Role of Neck Dissection in Locoregionally Advanced Head and Neck Cancer Treated with Primary Chemoradiotherapy
Received: 04-Dec-2015 / Accepted Date: 18-Jan-2016 / Published Date: 17-Feb-2016 DOI: 10.4172/2161-119X.1000220
Abstract
1.1. Introduction: Planned neck dissection after chemoradiotherapy (CRT) in locoregionally advanced head and neck cancer is controversial. The objective of the present study was to evaluate the influence of neck dissection on the long-term locoregional control and survival of patients with stage III-IV head and neck squamous cell carcinoma (HNSCC) after primary CRT.
1.2. Methods/Patients: We retrospectively analysed locoregional control, locoregional relapse-free survival (LRFS), and overall survival (OS) in 67 patients with locally-advanced HNSCC treated with exclusive CRT at our department between January 1998 and December 2013.
1.3. Results: Complete clinical response was achieved in 36 of 67 patients (53.7%), partial response > 50% in 17 pts (25.4%), stable disease in 3 (4.5%); 9 patients (13.4%) developed disease progression during treatment. At a median follow-up of 35 months, LRFS and OS were 100% in patients with complete response and neck dissection versus 77.9% and 79.8%, respectively, in patients who did not undergo neck dissection (p = ns). The only independent prognostic factor for locoregional control was complete response to CRT.
1.4. Conclusions: Patients who achieve a complete clinical response to CRT have a very low risk of isolated neck recurrence and, therefore, planned neck dissection may not be justified in such cases. Clinical and radiographic identification of patients with residual disease following CRT who could benefit from neck dissection remains challenging.
Keywords: Carcinoma, Squamous cell of head and neck, Chemoradiotherapy, Neck dissection, Neoplasm recurrence local
251300Introduction
Concomitant chemoradiotherapy (CRT) is one of the pillars of organ preservation in the treatment of advanced tumours of the head and neck. However, the need to perform neck dissection (ND) in patients with complete clinical and radiological response remains controversial. While some authors support cervical dissection to improve control in patients with N2 - N3 disease, other authors and centres do not routinely perform ND after CRT in those patients.
The main argument for systematic ND is the premise that this procedure reduces the risk of recurrence in patients with large pretreatment adenopathies and that any recurrence treated with salvage surgery in such patients will have a small likelihood of success. A review of the published literature reveals that the percentage of patients who achieve a complete clinical response in the neck after CRT varies widely, although the mean response rate is approximately 56%. In a phase II study carried out by Homma et al. in 41 patients with N2 disease, neck disease was successfully controlled by CRT in 44% of patients [1]. McHam et al. reviewed 109 patients with N2-N3 disease treated by CRT and found that neck disease was successfully controlled by CRT in 74% of patients [2]. Published reports indicate that patients who present complete clinical or radiographic response have a 20-30% incidence of pathologic disease at the time of dissection. Stenson et al. reported complete pathological response in 75% and 50%, respectively, of patients with N2 and N3 disease who underwent ND within 5-17 weeks after intensive concurrent CRT [3]. Although surgery can benefit some patients, it is worth noting that ND can contribute to morbidity in terms of pain and shoulder disability, with significant complications reported in 26% of patients. In studies that have evaluated the clinical (but not radiological) response of lymph nodes to treatment, the percentage of salvage cervical dissections with pathologic positivity is higher. It is also important to note that pathologic positivity does not necessarily translate into a subsequent neck failure, particularly when ND is performed early (2-6 weeks) after completion of CRT when tumour cell viability in pathologic specimens is uncertain. In 27 patients with complete clinical/radiologic response, Brizel et al. found a 26% pathologic positivity rate but a neck failure rate of only 4% [4]. Argiris et al. reported that 39% of patients with complete response after CRT harboured residual tumours in elective ND specimens, even though only 7% of patients with complete response (CR) who did not undergo ND experienced a neck recurrence; these authors concluded that, in the majority of cases, microscopic residual disease indicates nonviable tumours [5]. In a 2008 study carried out by our group, we reported a 100% control rate in 28 node-positive patients who presented a CR after radical treatment and did not undergo ND [6]. Given the context described above particularly the risk of significant morbidity associated with neck dissection and the fact that only a small percentage of patients with residual disease will go on to develop a recurrence our team has long preferred to take a wait-and-see approach in patients with complete nodal response to CRT, irrespective of the initial extent of nodal disease. However, the optimal treatment approach to patients with stage III-IV head and neck squamous cell carcinoma (HNSCC) after primary CRT remains unclear. For this reason, we carried out the present study to evaluate the influence of ND on long-term locoregional control and survival in this patient population.
Patients and Methods
We retrospectively reviewed the medical records of patients with squamous cell carcinoma of the oropharynx, hypopharynx, larynx, and oral cavity who received definitive radiotherapy at our institution (University Hospital La Princesa in Madrid, Spain) between January 1998 and December 2013. Patients who underwent ND or lymphadenectomy before radiation were excluded. Inclusion criteria were as follows: histologically-proven squamous cell carcinoma, tumour stage III/IV, ECOG 0-2 with normal renal and bone marrow function, absence of distant metastases, and treatment with curative intent. Variables evaluated included locoregional control, locoregional relapse-free survival (LRFS), and overall survival (OS).
Treatment modalities
Most of the patients in this study were treated with threedimensional conformal radiotherapy (3D-CRT), although in recent years intensity-modulated radiotherapy (IMRT) with sliding dynamic multileaf collimators was used. Computed tomography (CT)-based treatment planning was used for all patients. The gross tumour volume (GTV) encompassed the primary tumour and involved lymph nodes, and the clinical target volume (CTV) consisted of the GTV with a standard margin of 1 cm that was adjusted to account for natural barriers to tumour spread and nodal areas at high risk of containing microscopic disease. CTV margins were expanded by 3 mm to form the planning target volumes (PTV). The high-dose PTV received a total dose of 66 Gy in 30 fractions using IMRT or 70 Gy in 35 fractions using 3D-CRT. Platinum based chemotherapy or Cetuximab was administered in various treatment schemes to all patients.
Response assessment and follow up
Staging procedures included clinical examination, endoscopy/ laryngoscopy, contrast-enhanced neck CT or MRI and chest CT or X-ray. Eight weeks after completion of CRT, patients underwent a clinical examination/upper endoscopy and neck CT scan. Complete response was defined as no palpable tumour on physical examination, no evidence of local disease on radiographic examination, and no nodes > 1.5 cm and no nodes with irregular enhancement, round shape or a necrotic centre. In the event that any clinical or radiological abnormalities were found, patients were examined under anaesthesia and biopsies were performed. Our general policy was not to dissect the neck in patients with CR. However, the multidisciplinary team responsible for the patient made the final decision regarding whether to perform ND or not. Subsequently, patients were followed up with physical examination, and flexible endoscopy every 6-8 weeks in the first year after treatment, every 3 months for an additional 2 years, every six months for 5 years, and every year until final discharge at 10 years.
Statistical methods
All time-to-failure end points were calculated from the day of the final radiotherapy treatment. All-cause deaths are included in the overall survival estimates. Differences in locoregional control were tested by the Pearson chi-square test. Actuarial estimates for overall and disease-free survival were calculated by the Kaplan-Meier method and compared with the long-rank test. Cox’s proportional hazard model was used for multivariate analysis to assess the effect of patient characteristics and other prognostic factors of significance on the end points. Statistical significance was considered p<0.05. All statistical tests were performed using SPSS v.15 (Inc., Chicago, IL, USA).
Results
Treatment modalities
The study cohort comprised a total of 67 patients with a median follow up of 35 months. Patient and disease characteristics are detailed in Table 1. In 63 patients, 3D-CRT was administered to the primary tumour and involved nodes at a mean dose of 68 Gy (range 60-74 Gy) with 50 Gy to the elective nodal area. The remaining 4 patients received IMRT with concomitant boost technique in 30 fractions for a total of 66 Gy to the primary tumour and involved nodes and 54 Gy to the elective nodal area. The chemotherapy sequence was neoadjuvant taxol, platin, and 5-fluorouracil (TPF) followed by cetuximab in 1 patient and concurrent CRT in 66. Various chemotherapy regimens were administered, as follows: cisplatin, 75 mg/m2 every 21 days (45 patients); the same cisplatin regimen plus tirapazamine (6 patients); cisplatin with 5-fluorouracil (2 patients); cetuximab 200 mg/m2 weekly (10 patients); cisplatin with panitumumab (2 patients); and intraarterial-cisplatin in 1 patient.
| Characteristics | N (%) |
|---|---|
| Gender | |
| Male | 59 (88,1) |
| Female | 8(11,9) |
| Smoker | |
| yes | 64(95,5) |
| No | 3 (4,5%) |
| ECOG | |
| 0 | 41(61,2%) |
| 1 | 25(37,3) |
| 2 | 1(1,5%) |
| Primarysite | |
| Oropharynx | 41(62,7%) |
| Larynx | 14 (20,9%) |
| Hipopharynx | 11 (16,4%) |
| Oral cavity | 1(1,5%) |
| T | |
| 1 | 5 (7,5%) |
| 2 | 11 (16,4%) |
| 3 | 33 (49,3%) |
| 4 | 18 (27%) |
| N | |
| 1 | 13 (19,4%) |
| 2a | 8(11,9%) |
| 2b | 17 (25,4%) |
| 2c | 20 (29,9%) |
| 3 | 9 (13,4%) |
| Estadio | |
| III | 17 (25,4%) |
| IVa | 41 (61,2%) |
| IVb | 9 (13,4%) |
| Chemotherapy | |
| Cisplatin | 45(67%) |
| Cetuximab | 10(15%) |
| Cisplatin-Tirapazamine | 6(9%) |
| Cisplatin-Panitumumab | 2(3%) |
| Cisplatin-5FU | 2(3%) |
| Cisplatinintra arterial | 1(1,5%) |
| TPF-Cetuximab | 1(1,5%) |
| Radiotherapy | 69,3 Gy (64-74) |
Table 1: Patients and treatment characteristics.
Treatment response
After completion of CRT, patients were evaluated by superior panendoscope and CT after a median of 65 days. Complete response was defined as no palpable tumour on physical examination, no evidence of local disease on radiographic examination, and no nodes >1.5 cm or with irregular enhancement, round shape or a necrotic centre, partial response as tumor reduction >50%, and stable disease as not tumor change. Complete clinical and radiological response was achieved in 36 of 67 patients (53.7%), partial response, in 17 cases (25.4%), and stable disease in 3 (4.5%). Nine patients (13.4%) experienced disease progression during treatment and were excluded from the univariate and multivariate analysis. The decision to perform ND or not was individualized and influenced by comorbidities, treatment toxicity, unresectable/progressive disease or personal patient preferences; nevertheless, radiological response to CRT highly influenced this decision. ND was performed in 5 of the 36 patients with complete response and in 11 of 20 with partial response or stable disease (p= 0.058). Most patients who underwent ND received modified radical treatment. The median time from completion of CRT to neck dissection was 107 days. None of the 5 patients with complete response had evidence of residual disease in the pathological specimens. In contrast, 6 of 11 patients with partial or no response harboured viable tumour cells in the resected specimens.
Nodal control
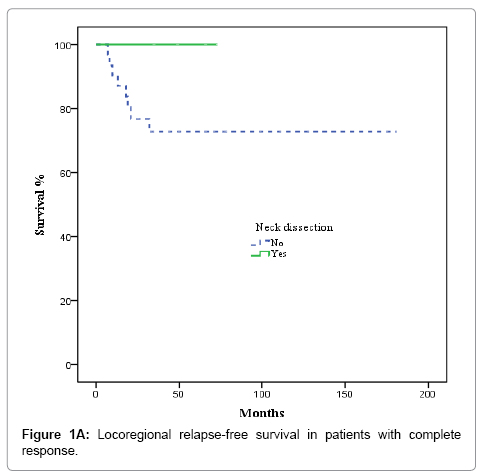
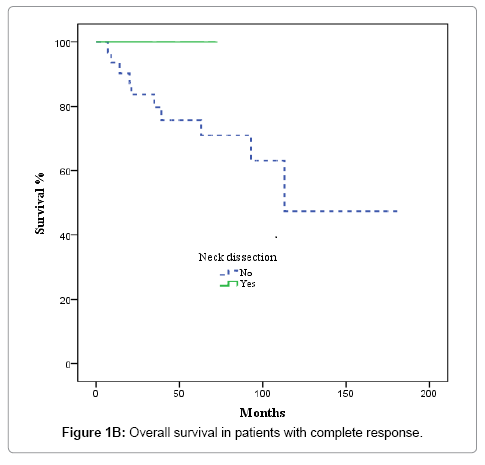
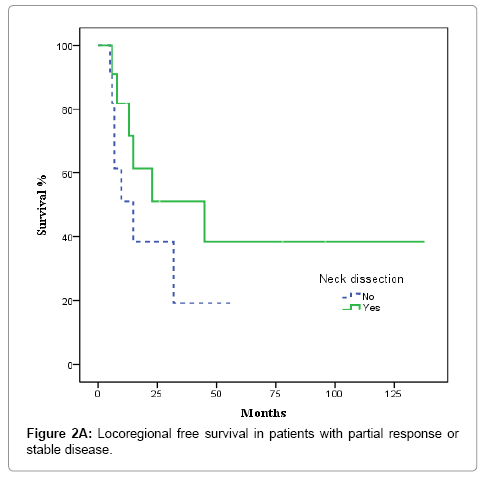
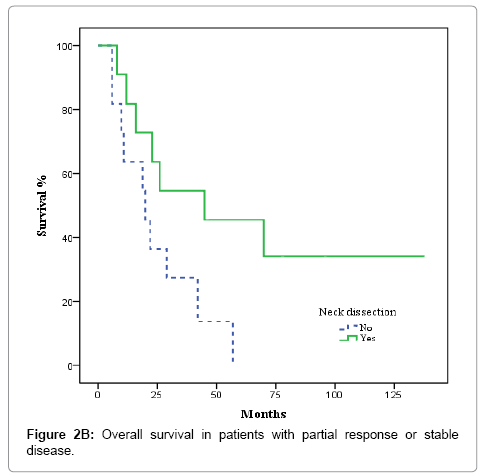
In the 36 patients with complete radiological response after CRT, 8 patients developed a recurrence, as follows: at the primary tumour site and lymph nodes (2 cases); the primary site alone (3 cases); bilateral nodal recurrence (1 case), and distant relapse (2 cases). No isolated homolateral regional recurrences were observed. No relapses were observed in the 5 patients with complete radiological response and ND. The actuarial 3-year LRFS rate was 100% for patients who underwent ND vs. 77.9% without ND (p = 0.22). OS was 100% for patients with ND vs. 79.8% for those who did not undergo ND, although this difference was not significant (p = 0.26) (Figures 1A and 1B). In the 20 patients with partial response or stable disease, 7 recurrences were recorded: 4 at the primary site and regionally; 2 at the primary site alone; and 1 case of distant recurrence. Of those 7 patients, 4 had undergone ND. The 3-year LRFS for patients with and without ND was 68.2% and 19.2%, respectively, while the corresponding OS rate was 54.5% vs 27.3% (p = 0.045) (Figures 2A and 2B). We evaluated the following to identify prognostic factors for locoregional control (Table 2): age, gender, primary tumour site, T/N classification, and CT response. On univariate analysis, only complete radiological response to chemotherapy was associated with better disease control (88.4% vs 55.9% p = 0.001). On multivariate analysis, complete response to chemotherapy was the only independent prognostic factor for locoregional control (Table 3).
Discussion
The present report is an update to our previous paper on the clinical outcomes of patients with node-positive HNSCC treated with definitive CRT [6]. Our present results confirm that high rates of nodal control can be achieved in selected patients (based on response to CRT) without the need to perform neck dissection. In patients with advanced N2-N3 disease, the benefits of elective post-radiotherapy ND regardless of the clinical response is still under debate. Authors who support neck dissection affirm that there are no definite criteria for complete nodal response, and complete radiological response correlates poorly with pathological response. Some studies have defined complete response as “no evidence of disease”. Nevertheless, assessing neck disease after CRT presents many difficulties and, moreover, the z criteria to determine what constitutes residual adenopathy have evolved over time [7-10]. According to the definition of response evaluation criteria in solid tumours (RECIST), nodes that shrink to <10mm on the short axis are considered normal [11]. However, some of the studies that have evaluated neck response after definitive CRT used criteria of <1 cm or ≤ 1.5 cm for maximum diameter without any focal abnormality [8,12]. In our study, we used the same criteria used by the University of Florida Health Science Center [12]. These criteria consider clinical and radiological response to be complete if there is no evidence of clinical disease in the panendoscope and residual visualised nodes in CT are < 1.5 cm in the short axis without irregular enhancement, round shape or necrotic centre.
The other point of controversy is that approximately 25% of patients with complete radiological response do not have complete pathological response [4,9]. According to some reports, the risk for residual tumour in ND specimens after CRT increases with initial cN-stage regardless of the clinical response. For example, in one series of 73 patients reported by Stenson et al. the risk for viable tumour cells in patients who had post-radiotherapy ND increased from 12% for initial N2b to 20% for N2c to 30% for N3[3]; rates up to 30-50%, have been reported for N3 [2,3]. Based on these pathological results, Stenson et al. concluded that, in patients with ≥ N2 disease after CRT, ND is necessary to eradicate residual disease. In our case, we did not find any residual disease in the pathological specimens from five patients with radiological CR. A relatively new imaging technique-[18F]-fluorodeoxyglucose positron emission tomography (FDG-PET)-has proven to be highly accurate in the detection of persistent and recurrent disease in HNSCC patients, with a low false-negative rate after CRT. In patients with residual lymphadenopathy but negative FDG-PET [10,12,13] it appears reasonable to omit ND. FDG PET was only introduced during the last years of our study and, consequently, we did not assess the results of FDG-PET here. Several studies report beneficial effects of postradiotherapy ND. Brizel et al. concluded that adjuvant modified ND confer a disease-free and overall survival advantage with acceptably low morbidity; in their study, overall survival was 77% in patients with N2/N3 disease with complete response plus ND compared to only 50% in patients with complete response but without ND [4]. Lavertu et al. found significantly better disease-specific survival in N2-N3 patients with complete response (p = 0.002). Disease-specific survival was not affected by ND (p = 0.40) but was significantly affected by viable tumour in the specimen (p = 0.03) [14]. Notwithstanding the evidence presented above, recent data provides support for the position that the strategy of planned ND is no longer justified in patients without clinically residual disease in the neck after CRT. More effective CRT regimens, together with improvements in response assessment, have further modified the paradigm of planned ND. In fact, both the University of Florida and the University of Chicago have changed their approach and now recommend ND only for patients with residual disease after CRT. The results of their studies showed no difference in outcomes regardless of whether patients underwent ND or not [3,15-17]. Ferlito et al. recently carried out a review of many recentlypublished studies on this topic. Those authors found that 24 studies indicate that regional control increased due to “planned” ND in patients with pre-treatment bulky neck disease. However, all of those 24 studies were retrospective and did not assess treatment response prior to surgery, although regional control rates were very good. In contrast, the same authors found that 26 other studies demonstrated no benefit from “planned” ND after complete clinical response. Based on their review, the authors concluded that there is now a large body of evidence based on long-term clinical outcomes that patients who have achieved a complete clinical (including radiologic) response to CRT have a low rate of isolated neck failure, and, therefore, the continued use of planned ND for these patients cannot be justified [18]. A retrospective study of 880 patients carried out by Thariat et al. confirmed these findings: those authors found no significant differences in regional control with or without neck dissection. Moreover, given the 92% 5-year neck control rate without ND after chemotherapy, there seems to be little justification for systematic neck dissection [19]. In our study, we found that the most important factor for locoregional control is radiological response rather than the pre-treatment nodal stage. None of our patients with complete response and without ND presented isolated homolateral nodal relapse during follow up. Consequently, we agree with the conclusions made by Thariat et al. and Ferlito et al. However, we found that-in line with Thariat et al. [19,20]. Our patients who did not exhibit complete response after CRT had significant better regional control and overall survival with ND. For this reason, in such patients, neck dissection remains a recommended treatment.
| Variable | Locoregional control | p value |
|---|---|---|
| Age | ||
| >60 | 68% | |
| <60 | 75,8 | 0,51 |
| Gender | ||
| Male | 74% | |
| Female | 62,5% | P:0,4 |
| Primarysite | ||
| Hypopharynx | 88,9% | |
| Larynx | 76,9% | |
| Oropharynx | 68,6% | |
| Oral cavity | 0% | P:0,2 |
| T | ||
| T1-2 | 85,7% | |
| T3-4 | 68,2% | P:0,2 |
| N | ||
| N1-2a | 70% | |
| N2b-3 | 73,6% | P:0,7 |
| Response | ||
| Complete | 83,3% | |
| < 50% orstable | 50% | P:0,008 |
| Neckdissection | ||
| Yes | 81,2% | |
| No | 69% | P:0,3 |
Table 2: Univariate analysis for locoregional control.
| HR | p value | 95,0% IC | ||
|---|---|---|---|---|
| Inferior | Superior | |||
| CT response Others vs Complete |
6,102 | 0,002 | 1,955 | 19,052 |
| T stage T3-4 vs T1-2 |
1,144 | 0,872 | 0,224 | 5836 |
| Primary site Oropharynx vs Others |
1,752 | 0,352 | 0,537 | 5,709 |
Table 3: Multivariate Cox regression model with no locoregional relapse as end point.
Conclusion
Patients who achieve a complete clinical response to CRT, regardless of initial nodal stage, have a very low risk of an isolated neck recurrence and such patients can be spared from planned ND. In order to better identify patients with residual anatomical abnormalities who do not harbour viable tumour cells, it will first be necessary to achieve a consensus regarding the definition of “complete response”. This new definition will require the integration of newer, more sophisticated imaging techniques. In patients with residual viable tumour cells, neck dissection appears to significantly improve overall survival.
References
- Homma A,Furuta Y, Oridate N, Suzuki F, Higuchi E, et al. (2006) "Watch-and-see" policy for the clinically positive neck in head and neck cancer treated with chemoradiotherapy. Int J ClinOncol 11: 441-448.
- McHam SA,Adelstein DJ, Rybicki LA, Lavertu P, Esclamado RM, et al. (2003) Who merits a neck dissection after definitive chemoradiotherapy for N2-N3 squamous cell head and neck cancer? Head Neck 25: 791-798.
- Stenson KM,Haraf DJ, Pelzer H, Recant W, Kies MS, et al. (2000) The role of cervical lymphadenectomy after aggressive concomitant chemoradiotherapy: the feasibility of selective neck dissection. Arch Otolaryngol Head Neck Surg 126: 950-956.
- Brizel DM,Prosnitz RG, Hunter S, Fisher SR, Clough RL, et al. (2004) Necessity for adjuvant neck dissection in setting of concurrent chemoradiation for advanced head-and-neck cancer. Int J RadiatOncolBiolPhys 58: 1418-1423.
- Argiris A,Stenson KM, Brockstein BE, Mittal BB, Pelzer H, et al. (2004) Neck dissection in the combined-modality therapy of patients with locoregionally advanced head and neck cancer. Head Neck 26: 447-455.
- Lopez R M, Cerezo P L, Martin M M, Counago L F (2008) Neck dissection after radiochemotherapy in patients with locoregionally advanced head and neck cancer.ClinTranslOncol 10: 812-816.
- Chan AW,Ancukiewicz M, Carballo N, Montgomery W, Wang CC (2001) The role of postradiotherapy neck dissection in supraglottic carcinoma. Int J RadiatOncolBiolPhys 50: 367-375.
- Corry J, Peters L, Fisher R,Macann A, Jackson M, et al. (2008) N2-N3 neck nodal control without planned neck dissection for clinical/radiologic complete responders-results of Trans Tasman Radiation Oncology Group Study 98.02. Head Neck 30:737-742.
- Goguen LA, Posner MR, Tishler RB, Wirth LJ, Norris CM, et al. (2006) Examining the need for neck dissection in the era of chemoradiation therapy for advanced head and neck cancer. Arch Otolaryngol Head Neck Surg 132: 526-531.
- Eisenhauer EA,Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247.
- Liauw SL, Mancuso AA, Amdur RJ, Morris CG, Villaret DB, et al. (2006) Postradiotherapy neck dissection for lymph node-positive head and neck cancer: the use of computed tomography to manage the neck. J ClinOncol 24: 1421-1427.
- Stenson KM,Huo D, Blair E, Cohen EE, Argiris A, et al. (2006) Planned post-chemoradiation neck dissection: significance of radiation dose. Laryngoscope 116: 33-36.
- Chen AM,Khodayari B, Daly ME, Farwell G,Luu Q, et al. (2013) Observation Versus Neck Dissection for Residual, PET-Negative Lymphadenopathy After Chemoradiotherapy for Head-and-Neck Cancer. PractRadiatOncol 3: S5.
- Khodayari B, Daly ME, Bobinski M, Farwell DG, Shelton DK, et al. (2014) Observation versus neck dissection for positron-emission tomography-negative lymphadenopathy after chemoradiotherapy. Laryngoscope 124: 902-906.
- Yao M,Luo P, Hoffman HT, Chang K, Graham MM, et al. (2007) Pathology and FDG PET correlation of residual lymph nodes in head and neck cancer after radiation treatment. Am J ClinOncol 30: 264-270.
- Lavertu P, Adelstein DJ, Saxton JP, Secic M, Wanamaker JR, et al. (1997) Management of the neck in a randomized trial comparing concurrent chemotherapy and radiotherapy with radiotherapy alone in resectable stage III and IV squamous cell head and neck cancer. Head Neck19:559-566.
- Mendenhall WM, Million RR, Cassisi NJ (1986) Squamous cell carcinoma of the head and neck treated with radiation therapy: the role of neck dissection for clinically positive neck nodes. Int J RadiatOncolBiolPhys 12: 733-740.
- Langerman A, Plein C, Vokes EE, Salama JK, Haraf DJ, et al. (2009) Neck response tochemoradiotherapy: complete radiographic response correlateswithpathologic complete response in locoregionallyadvanced head and neckcancer. ArchOtolaryngol Head NeckSurg 135: 1133-1136.
- Ferlito A, Corry J, Silver CE, Shaha AR, Thomas Robbins K, et al. (2010) Plannedneckdissectionforpatientswith complete response tochemoradiotherapy: a concept approachingobsolescence. Head Neck 32: 253-261.
- Thariat J, Ang KK, Allen PK, Ahamad A, Williams MD, et al. (2012) Prediction of neckdissectionrequirementafterdefinitiveradiotherapyfor head-and-necksquamouscell carcinoma. Int J RadiatOncolBiolPhys 82: e367-374.
Citation: Martin M, García J, Lopez M, Hinojar A, Manzanares R, et al. (2016) Role of Neck Dissection in Locoregionally Advanced Head and Neck Cancer Treated with Primary Chemoradiotherapy. Otolaryngology 6:220. DOI: 10.4172/2161-119X.1000220
Copyright: © 2016 Martin M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 11318
- [From(publication date): 2-2016 - Aug 25, 2025]
- Breakdown by view type
- HTML page views: 10385
- PDF downloads: 933