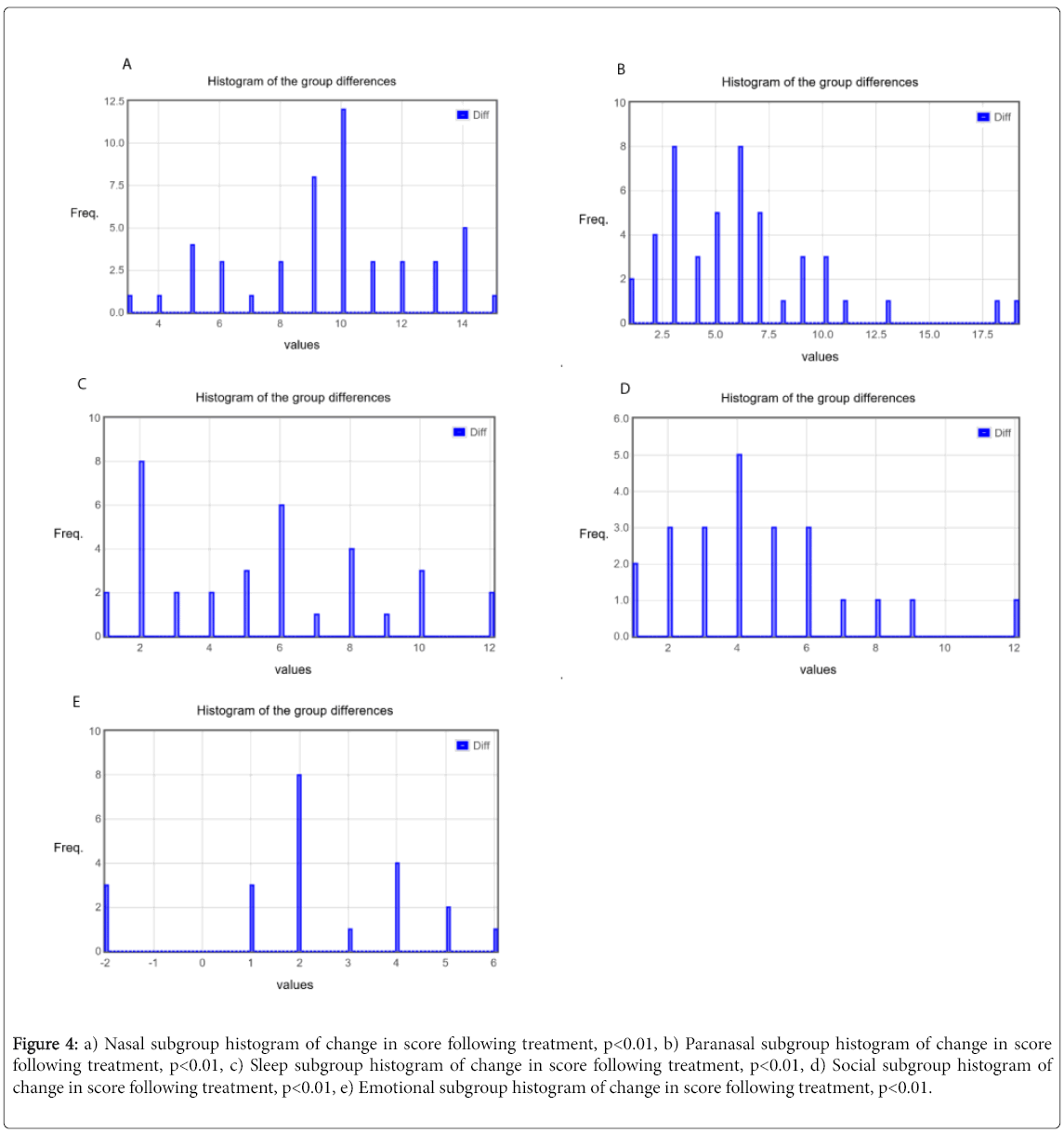
The mean and standard deviations were calculated for before and after treatment responses and shown in Figure 3. Similar calculation was then carried out for all five subgroups as shown in Table 1 with the changes in each subgroup before and after treatment delineated in Figures 4a-4e. There was a greater than 50% decrease in symptomology post treatment with all subgroups showing improvement, though the improvement was greatest in the nasal subgroup.
| Subgroup | Mean before treatment (Standard Deviation) | Mean after treatment (Standard Deviation) | p value |
|---|---|---|---|
| Nasal | 15.69 (4.04) | 6.08 (2.43) | <0.01 |
| Paranasal | 9.40 (5.54) | 3.56 (2.95) | <0.01 |
| Sleep | 6.29 (6.82) | 2.44 (3.93) | <0.01 |
| Social | 4.10 (5.15) | 1.90 (3.14) | <0.01 |
| Emotional | 1.83 (2.36) | 0.83 (1.36) | <0.01 |
Table 1: Representing total subgroup mean and standard deviation for each subgroup before and after treatment with p value quoted.
Figure 4: a) Nasal subgroup histogram of change in score following treatment, p<0.01, b) Paranasal subgroup histogram of change in score following treatment, p<0.01, c) Sleep subgroup histogram of change in score following treatment, p<0.01, d) Social subgroup histogram of change in score following treatment, p<0.01, e) Emotional subgroup histogram of change in score following treatment, p<0.01.
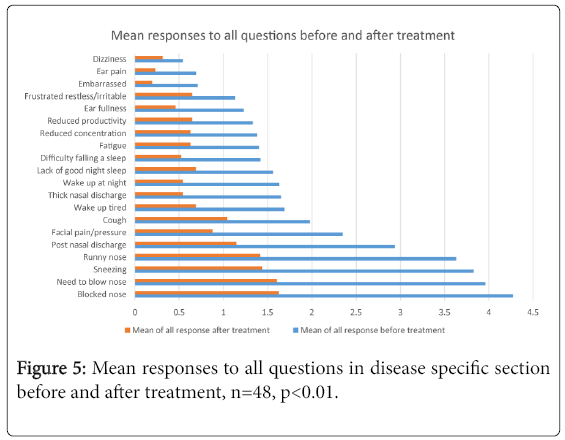
Key symptoms
Figure 5 represents the mean responses to each question before and after treatment, showing the improvement in all symptoms post treatment whilst also showing what the most severe symptoms patients presented with were (below, in descending order), this order remained the same post treatment.
1st Blocked nose
2nd Need to blow nose
3rd Sneezing
4th Runny nose
5th Post nasal discharge
The questionnaire asked patients to report the top five symptoms they felt were most important to them in terms of impact on their health. All responses to this were collated and the top 5 symptoms patients perceived to be of greatest importance to them were the same as the above list.
Subgroup Correlation
Spearman’s Rank Correlation Coefficient test was used to determine correlations between the subgroup as defined in the Method section. The strength of the correlation is represented by the R value; a score of greater than 0.30 is considered a positive, an R score of greater 0.50 considered moderately strong positive correlation whilst a score over 0.70 in strength is considered a strongly positive correlation [14].
• The MSNOT-20 correlates strongest with the sleep subgroup (R=0.90, p<0.05) followed by social (0.85, p<0.05), paranasal (0.78, p<0.05) and emotional (0.76, p<0.05)
• Paranasal subgroup correlates positively with sleep (0.62, p<0.05) followed by social (0.52, p<0.05) and emotional subgroups (0.46, p<0.05)
Skin prick test results and correlation with overall score
The breakdown of the skin prick test results for the entire cohort (n=48) are shown below:
• House dust mite: n=29 individuals positive on skin prick testing
• Grass pollen: n=37 individuals positive on skin prick testing
• Tree pollen: n=17 individuals positive on skin prick testing
• Mould: n=5 individuals positive on skin prick testing
• Aspergillus: n=3 individuals positive on skin prick testing
• Cat: n=16 individuals positive on skin prick testing
• Dog: n=13 individuals positive on skin prick testing
Table 2 shows the improvement in MSNOT-20 score and Nasal subgroup score before and after treatment and has been split by seasonal (grass or tree pollen in this study) and perennial (allergens present all year round, this study included house dust mite, mould, aspergillus, cat and dog) allergens. The results show that the total MSNOT-20 was reduced by two and a half times through the treatment in the seasonal allergens, this was also similar in the nasal score. A considerable improvement in MSNOT-20 score and Nasal subgroup was also noted for perennial allergens.
| Allergen | MSNOT-20 score before | MSNOT-20 score after | Nasal subgroup before | Nasal Subgroup after |
|---|---|---|---|---|
| Seasonal Allergen | ||||
| Grass pollen | 41 | 16.54 | 15.89 | 6.19 |
| Tree Pollen | 45.29 | 17.82 | 17.71 | 7.12 |
| Perennial Allergen | ||||
| House dust mite | 41.41 | 16.9 | 14.66 | 5.79 |
| Mould | 51.4 | 16.8 | 14.8 | 4 |
| Aspergillus | 30.33 | 14.67 | 14 | 5.67 |
| Cat | 48.44 | 19.5 | 16.81 | 6.81 |
| Dog | 49.08 | 19.92 | 15.85 | 5.85 |
Table 2: MSNOT-20 before and after treatment and Nasal subgroup before and after treatment split by individual allergen (as confirmed by skin prick test), n=48, p<0.01.
Discussion
Summary
There was improvement in overall symptom severity and in all subgroups following treatment in patients with seasonal Allergic Rhinitis and perennial Allergic Rhinitis who had failed community treatment and been referred to secondary care. It can take approximately 6 weeks from GP referral to presentation at clinic; our results showed that patients had a high burden of symptoms at presentation to clinic indicating inadequacy of initial treatment and unlikelihood of symptom resolution independent of our interventions.
In subgroup analysis, the biggest improvement post treatment was seen in the nasal subgroup, followed by the paranasal subgroup showing the effective beneficial relief this local agent is able to achieve. Though it is a case that all subgroups improved this was small (but significant) in the emotional subgroup indicating the variety of different causes of impairment in this domain and importance of assessing the impact of treatment based on the overall response.
This study also demonstrated the widespread impact that this condition has; as seen by the fact that some of the strongest correlation noted with a high MSNOT-20 score (i.e., most symptomatic) is with the sleep and social subgroups. This study also represents the dominant role of paranasal symptoms in Allergic Rhinitis, which also responded to the treatment provided here. Paranasal subgroup itself correlated with sleep and social subgroups as well as the emotional subgroup.
This study has shown that the MSNOT-20 is able to detect objectively the most severe symptoms of the patient (among others) and correlate this with what the patients perceive to be their most troublesome symptoms, ensuring this very important aspect of management is noted as early as possible. In this study, ‘Blocked nose’ was the most severe symptom both objectively and as perceived by the patient as most impacting on their health, this is decreased by more than two-and-a-half times through this intranasal effective treatment in patients who had initially failed treatment through their GP.
The majority of our patients were within the young working age group which is likely to have impact on occupation, among others, however Section 3 of the MSNOT-20 questionnaire which addresses this was not reviewed in this analysis, as were data on ethnicity, housing, smoking due to incomplete results available allowing for scope to further look into this.
Skin prick testing revealed our cohort had sensitisation to multiple allergens, which is not uncommon in clinical practise. One study of 200 patients with medically unresponsive chronic or recurrent rhinosinusitis revealed that 52% of these had multiple allergy sensitivities however this did not appear to determine the severity of sinus disease as seen on imaging, mitigating impact on clinical severity [15].
Strength and limitations
This study was able to elicit large improvements in the key domains of nasal and overall symptomatology, with improvements in all domains noted in patients with proven Allergic Rhinitis, this was in spite of the relatively limited sample size. A larger sample size may also allow us to explore the role of family history and symptom severity, to correlate with current research identifying genetic factors, notably genes involved in epithelial barrier/regulatory function, which may be involved in allergic rhinitis [16]. It was also difficult to carry out comprehensive analyses on some aspects e.g. Section 3 of the questionnaire due to some incomplete data.
Comparison with existing literature
Treatment of Allergic Rhinitis is a combination of lifestyle changes (by reducing exposure to the allergens) and medical therapy including nasal irrigation. One meta-analysis (n=2,267) showed that intranasal corticosteroids in patients with Allergic Rhinitis provides significantly greater relief of nasal congestion than oral antihistamines [17].
It is combination therapy which has proven to not only improve symptomatology but also be found to be more convenient and effective when used by patients [18,19].
Implications for research and/or practice
Primary care setting has an increasing demand of medications used in Allergic Rhinitis [11]. This combination therapy has proven to be effective at decreasing disease burden and relief from its associated impact on sleep, social and emotional domains. Its effects have been proven in a cohort of patients with confirmed Allergic Rhinitis who had initially failed treatment at the primary care level and as such is a viable option available to the community setting.
Future research into correlation of familial and demographic variables on disease and severity with a more comprehensive assessment of financial and occupational complications of this very prevalent disease are warranted.
Conclusion
There was significant improvement in Allergic Rhinitis symptoms with all five subgroups having improvements in symptomatology. The nasal subgroup in particular responded well in patients with both seasonal allergens and perennial allergens. This treatment has been effective where subjects had previously failed community therapy.
Conflict of Interest
A.S.S has received fees from Meda Pharmaceuticals. Meda Pharmaceuticals had no role in study design, collection, analysis, interpretation of data, writing the report or decision to submit for publication.
Funding
A.S.S received fees from Meda Pharmaceuticals.
References
- Baena-Cagnani CE, Canonica GW, Helal MZ, Gómez RM, Compalati E, et al. (2015) The international survey on the management of allergic rhinitis by physicians and patients (ISMAR). World Allergy Organ J 8: 1.
- Sami AS, Scadding G (2013) Management of allergic rhinitis in schools. British Journal of School Nursing 8: 119-123.
- Sami AS, Malik M, Amjad M, Howarth P (2013) Rhinitis, sinusitis and ocular disease–2092. The MSNOT-20 questionnaire: Repeatability and disease analysis of rhinitis/rhinosinusitis. World Allergy Organ J 6: 1.
- Bousquet J, Khaltaev N, Cruz A, Denburg J, Fokkens W, et al. (2008) Allergic rhinitis and its impact on asthma (ARIA) 2008. Allergy 63: 8-160.
- Kariyawasam HH, Scadding GK (2010) Seasonal allergic rhinitis: Fluticasone propionate and fluticasone furoate therapy evaluated. J Asthma Allergy 3: 19-28.
- Canonica GW, Bousquet J, Mullol J, Scadding GK, Virchow JC (2007) A survey of the burden of allergic rhinitis in Europe. Allergy 85: 17-25.
- Meltzer EO, Gross GN, Katial R, Storms WW (2012) Allergic rhinitis substantially impacts patient quality of life: Findings from the Nasal Allergy Survey Assessing Limitations. J Fam Pract 61: S5-S10.
- Smith HE, Hogger C, Lallemant C, Crook D, Frew AJ (2009) Is structured allergy history sufficient when assessing patients with asthma and rhinitis in general practice? J Allergy Clin Immunol 123: 646-650.
- Bousquet P, Chinn S, Janson C, Kogevinas M, Burney P, et al. (2007). Geographical variation in the prevalence of positive skin tests to environmental aeroallergens in the European Community Respiratory Health Survey I. Allergy 62: 301-309.
- Baena-Cagnani CE, Gómez RM (2009) Is the prevalence of allergy continuously increasing? Allergy frontiers: Epigenetics, allergens and risk factors. Springer, pp: 17-31.
- Ghouri N, Hippisley-Cox J, Newton J, Sheikh A (2008) Trends in the epidemiology and prescribing of medication for allergic rhinitis in England. J R Soc Med 101: 466-472.
- Walker SM, Durham SR, Till SJ, Roberts G, Corrigan CJ, et al. (2011) Immunotherapy for allergic rhinitis. Clin Exp Allergy 41: 1177-1200.
- Sami AS, Scadding GK (2014) Rhinosinusitis in secondary school children-part 2: Main project analysis of MSNOT-20 Young Persons Questionnaire (MSYPQ). Rhinology 52: 225-230.
- Mukaka MM (2012) Statistics corner: A guide to appropriate use of correlation coefficient in medical research. Malawi Med J 24: 69-71.
- Emanuel IA, Shah SB (2000) Chronic rhinosinusitis: allergy and sinus computed tomography relationships. Otolaryngol Head Neck Surg 123: 687-691.
- Portelli MA, Hodge E, Sayers I (2015) Genetic risk factors for the development of allergic disease identified by genome-wide association. Clin Exp Allergy 45: 21-31.
- Nathan RA (2008) The pathophysiology, clinical impact, and management of nasal congestion in allergic rhinitis. Clin Ther 30: 573-586.
- Carr W, Bernstein J, Lieberman P, Meltzer E, Bachert C, et al. (2012) A novel intranasal therapy of azelastine with fluticasone for the treatment of allergic rhinitis. J Allergy Clin Immunol 129: 1282-1289.
- Meltzer EO, LaForce C, Ratner P, Price D, Ginsberg D, et al. (2012) MP29-02 (a novel intranasal formulation of azelastine hydrochloride and fluticasone propionate) in the treatment of seasonal allergic rhinitis: A randomized, double-blind, placebo-controlled trial of efficacy and safety. Allergy Asthma Proc 33: 324-332.
Citation: Sami AS, Ahmed AS, Ahmed N (2017) ne in Allergic Rhinitis, A Questionnaire Based Study in ENT Outpatients. Otolaryngol (Sunnyvale) 7:294. DOI: 10.4172/2161-119X.1000294
Copyright: © 2017 Sami AS, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5935
- [From(publication date): 0-2017 - Jul 31, 2025]
- Breakdown by view type
- HTML page views: 4946
- PDF downloads: 989