Review of Parasites and Pathologies of the Main Bivalve Species of Commercial Interest of Argentina and Uruguay, Southwestern Atlantic Coast
Received: 11-Jul-2017 / Accepted Date: 29-Aug-2017 / Published Date: 31-Aug-2017
Abstract
A systematic review of the parasites and pathologies of the main important commercially bivalve species from Argentina and Uruguay has been done. Bacteria, algae, Perkinsozoa, Haplosporidia, Ciliophora, Apicomplexa, Turbellaria, Trematoda, Cestoda and Nemertea have been found infecting natural populations of bivalve species. Disseminated neoplasia has been recorded in mussels from culture causing gonadal castration and digestive gland atrophy. In particular, mortalities from cultured oysters have been associated predominately to Bonamia exitiosa. In some cases, parasites have been shown to reduce the condition index of the host. This review provides information on the individual and population effects of these conditions as well as providing suggestions for future research.
Keywords: Protozoans; Metazoans; Mollusc diseases; Shellfish; Southweast atlantic ocean
66875Introduction
Along the Argentinean coast several bivalve species have an important economic value [1]. In Buenos Aires province, the yellow clam Amarilladesma mactroides (Mesodesmatidae) was an important economic resource during the decades of 1940 and 1950. After a maximum extraction of 1078.80 ton in 1953 [2] the production declined due to overexploitation, consequently the commercial harvest was prohibited in 1958 [3] and the fisheries targeted other species as cockles [4]. By other hand, the artisanal fisheries of the north Patagonian gulfs have been operating mainly on the Tehuelche scallop Aequipecten tehuelchus (Pectinidae). Following the marked decline due to overexploitation during the 1995s, the fisheries continued operating targeting other resources [5]. These mainly included the mussel Mytilus platensis and the ribbed mussel Aulacomya atra (Mytilidae), the Puelche oyster Ostrea puelchana (Ostreidae), the “geoduck” Panopea abbreviata (Hiatellidae), the razor clam Ensis macha (Pharidae) and the venerid Leukoma antiqua and Amiantis purpurata clams. In Uruguay, fishing companies pointed out their interest in exporting the venerid clam, Pitar rostratus as a fresh product to Europe; therefore a survey about its health status was conducted to confirm that these populations were free of pathogens included in the OIE (World Organisation for Animal Health) list of notifiable diseases [6].
The aquaculture industry is now the single fastest-growing food production process in the world [7]. Although Argentina and Uruguay have a vast platform and more than 5000 km of Atlantic coast favourable for the oceanic and sustainable culture, current aquatic production volumes are not relevant when compared to the growth of aquaculture at the regional and global levels [7]. In Argentina, bivalve molluscs occupy the third place in aquaculture production. According to their importance, culture products include Japanese Pacific oyster, Magallana (=Crassostrea ) gigas and the mussel, M. platensis . Their production has reached the market since the year 2000, and by 2003, 80 tonnes were commercialized, which is equivalent to 5 percent of total aquaculture production, showing at present a sustained growth [7]. Particularly, the Puelche oyster O. puelchana , constitutes an important resource, since its flesh is similar to the European oyster O. edulis . Its fishery has been closed in San Matías gulf for the last several years [8], but its culture, including seed production, was successful until the appearance of the pathogen Bonamia exitiosa , which has caused an OIE notifiable aquatic disease leading to mass mortalities of oysters from culture [9,10].
Epizootic diseases have been shown to cause catastrophic mortality among marine bivalves, which can severely affect fisheries and aquaculture activities around the world [11]. Some of these diseases have become a serious constraint on the development and sustainability of shellfish farming and fishing [12]. Currently, the main cause of epizootic outbreaks is thought to be the transfer of infectious agents through the transport of live shellfish [13]. According to OIE, notifiable diseases are ones of socioeconomic and/or public health importance within countries or those that are significant in the international trade of aquatic animals and their products. The OIE has identified a series of diseases that can drastically affect the survival and growth of bivalve mollusc of economic importance. The current list [14] of notifiable bivalve’s diseases and the causative pathogens comprises: marteiliosis (Marteilia refringens), bonamiosis (Bonamia ostreae and B. exitiosa ) and perkinsosis (Perkinsus marinus and P. olseni ).
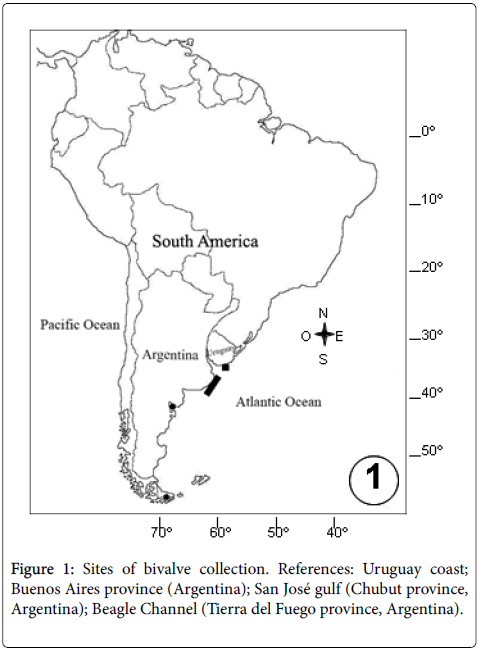
In Argentina, the health status of several bivalve species of commercial interest regarding their parasitofauna and pathologies has been well documented: the yellow clam A. mactroides encompassing the entire geographic range distribution (32° 12´S, 52° 11´W Brazil-40° 55´S, 62° 25´W Argentina) [15-19]; the Tehuelche scallop A. tehuelchus , the mussel M. platensis , the ribbed mussel A. atra , the Puelche oyster O. puelchana , the false oyster Pododesmus rudis (Anomiidae), and the geoduck P. abbreviata , razor E. macha and venerid L. antiqua clams from natural populations of San José gulf (42° 25’S, 64° 07’W- Chubut province); Amiantis purpurata from San Matías gulf (41° 30´S, 64° 15'W- Río Negro province); the stout razor clam Tagelus plebeius (Solenocurtidae) from Mar Chiquita (37° 46´S, 57° 27´W- Buenos Aires province); M. platensis from culture from Beagle Channel (54° 47´S, 68° 14´W-Tierra del Fuego province) (Tables 1 and 2). Instead, little is known about the parasites and pathologies of bivalve populations in Uruguay, where only the venerid P. rostratus clams have been object of study (Figure 1).
| Parasite | Host |
|---|---|
| Prokaryotic microorganisms | Aequipecten tehuelchus, Mytilus platensis, Aulacomya atra, Ostrea puelchana, Pododesmus rudis[23,33,45]; Panopea abbreviata [46]; Ensis macha [34]; Pitar rostratus [6]. |
| Coccomyxa parasitica | M. platensis [40,41]; P. abbreviata, E. macha [42]. |
| Trichodina-like ciliate | Amarilladesma mactroides [2,15,19]; A. tehuelchus [23,33]. |
| Other ciliates | A. tehuelchus, M. platensis, A. atra [33,45]; P. abbreviata [45]; E. macha [34]. |
| Nematopsis-like | A. tehuelchus, P. rudis[23,33]; P. rostratus [6]. |
| Porospora-like | P. abbreviata [46]. |
| Coccidean | A. mactroides [15,16]; P. rudis [23]; E. macha[34]; P. rostratus [6]. |
Table 1: List of microbial and protozoan parasites reported in the main important commercially bivalve species from Argentina and Uruguay.
| Parasite | Host (reference) |
|---|---|
| Paravortex nicolli (Turbellaria, Graffillidae) | Mytilus platensis[52]. |
| Paravortex mesodesma (Graffillidae) | Amarilladesma mactroides [19,51]. |
| Paravortex panopea (Graffillidae) | Panopea abbreviata, Ensis macha [53]. |
| Paravortex sp. (Graffillidae) | Pododesmus rudis [33]. |
| Trematode sporocyst (Gymnophallidae) | Leukoma antiqua [33]. |
| Trematode sporocyst (Fellodistomidae) | Tagelus plebeius [61]. |
| Trematode sporocyst (Monorchiidae) | Amiantis purpurata [54,55]. |
| Trematode sporocyst (Aporocotylidae) | A. purpurata [55]; E. macha [34]. |
| Trematode sporocyst (Bucephalidae) | M. platensis, Aulacomya atra [59]. |
| Trematode unidentified metacercaria | M. platensis [45]; Pitar rostratus [6]. |
| Trematode metacercaria (Renicolidae) | M. platensis, A. atra [59]. |
| Trematode sporocyst/metacercaria (Gymnophallidae) | M. platensis, A. atra [60]; L. antiqua [33]; T. plebeius [61]. |
| Larval cestode (Tetraphyllidea) | Aequipecten tehuelchus, L. antiqua[33]. |
| Larval nematode (Spirurinae) | T. plebeius[61]. |
| Malacobdella arrokeana (Malacobdellidae) | P. abbreviata[68]. |
Table 2: List of metazoan parasites reported in the main important commercially bivalve species from Argentina and Uruguay.
The present review comprised microbial, protozoan and metazoan parasites affecting the main important commercially bivalve species in Argentina and Uruguay.
Parasites Included in the OIE List of Notifiable Diseases
Perkinsus olseni
Protozoan parasites of the genus Perkinsus are known to infect many species of marine molluscs including oysters, abalones, clams, scallops, cockles, and mussels. The systematic affinities of the genus Perkinsus was controversial from its beginning. Initially, it was placed in the phylum Apicomplexa [20] subsequent phylogenetic studies support its inclusion as a unique group of Alveolates [21]. There are presently seven accepted species, P. marinus , P. olseni (=P. atlanticus ), P. qugwadi , P. chesapeaki (=P. andrewsi ), P. mediterraneus , P. honshuensis , and P. beihaiensis [22].
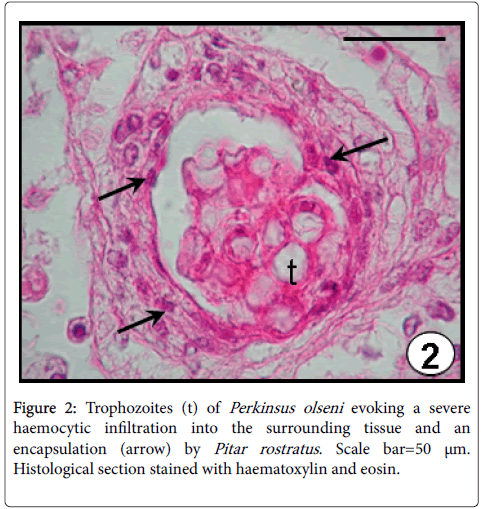
Perkinsus olseni has been reported for the first time in South America infecting natural populations of P. rostratus from Uruguay with a prevalence of 22% [6]. The occurrence was observed mainly in the connective tissue surrounding the intestine, as well as the mantle and gills. A severe haemocytic infiltration and an encapsulation of the trophozoites were evidenced as host defence responses (Figure 2). Perkinsus olseni has been detected infecting several clam and oyster species from Australia, New Zealand, South Korea, Japan, China, Portugal, Spain and Italy [22].
None species of Perkinsus was found infecting the bivalve species studied along the Patagonian coats of Argentina (A. tehuelchus , M. platensis , A. atra , O. puelchana , P. rudis , P. abbreviata , E. macha and L. antiqua ) neither by histological examination nor by culture in Ray’s fluid thioglycollate medium and immunoassays [23]. The absence of this parasite may be related to the colder water (<20°C), unfavourable for its development, since proliferation of all Perkinsus species occurred in temperatures over 20°C [24].
Bonamia exitiosa
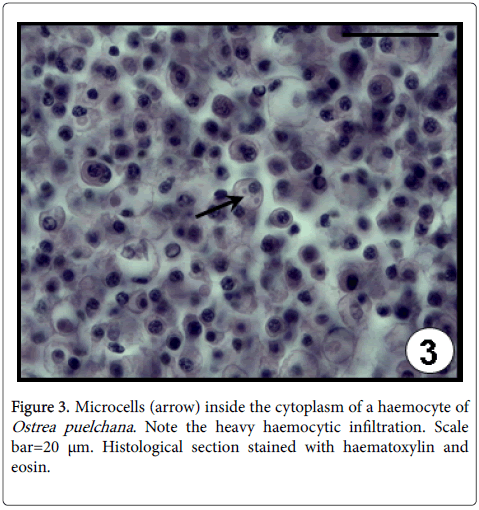
Species of Bonamia are haplosporidian protozoan parasites [25] which infect haemocytes of several oyster species, inducing physiological disorders and eventually causing the death of the animal [26,27]. Three Bonamia species have been identified worldwide, B. ostreae in Europe and North America; B. perspora from North America and B. exitiosa from North and South America, Australia, New Zealand and the Mediterranean sea [10]. In Argentina, B. exitiosa has been blamed for mass mortalities events among O. puelchana culture in San Antonio bay (San Matías gulf) during 1996 [9,10]. Most of the microscopic signs of Bonamia spp. infection are the haemocytic infiltration in all the connective tissue (systemic infiltration) (Figure 3), the dissociated appearance of the connective tissue containing haemocytes with picnotic nuclei and the intracellular location of the microcells (1-8 parasites in one haemocyte) [9].
Infection radiated from culture area, since 1996, through the oyster populations of San Matías gulf, with highest annual values in austral autumn (from March to May) and lower levels in austral spring/early summer (from September to November) [28]. Bonamia exitiosa has also been recorded infecting several oyster species from New Zealand, Australia, northeast of Spain, Chile and North Carolina in USA [29].
Other parasites and pathologies
Several microbial, protozoan parasites (Table 1), metazoans (Table 2) and pathologies not included in the OIE list have been detected causing both a negative effect in the condition of their hosts or evoking inflammatory/encapsulation responses as well as host castration.
Prokaryotic microorganisms
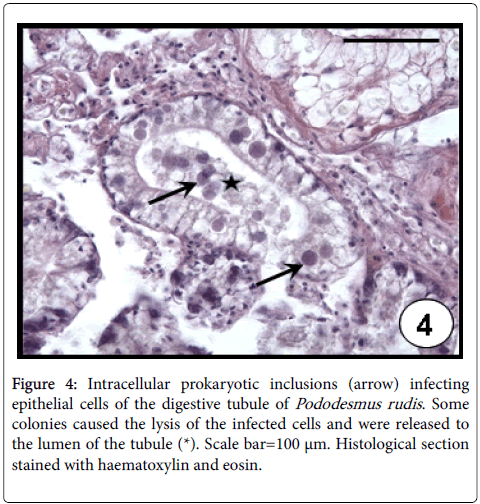
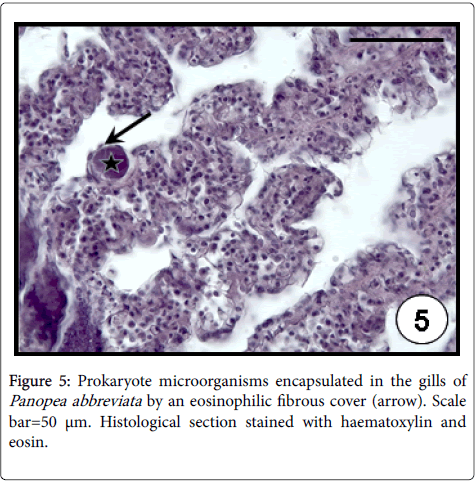
Rickettsia -like (RLO´s) and Chlamydia -like organisms have been reported in several marine bivalve molluscan [30]; although in most cases of mortality, the detected prokaryotes have been associated with RLO´s. Rickettsiae and Chlamydiae species are commonly distinguished by using transmission electron microscopy, taking into account their morphology and division system. Few intracellular prokaryotic organisms infecting aquatic animals have been sufficiently characterized for precise taxonomic placement [31]. In the case of marine invertebrates, the principal problem in the study of intracellular obligate parasites is the absence of cell lines. Therefore, without ultrastructural data it is only possible to name these inclusions as ‘prokaryotic microorganisms’ [32]. Prokaryotic microorganisms were found infecting both epithelial cells of digestive tubule (Figure 4) or intestine in almost of all studied bivalve species (A. mactroides , A. tehuelchus , M. platensis , O. puelchana , P. rudis , P. abbreviata , E. macha ) on the Argentinean coasts [23,33,34]. These occurred as small, rounded intracellular basophilic colonies, evoking the lysis of infected cells of the digestive tubule. The highest values of prevalence (41%) and intensity of infection (95 colonies per histological section) were recorded in P. rudis . Prokaryote microorganisms were detected in gills by light microscopy from histological sections in several bivalve species. They are basophilic and Gram-negative, but their intracellular location is not clearly defined by light microscopy; they are coated by an eosinophilic fibrous cover, which is interpreted as an encapsulation host response [35]. Despite their intracellularity being unclear, these “cysts” were reported as rickettsial-like organisms in the gills of M. edulis and Tridacna crocea clam [35,36]. On the Argentinean coasts, these were found as encapsulated large inclusions in the gills of M. platensis , A. atra and P. abbreviata (Figure 5), indicating a host defence mechanism by the hosts. The highest values of prevalence (23%) and intensity of infection (47 cysts per histological section) were recorded in P. abbreviata . Infections by prokaryotic microorganisms have been associated with mortalities in bivalve populations worldwide [37].
Figure 4: Intracellular prokaryotic inclusions (arrow) infecting epithelial cells of the digestive tubule of Pododesmus rudis . Some colonies caused the lysis of the infected cells and were released to the lumen of the tubule (*). Scale bar=100 μm. Histological section stained with haematoxylin and eosin.
Coccomyxa parasitica
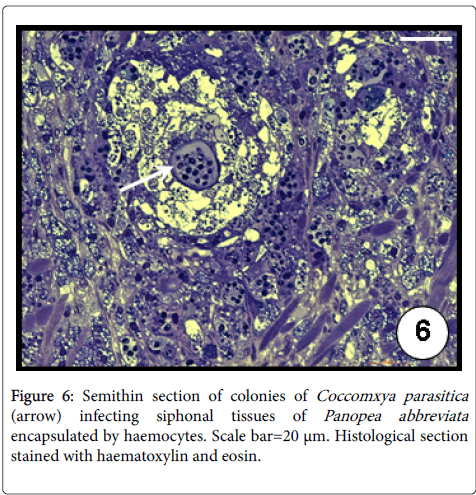
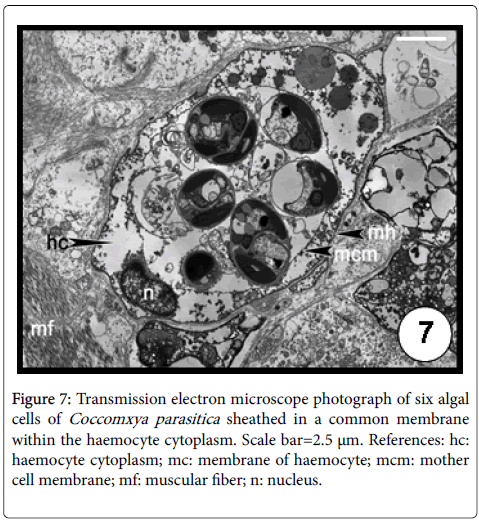
The intracellular green alga C. parasitica was first described infecting the giant scallop Placopecten magellanicus (Pectinidae) from Newfoundland, Canada [38]. Then it was later recorded in M. platensis from the North Sea and Malvinas (Falkland) Islands [39] and from San José gulf (Argentina) [40,41]. Recently, C. parasitica was identified by transmission electron microscope and molecular analysis for the first time in the geoduck, P. abbreviata and in the razor clam, E. macha in Argentina [42]. The infection occurred exclusively in the distal section of the siphonal tissues evoking a severe inflammation response of the host evidenced by both haemocytic infiltration and encapsulation (Figure 6). Up to six algal cells ensheated in a common membrane were observed in a single haemocyte (Figure 7). The authors found lower condition index values in infected geoducks than in uninfected ones, associated to energetic cost required in the defence mechanisms. The prevalence in geoducks was of 51% whilst only 1 specimen was infected out 480 razor clams examined, suggesting its role as an occasional host because the siphons of this clam species remain buried, not being exposed to the sunlight [42].
Protozoans
Ciliophora
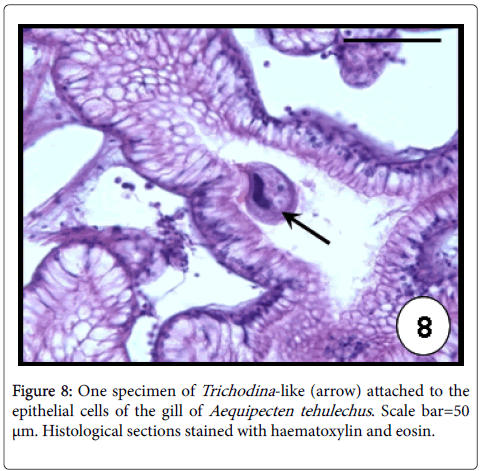
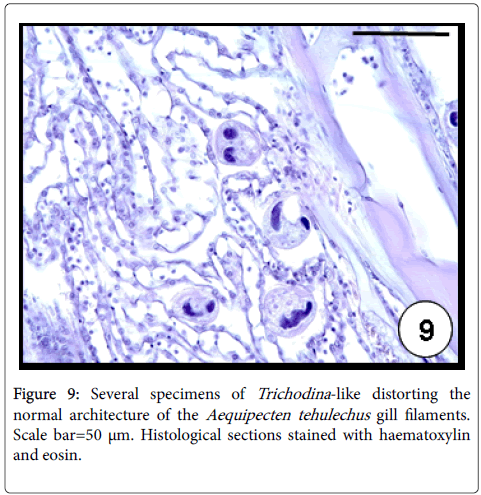
Trichodina -like: Ciliates of the genus Trichodina are common inhabitants of the bivalve surface gills and infrequently of the labial palps [43]. They are usually considered harmless commensals when occurred in low intensities, although mass mortalities were associated to severe infections as the case of cockles and oysters [43,44]. In Argentina, Trichodina -like ciliates were found infecting A. mactroides and A. tehuelchus [33]. These protozoans occurred as disc-shaped characterized by a circlet of eosinophilic denticles, ciliary fringes and a horse-shoe shaped nucleus (Figure 8). High values of prevalence (98%) and intensity of infection (564 ciliates per histological section) were recorded in A. tehuelchus causing a distortion of the natural architecture of the gill filaments (Figure 9) [23].
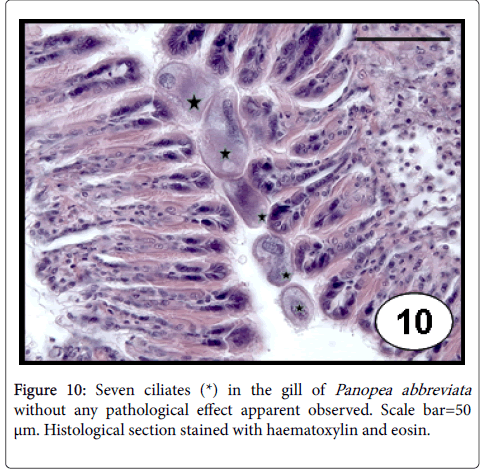
Other ciliates: Unidentified ciliate species were recorded on the surface of the gill filaments of scallops, mussels and ribbed mussels. These protozoans are easily identified in histological sections by having one macronucleus and several micronucleus [33,45]. Ciliates with ovalshaped, one basophilic nucleus macronucleus, several vacuoles, a cytostome and dense ciliature were recorded in gills and often in labial palps of E. macha , without cause any specific host response [34]. Ciliates of oval or rounded shape, with surface densely ciliated and a large polymorphic macronucleus were reported in gills of P. abbreviata . The relationship to the gill epithelium seemed to be superficial and no particular host response was apparent (Figure 10). The highest values of prevalence (79%) and intensities of infection (147 ciliates per histological section) were recorded in the austral spring season showing a negative relationship with the shell size [46].
Apicomplexa
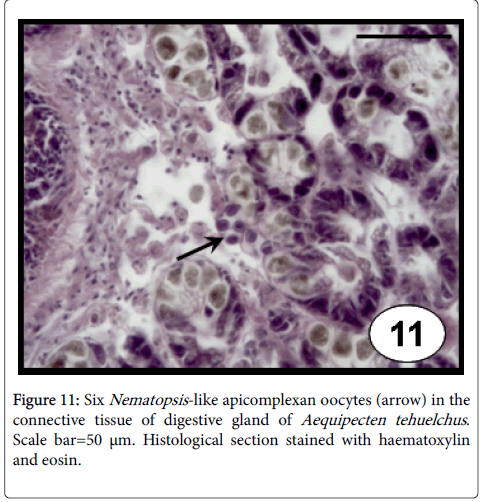
Gregarines: Nematopsis -like gregarines include oocysts bounded by a single thickened membrane with a basophilic vermiform sporozoite inside (Figure 11), were recorded in the connective tissue of almost all the organs of A. tehuelchus and P. rudis , associated usually with a focal, haemocytic infiltration, without significant health effects [33] as well as in the epithelial cells of the intestine of P. rostratus [6]. The prevalence was associated to the shell length of A. tehuelchus , indicating that bigger scallops were more frequently infected by this gregarine species [23]. These protozoans were reported infecting several bivalve species worldwide, without apparent damage to the hosts [43].
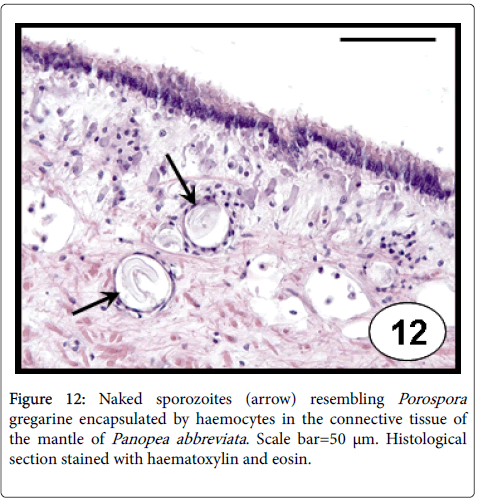
Porospora -like gregarines occurred as naked sporozoites (Figure 12), mainly in the connective tissue of mantle of P. abbreviata [46]. The presence of this protozoan evoked an inflammatory host response as haemocytic infiltration and encapsulation. The studies reporting the pathogenicity of Porospora spp. in bivalves are scarce; just their presence in natural populations of the venerid clam Callista chione was reported [47].
Coccideans
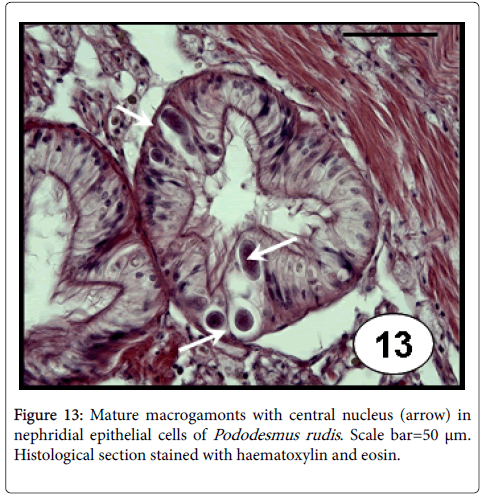
Meronts and macrogamonts stages of unidentified coccidians were reported leading to hypertrophy of the epithelial cells of the nephridal tubules causing the lysis of them and the release of the parasites into the lumen of the nephridal tubule of A. mactroides . This apicomplexan protozoan showed a high prevalence (64%) although low mean intensity of infection (1.64) [15,16]. Different stages of a coccidian aplicomplexan (trophozoites, large and crescent-shaped macrogamonts, and both mature and immature meronts containing merozoites) were also observed in the lumen of nephridia of E. macha [34]. Coccideans were associated with the presence of ‘‘brown cells’’ in the tissues of infected clams, although no haemocyte response elicited by the host was reported. Merogonic and gamontogonic stages including immature meronts, merozoites, trophozoites, microgamonts and macrogamonts (Figure 13) were identified infecting the nephridia epithelium of P. rudis , although less frequently in epithelial cells of both digestive tubule and intestine [23]. These infections were reported to induce light to moderate lesions, evidencing a hypertrophy of the infected nephridial cells, causing the lysis of the host cells and the release of the parasites, occupying the space of the lumen of nephridia. Although the prevalence recorded in this bivalve species was low (4%), the mean intensity reached high value (37 per histological section). Nephridia of P. rostratus were found obstructed by different stages of an unidentified coccidian and, in heavy infections a few macrogamonts were also seen in the connective tissue that surrounds the intestine [6]. These heavy infections are considered to cause severe renal dysfunction [48]. Furthermore, the coccidian parasite of the genus Aggregata is known for play a major role in the collapse of the Iceland scallop, Chlamys islandica [49]. In addition to causing mortality, the authors pointed out that the infections significantly impacted the gonadal development, which contributes further to the collapse of the stock in the form of lower larval recruitment.
Metazoans
Turbelleria: “Turbellaria” of the genus Paravortex parasitize mainly marine bivalves, but some are associated with fish [50]. In the Northern Hemisphere (i.e., Europe and USA) five species were described [51], while in South America three species from three distinct host species were described: P. nicolli from M. platensis from Buenos Aires province, Argentina [52], P. mesodesma from A. mactroides in northern Uruguay and in Buenos Aires province, Argentina [19,51], and P. panopea from P. abbreviata and E. macha [53]. Since the graffilids lack endogenous digestive enzymes, they use their host enzymes to feed on partially digested host’s food, and their food reserves consist mainly on glycogen; these facts represent physiological adaptations to a parasite mode of life. This high degree of metabolic dependence may be responsible for the high specificity observed in the species of Paravortex [53]. Unidentified Paravortex species were also reported occupying the intestine lumen of P. rudis , without evidence of direct physical damage [33].
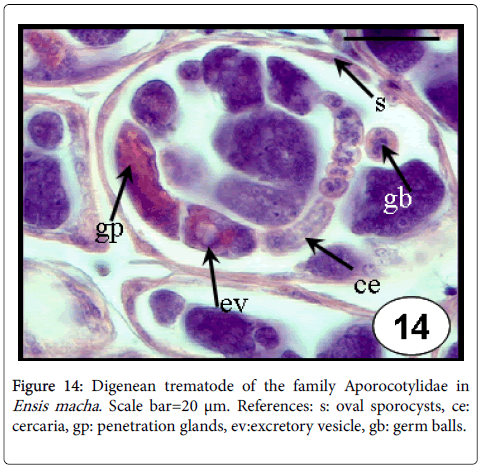
Digenea: Digeneans typically have a dramatic effect on the health of their molluscan first intermediate host. Intramolluscan stages live within the haemocoel and occupy the gonadal space causing castration [50]. Their larvae have been reported in almost all bivalves [50]. Three different sporocysts with developing cercariae, two belonging to the family Monorchiidae and the other to the Aporocotylidae, were found in the purple clam, A. purpurata [54,55]. In one case, of 21 infected clams, 17 individuals had no gametes in the gonads, and the remaining 4 individuals that did produce gametes harboured just a few sporocysts with poorly developed cercariae [54]. In the same way, sporocysts of the family Aporocotylidae were reported to cause complete castration in one specimen of the razor clam E. macha (Figure 14) [34].
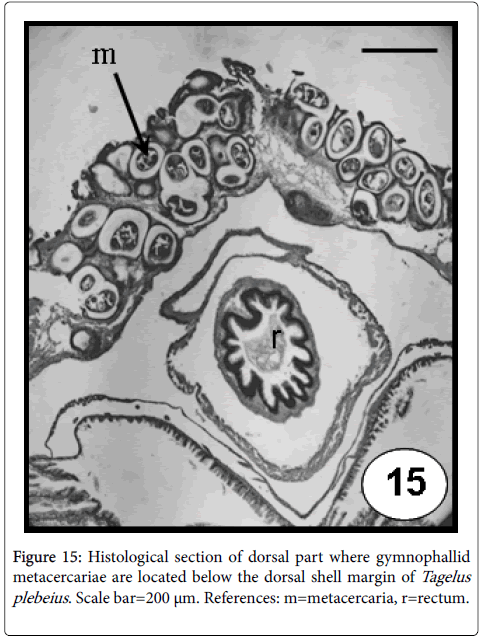
The family Gymnophallidae Odhner, 1905 is a small and homogeneous entity of marine digeneans [56,57]. Most members use molluscs as first intermediate hosts and with rare exceptions, charadriiform and anseriform birds as definitive hosts [56,57]. Gymnophallids use bivalves as first host causing castration and mainly the same or other bivalve species as second intermediate host, by hosting the metacercarial stage; eliciting diverse reactions in the mantle and valve [50]. When the bivalve is infected by the metacercarial stage and is eating raw, the may cause a zoonotic disease. For example, Gymnophalloides seoi was described from a human case with pancreatitis; the patient got infected by eating raw Japanese oyster [58]. In Argentina, the mytilids, M. platensis and A. atra from Buenos Aires and Chubut provinces (Argentina) were found infected by Gymnophallus australis [59,60]. Likewise, the stout razor clam T. plebeius was found acting as first intermediate host for two digenetic trematode species of the families Gymnophallidae and Fellodistomidae, by hosting sporocysts (mainly in gonad and digestive gland that results in the replacement of host tissues) and as second intermediate host by housing a gymnophallid metacercaria (Figure 15). The valves of the infected stout razor clams showed abnormal calcifications and strong orange colorations [61-63]. Specimens of the L. antiqua living on the intertidal beds on the Patagonian coast were found infected with an unidentified gymnophallid metacercaria, eliciting pits in the inner valve surface [33].
Cestoda: Larval cestodes have been reported from a great variety of marine invertebrates, including molluscs; their adults are parasites of elasmobranches [64]. Plerocercoids larval were found in both A. tehuelchus and L. antiqua [33]; however due to the absence of taxonomically important characteristics, their identification beyond the order level was not possible [30]. In A. tehuelchus , the larvae were found in the intestine lumen without apparent reaction, while in L. antiqua , a thick capsule formed by an inner wall composed of aggregated haemocytes and an outer thicker and formed by fibroblastlike cells concentrically ringed around the parasite [33].
Nemertea: The family Malacobdellidae comprises a single genus, Malacobdella , with 6 species inhabiting the mantle cavity of bivalves [65,66]. They are usually considered as entocommensals, although other studies supported their parasitic life style [67]. A histopathological study of mantle tissues on the commercial geoduck, P. abbreviata hosting the nemertean, Malacobdella arrokeana , was carried out to evaluate the possible damage of host mantle tissues elicited by the nemertean attachment structure [68]. This nemertean was found in high prevalence, almost in the total of the geoducks studied (P=99.4%, n=657), varying between 0 and 191 individuals (adult and young nemerteans) per clam. This study revealed that the vacuum force generated by the nemertean sucker elicited a moderate deformation of epithelial cells and an inflammatory response affecting the connective tissue between inner and outer mantle epithelium, just below the point of attachment [68].
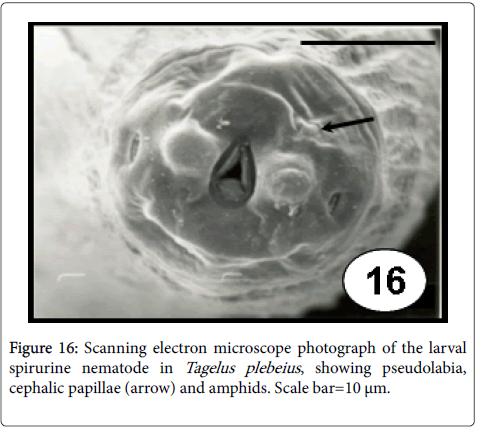
Nematoda: Although nematodes are uncommon as bivalve parasites, few species of the orders Ascaridoidea and Spirurida could be found as larval stages without specificity for their site infection. Indeed, they frequently appear in low prevalence and intensities of infection, eliciting different kind of capsules [50]. In T. plebeius , spirurina larval nematodes (Figure 16) were found free or individually surrounded by a capsule, mainly in the muscular wall of visceral mass (48%), but also in labial palps (28%), siphon retractor muscles (8%), adductor muscles (8%), radial muscles of the mantle border (4%), mantle (4%) [61]. These histopathology results revealed that the degree of tissue reaction elicited by the presence of this parasite varied from no reaction to the formation of a thick capsule.
Disseminated neoplasia
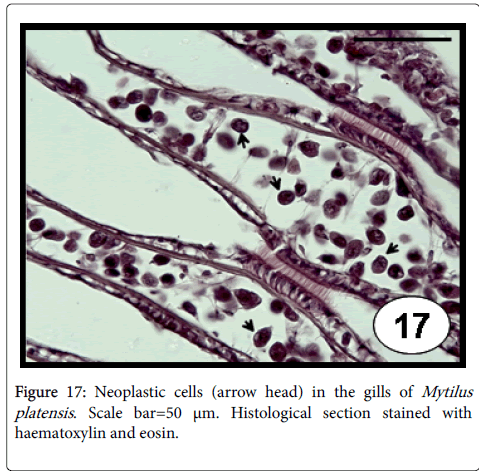
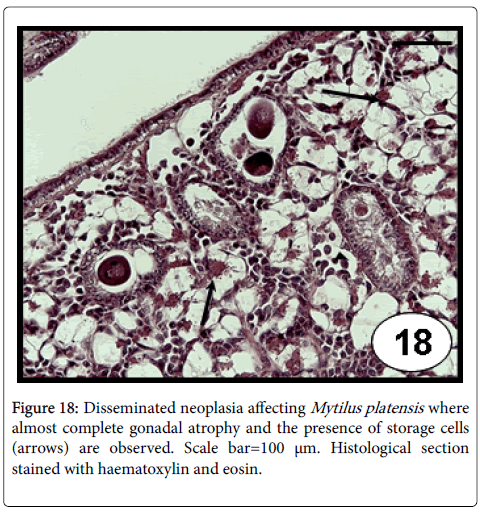
This disease consists of a proliferation of abnormal circulating cells of unknown origin (likely haemocytes), with a progressive and replaced of normal cells by the neoplastic cells (involving loss of the normal architecture of tissues and organs) that often causes the death [69]. Disseminated neoplasia in bivalve mollusc species is characterized by the presence of large (2-4 times the diameter of normal haemocytes), neoplastic circulating cells that have a hyperchromatic and often pleomorphic nucleus containing 1 or more prominent nucleoli [70], and a high nuclear to cytoplasmic volume ratio in neoplastic cells [71]. The disease can be transmitted to naive animals by experimental transplantation of haemocytes [72,73]. A viral cause has been suspected, but no infectious agent has been confirmed [72,73]. The detection of reverse transcriptase activity in neoplastic cells [74] suggested the possibility of retroelement involvement, called73 Steamer , which its DNA copy number per genome was found at enormously high levels in neoplastic cells of the softshell clam Mya arenaria [75]. Recently, using three types of DNA markers (sequences of Steamer integration sites, mitochondrial DNA single-nucleotide polymorphisms (SNPs) and polymorphic microsatellite alleles), the genotype of neoplastic cells was found to be different from that of normal cells of the host, whereas the neoplastic cells of clams from dispersed locations in Canada and North eastern USA all had nearly identical genotype, suggesting that disseminated neoplasia is spreading among animals in the marine environment as a clonal transmissible cell derived from a single original clam [76]. Disseminated neoplasia in cultured M. platensis (Figure 17) from the Beagle Channel (Tierra del Fuego province) in Southern Argentina is the only record of this disease in marine bivalve species from Argentina and Uruguay [45]. All diseased individuals were female and in advanced stages of the disease, the gonad was represented only by a few atrophied follicles invaded by neoplastic cells (Figure 18). Moreover, atrophied digestive tubules were observed; the high prevalence reported indicates an epizootic level.
Conclusions and Future Directions
This review has covered all the parasites and pathologies of the main commercially bivalve species from Argentina and Uruguay, considering the effect of these on both individual and population health. Based on reported morphological alterations, bonamiosis, perkinsosis and disseminated neoplasia, were the most severe diseases. Nevertheless, the rest of the parasites are not considered as severe pathogens, since none caused significant damage to their hosts and none appears to be a problem to the fishery as well as to future farming, based on either low infestation levels or low pathological effects. Paid attention should be addressed to the venerid clams and mussel’s consumption, since its infection by the gymnophallid digenean parasite is considered of zoonotic importance.
The research effort of the countries of Latin America and the Caribbean focuses on detecting notifiable diseases according to the OIE [77]. This fact is partly due to attempts to consolidate with international market rules for fresh exported products and that the most scientific knowledge, as well as the diagnostic methods has been developed for the diseases that have traditionally affected countries with historical shellfish culture development, mainly Spain, France and the United States. A few cases reporting the viral pathogens associated to mass mortalities and bivalve diseases would be due to lack of tools for its study, primarily, the absence of cell lines and the expensive manipulation of transmission electron microscopy [78]. Causes and mechanisms of mass mortalities events in natural populations are very difficult to ascertain, and they are probably due to a combination of factors [79]; the causes of the mass mortalities episodes affecting the yellow clam Amarilladesma mactroides, throughout its geographic range distribution remain unknown. The future studies would be addressed to investigate the co-infection of virus (OsHV-1) and different Vibrio species, since protozoans as causative agents of the mortalities have not been detected.
Furthermore, we consider that each country should be focused on investigating the parasites and diseases that are proper or affect to their bivalve species along a regional scale by developing and using specific diagnostic methods for these diseases, instead of focusing exclusively to detect diseases that are notifiable by the developed countries.
Acknowledgements
Authors are member of CONICET. Financial support was provided by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT) (Préstamo BID PICT 2013- 1702, 2582) and Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) (PIP 0630/14).
References
- Ciocco NF, ML Lasta, Bremec CS (1998) PesquerÃas de bivalvos: Mejillón, vieiras (tehuelche y patagónica) y otras especies. Los moluscos de interés pesquero. Cultivos y estrategias reproductivas de bivalvos y equinoideos. In: El Mar Argentino y sus recursos pesqueros, Boschi E (Ed). Instituto Nacional de Investigación y Desarrollo Pesquero (INIDEP): Mar del Plata, Argentina pp: 143-166.
- Coscarón S (1959) La ‘‘almeja amarilla’’ (Mesodesma (T.) mactroides Deshayes) de la costa de la provincia de Buenos Aires. Agro 1:1-66.
- Olivier SR, Penchaszadeh PE (1968) Evaluación de los efectivos de almeja amarilla (Mesodesma mactroides Desh. 1854) en las costas de la provincia de Buenos Aires. Proyecto de Desarrollo Pesquero-FAO. Serie Informes Técnicos 8: 1-10.
- ElÃas I, Carozza C, Di Giácomo EE, Isla MS, (Lobo) Orensanz JM, et al. (2009) Coastal Fisheries of Argentina. In: Coastal Fisheries in Latin America and the Caribbean, Salas S, R Chuenpagdee, A Charles, JC Seijo Ed. FAO. Publication, Rome pp: 13-47.
- Ciocco N (2000) Almeja Panopea, un nuevo recurso pesquero para el Mar Argentino. Infopesca Internacional 6: 36-39.
- Cremonte F, Balseiro P, Figueras A (2005) Occurrence of Perkinsus olseni (Protozoa: Apicomplexa) and other parasites in the venerid commercial clam Pitar rostrata from Uruguay (Southwest Atlantic coast). Dis Aquat Organ 64: 85-90.
- Food and Agriculture Organization of United Nations (2014) National Aquaculture Sector Overview. Argentina. National Aquaculture Sector Overview Fact Sheets. Text by Wicki GA. In: FAO Fisheries and Aquaculture Department, Rome.
- Lasta ML, Ciocco NF, Bremec CS, Roux AM (1998) Moluscos bivalvos y gasterópodos. En: El Mar Argentino y sus recursos pesqueros. Tomo 2. Los moluscos de interés pesquero. Cultivos y estrategias reproductivas de bivalvos y equinoideos, Boschi EE (Ed). Instituto Nacional de Investigación y Desarrollo Pesquero pp: 115- 142.
- Kroeck MA, Montes J (2005) Occurrence of the haemocyte parasite Bonamia sp. in flat oysters Ostrea puelchana d´Orbigny farmed in San Antonio Bay (Argentina). Dis Aquat Organ 63: 231-235.
- Hill KM, Stokes NA, Webb SC, Hine PM, Kroeck MA, et al. (2014) Phylogenetics of Bonamia parasites based on small subunit and internal transcribed spacer region ribosomal DNA sequence data. Dis Aquat Organ 110: 33-54.
- Fisher WS (1988) Disease processes in marine bivalve molluscs. American Fisheries Society, Special Publication 18, Bethesda, Maryland.
- Villalba A, D Iglesias, A Ramilo, S Darriba, JM Parada, et al. (2014) Cockle Cerastoderma edule fishery collapse in the RÃa de Arousa (Galicia, NW Spain) associated with the protistan parasite Marteilia cochillia. Dis Aquat Organ 109:55-80.
- Figueras A, Novoa B (2004) What has been going on in Europe in bivalve pathology? Bull Eur Ass Fish Pathol 24:16.
- World Organisation for Animal Health (2014) Old classification of diseases notifiable to the OIE, list B. OIE, Paris.
- Cremonte F, Figueras A (2004) Parasites as possible cause of mass mortalities of the presently critically endangered clam Mesodesma mactroides on the Southwest Atlantic coast. Bull Eur Ass Fish Pathol 24: 166-171.
- Fiori SM, Cazzaniga NJ (1999) Mass mortality of the yellow clam, Mesodesma mactroides (Bivalvia: Mactracea) in Monte Hermoso beach, Argentina. Biol Conserv 89: 305-309.
- Carvalho YBM, da Silva JJS, Raibenberg FC, Poersch LH, Romano LA (2016) Use of polymerase chain reaction for bivalve pathogen surveillance in the yellow clam Mesodesma mactroides. J Aquat Animal Health 28: 114-117.
- Carvalho YBM, Poersch LH, Romano LA (2013) Rickettsia associated mortality of the yellow clam Mesodesma mactroides (Bivalvia: Mesodesmatidae) in southern Brazil. Malacologia 56: 301-307.
- Vázquez N, Fiori S, Arzul I, Carcedo C, Cremonte F (2016) Mass mortalities affecting populations of the yellow clam Amarilladesma mactroides along its geographic range. J Shellfish Res 35: 739-745.
- Levine ND (1978) Perkinsus gen. n. and other new taxa in the protozoan phylum Apicomplexa. J Parasitol 64: 549.
- Zhang H, DA Campbell, NR Sturm, CF Dungan, Lin S (2011) Spliced leader RNAs, mitochondrial gene frameshifts and multi-protein phylogeny expand support for the genus Perkinsus as a unique group of Alveolates. PLoS ONE 6 e19933.
- Villalba A, Gestal C, Casas SM, Figueras A (2011) Perkinsosis en Moluscos. In: Enfermedades de moluscos bivalvos de interés en acuicultura, Figueras A, Novoa B Ed. Publicaciones CientÃficas y Tecnológicas de la Fundación Observatorio Español de Acuicultura, Madrid pp: 181-205.
- Vázquez N (2012) PatologÃas que afectan a poblaciones comercialmente explotadas de moluscos bivalvos del litoral norpatagónico y la vinculación con sus historias de vida. PhD thesis, pp: 243, Universidad Nacional del Comahue, Argentina.
- de Montaudouin X, Paul-Pont I, Lambert C, Gonzalez P, Raymond N, et al. (2010) Bivalve population health: multistress to identify hot spots. Marine Pollut Bull 60: 1307-1318.
- Carnegie RB, Cochennec-Laureau N (2004) Microcell parasites of oysters: Recent insights and future trends. Aquat Living Resour 17: 519-528.
- Dinamani P, Hine PM, Jones JB (1987) Occurrence and characteristics of the haemocyte parasite Bonamia sp. in the New Zealand dredge oyster Tiostrea lutaria. Dis Aquat Organ 3:37-44.
- Cranfield HJ, Dunn A, Doonan LJ, Michael KP (2005) Bonamia exitiosa epizootic in Ostrea chilensis from Foveaux Strait, southern New Zealand between 1986 and 1992. ICES J of Marine Sci 62: 3-13.
- Kroeck M, Semenas L, Morsan EM (2008) Epidemiological study of Bonamia sp. in the native flat oyster, Ostrea puelchana from San MatÃas Gulf (NW Patagonia, Argentina). Aquacult 276: 5-13.
- Abollo E, Villalba A (2011) Enfermedades causadas por parásitos del grupo Haplosporidia. In: Enfermedades de moluscos bivalvos de interés en acuicultura, Figueras A, Novoa B Ed. Publicaciones CientÃficas y Tecnológicas de la Fundación Observatorio Español de Acuicultura, Madrid pp: 285-328.
- Lauckner G (1983) Introduction: Bivalvia to Scaphopoda. In: of Marine Animals. Vol 2. Introduction Bivalvia to Scaphopoda Diseases, Kinne O Ed. Hamburg pp: 477-977.
- Fryer JL, Lannan CN (1994) Rickettsial and chlamydial infections of fresh water and marine fishes, bivalves, and crustaceans. Zoological Studies 33: 95-107.
- Darriba S, M Ruiz, López C (2012) Phage particles infecting branchial Rickettsiales-like organisms in banded carpet shell Polititapes virgineus (Bivalvia) from Galicia (NW Spain). Dis Aquat Organ 100: 269-272.
- Cremonte F, Figueras A, Burreson EM (2005b) A histopathological survey of some commercially exploited bivalve molluscs in northern Patagonia, Argentina. Aquaculture 249: 23-33.
- Vázquez N, Pérez Bruno E, Márquez F, Van der Molen S, Gilardoni C, et al. (2013) A histopathological survey of the razor clam Ensis macha (Pharidae) along the Patagonian Argentina coast. J Invertebr Pathol 12: 253-259.
- Gulka G, Wen Chang P (1985) Pathogenicity and infectivity of a rickettsia-like organism in the sea scallop Placopecten magellanicus. J Fishes Dis 8: 309-318.
- Goggin CL, Lester RJG (1990) Rickettsiales-like infection in the gills of Tridacna crocea from the Great Barrier Reef. J Invertebr Pathol 56: 135-138.
- Romalde JL, Prado S (2011) Enfermedades bacterianas en moluscos bivalvos. In: Enfermedades de moluscos bivalvos de interés en Acuicultura, Figueras A, Novoa B (Eds). Publicaciones CientÃficas y Tecnológicas de la Fundación Observatorio Español de Acuicultura, Madrid pp: 93-134.
- Stevenson RN, South GR (2007) Coccomyxa parasitica sp. nov. (Coccomyxaceae, Chlorococcales), a parasite of giant scallops in Newfoundland. British Phycol J 9: 319-329.
- RodrÃguez F, Feist SW, Guillou L, Harkestad LS, Bateman K, et al. (2008) Phylogenetic and morphological characterisation of the green algae infesting blue mussel Mytilus edulis in the North and South Atlantic oceans. DisAquat Organ 81: 231-240.
- Boraso de Zaixso A, Zaixso H (1979) Coccomyxa parasitica Stevenson and South endozoica en Mytilus edulis. Physis 38: 131-136.
- Bala LO (1995) Especificidad y prevalencia de la endobiosis de Cocccomyxa parasitica (Chlorophyta: Chlorococcales) en Mytilus edulis platensis (Mollusca: Bivalvia). Naturalia Patagónica 3: 1-9.
- Vázquez N, Rodriguez F, Ituarte C, Klaich J, Cremonte F (2010) Host–parasite relationship of the geoduck Panopea abbreviata and the green alga Coccomyxa parasitica in the Argentinean Patagonian coast. J Invertebr Pathol 105: 254-260.
- Bower SM, McGladdery SE, Price IM (1994) Synopsis of infections diseases and parasites of commercially exploited shellfish. Annual Review of Fish Diseases 4: 1-199.
- Boussaïd B, Grippari JL, Renault T, Tige G, Dorange G (1999) Trichodina sp. infestation of Crassotrea gigas oyster gills in Brittany, France. JInvertebr Pathol 73: 339-342.
- Cremonte F, Vázquez N, Silva MR (2011) Gonadal atrophia caused by disseminated neoplasia in Mytilus chilensis cultured in the Beagle Channel, Argentina. J Shellfish Res 30: 1-5.
- Vázquez N, Ituarte C, Cremonte F (2015) A histopathological study of the geoduck clam Panopea abbreviata from San José Gulf, North Patagonia, Argentina. J Marine Biologi Ass United Kingdom 95:1173-1181.
- Canestri-Trotti G, Baccarani EM, Paesant F, Rurolla E (2000) Monitoring of infections by Protozoa of the genera Nematopsis, Perkinsus and Porospora in the smooth venus clam Callista chione from he North-Western Adriatic Sea (Italy). Diseases of Aquatic Organisms 42: 157-161.
- Carballal M, Iglesias D, Santamarina J, Ferro-Soto B, Villalba A (2001) Parasites and pathologic conditions of the cockle Cerastoderma edule populations of the coast of Galicia (NW Spain). J Invertebr Pathol 78: 87-97.
- Kristmundsson Ã, Erlingsdóttir Ã, Freeman MA (2015) Is an Apicomplexan Parasite Responsible for the Collapse of the Iceland Scallop (Chlamys islandica) Stock? PLoS ONE 10: e0144685.
- Cremonte F (2011) Enfermedades de moluscos bivalvos de interés comercial causadas por metazoos In: Enfermedades de moluscos bivalvos de interés en acuicultura, Figueras A, Novoa B Ed. Publicaciones CientÃficas y Tecnológicas de la Fundación Observatorio Español de Acuicultura, Madrid pp: 331-396.
- Brusa F, Ponce de León R, Damborenea C (2006) A new Paravortex (Platyhelminthes, Dalyellioida) endoparasite of Mesodesma mactroides (Bivalvia, Mesodesmatidae) from Uruguay. Parasitology Research 95: 566-571.
- Szidat L (1965) Los parásitos de los mitÃlidos y los daños por ellos causados. II. Los parásitos de Mytilus edulis platensis (Orb.) (mejillón del Plata). Com. Mus. Arg. Cien. Nat. Bernardino Rivadavia, Parasitology 1: 1-16.
- Brusa F, Vázquez N, Cremonte F (2011) Paravortex panopea n. sp. (Platyhelminthes: Rhabdocoela) on clams from the northern Patagonian coast, Argentina: pathogeny and specificity. Helminthologia 48: 94-100.
- Cremonte F, Vázquez N, Silva MR (2011) Gonadal atrophy caused by disseminated neoplasia in Mytilus chilensis cultured in the Beagle Channel, Argentina. J Shellfish Res 30: 845-849.
- Gilardoni C, Etchegoin J, Diaz JI, Ituarte C, Cremonte F (2011) A survey of larval digeneans in the commonest intertidal snails from Northern Patagonian coast, Argentina. Acta Parasitologica 56: 163-179.
- Bartoli P (1974) Recherches sur les Gymnophallidae F.N. Morozov, 1955 (Digenea) parasites d´oiseaux des côtes de Camargue: systématique, biologie et ecologie, Université des Sciences Dáix-Marseille, Marseille, pp. 338.
- Cremonte F, Gilardoni C, Pina S, Rodrigues P, Ituarte C (2015) Revision of the family Gymnophallidae Odhner, 1905 (Digenea) based on morphological and molecular data. Parasitology International 64: 202-210.
- Lee SH, Chai JY, Hong ST (1993) Gymnophalloides seoi n. sp. (Digenea: Gymnophallidae), the first report of human infection by a gymnophallid. J Parasitol 79: 677-680.
- Cremonte F (1999) Estudio parasitológico de bivalvos que habitan ambientes marinos y mixohalinos en Argentina, Universidad Nacional de La Plata, Argentina, pp: 196.
- Cremonte F, Vázquez N, Ituarte C (2008) The development from metacercaria to adult of Gymnophallus australis Szidat, 1962 (Digenea: Gymnophallidae) from Patagonian coast (Argentina), with an emended diagnosis of the genus Gymnophallus Odhner, 1905. SystParasitol 69: 23-31.
- Vázquez N, Ituarte C, Navone G, Cremonte F (2006) Parasites of the stout razor clam Tagelus plebeius (Psammobiidae) from the Southwestern Atlantic Ocean. J Shellfish Res 25: 877-886.
- Ituarte C, Cremonte F, Scarano A (2009) Tissue reaction of Tagelus plebeius Lightfoot (Bivalvia, Psammobiidae) against larval digeneans in mixohaline habitats connected to the Southwestern Atlantic Ocean. J Marine Biol Ass United Kingdom 89: 569-577.
- Lomovasky B, Gutiérrez J, Iribarne O (2005) Identifying repaired shell damage and abnormal calcification in the stout razor clam Tagelus plebeius as a tool to investigate its ecological interactions. Journal of Sea Research 54: 163-175.
- Cheng TC (1967) Marine Molluscs as Hosts for Symbiosis with a Review of Known Parasites of Commercially Important Species, Academic Press Inc., London, pp:424.
- Ivanov V, Bigatti G, Penchaszadeh PE, Norenburg JL (2002) Malacobdella arrokeana (Nemertea; Bdellonemertea), a new species of nemertean from Southwest Atlantic Ocean entocommensal in Panopea abbreviata (Bivalvia; Heterodonta; Hiatellidae) in Argentina. Proceedings of the Biological Society of Washington 115: 359-367.
- Jensen K, Sadeghian PS (2005) Nemertea. In: Marine Parasitology, Rohde K (Ed). Australia pp: 205-210.
- Sundet JH, Jobling M (1985) An investigation of the interactions between the nemertine Malacobdella grossa, and its bivalve host, Arctica islandica. In: Institute of Fisheries, Norway, Gray JS, Christiansen ME (Eds). Marine Biology of Polar Regions and Effects of Stress on Marine Organisms, pp: 185-197.
- Vázquez N, Bigatti G, Ituarte C, Cremonte F (2009) Attachment of the nemertean Malacobdella arrokeana to the mantle of the geoduck Panopea abbreviata and survival outside the host. J Shellfish Res 28: 759-761.
- Carballal MJ, Bruse JB, Iglesias D, Villalba A (2015). Neoplastic diseases of marine bivalves. J Invertebr Pathol 131: 83-106.
- Barber BJ (2004) Neoplastic diseases of commercially important marine bivalves. Aquat Living Res 17: 449-466.
- Mix MC (1975) Proliferative characteristics of atypical cells in native oysters (Ostrea lurida) from Yaquina Bay, Oregon. J Invertebr Pathol 26: 289-298.
- McLaughlin SM (1991) Transmission studies of sarcoma in the softshell, Mya arenaria. In: Invertebrate Neoplasia: Initiation and Promotion Mechanisms, Rosenfield A, Kern FG, Keller BJ Ed. U.S. Department of Commerce, Woods Hole, MA, pp: 21-22.
- Weinberg JR, Leavitt DF, Lancaster BA, McDowell Capuzzo J (1997) Experimental field studies with Mya arenaria (Bivalvia) on the induction and effect of hematopoietic neoplasia. J Invertebr Pathol 69: 183-194.
- House ML, Kim CH, Reno PW (1998) Soft shell clams Mya arenaria with disseminated neoplasia demonstrate reverse transcriptase activity. Diseases of Aquatic Organisms 34: 187-192.
- Arriagada G, Metzger MJ, Muttray AF, Sherry J, Reinisch C, et al. (2014) Activation of transcription and retrotransposition of a novel retroelement, Steamer, in neoplastic haemocytes of the mollusk Mya arenaria. Proceedings of the National Proc Natl Acad Sci U S A 111: 14175-14180.
- Metzger MJ, Reinisch C, Sherry J, Goff SP (2015) Horizontal transmission of clonal cancer cells causes leukemia in soft-shell clams. Cell 161: 255-263.
- Cáceres MartÃnez J, Vásquez-Yeomans (2008) La patologÃa en moluscos bivalvos: principales problemas y desafÃos para la producción de bivalvos en América Latina. In: Estado actual del cultivo y manejo de moluscos bivalvos y su proyección futura: factores que afectan su sustentabilidad en América Latina, A. Lovatelli, A FarÃas e I Uriarte (Eds). Taller Técnico Regional de la FAO, Puerto Montt, Chile. FAO Actas de Pesca y Acuicultura. No. 12. Roma, FAO. pp: 327-337.
- Figueras A, Novoa B (2011) Enfermedades de moluscos bivalvos de interés en acuicultura. Madrid: Publicaciones CientÃficas y Tecnológicas de la Fundación Observatorio Español de Acuicultura, pp: 543.
- Montagna PA, Stockwell DA, Kalke RD (1993) Dwarf surf clam Mulinia lateralis (Say, 1822) populations and feeding during the Texas brown tide event. J Shellfish Res 12: 433-442.
Citation: Vázquez N, Cremonte F (2017) Review of Parasites and Pathologies of the Main Bivalve Species of Commercial Interest of Argentina and Uruguay, Southwestern Atlantic Coast. Arch Parasitol 1:112.
Copyright: © 2017 Vázquez N, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 15850
- [From(publication date): 0-2017 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 14750
- PDF downloads: 1100