Serotonin and its Functions as Gastrointestinal Hormone
Received: 14-Nov-2017 / Accepted Date: 23-Nov-2017 / Published Date: 30-Nov-2017 DOI: 10.4172/2161-069X.1000537
Abstract
Background: Serotonin plays the role of critical signal molecule in great number of physiologic processes and pathologic symptoms of gastrointestinal tract. As a gastrointestinal hormone serotonin takes place in the regulation of smooth muscle motor activity and glandular secretion. It affects the gastrointestinal sensation of pain, nausea and vomiting. The aim of our study is to determine the presence and localization of serotonin-producing EC cells and distribution of serotonin receptor 5-HTR3 in the human stomach.
Material and methods: Biopsy specimens from 25 patients aged 40-70 years: body and antral part of the stomach are studied by immunohistochemical reactions for serotonin, serotonin receptor 5-HTR3; and transmission electron microscopy.
Results: The serotonin-producing cells are found between columnar cells of gastric epithelium, in the fundic and pyloric glands of the gastric mucosa. Their ultrastructural characteristics show the presence of EC1 and EC2 enterochromaffin cells. The expression of serotonin receptor 5-HTR3 in covering epithelium, glandular and interstitial cells shows the possibility serotonin to affect gastric mucosa secretion. The intensive expression of 5-HTR3 receptors in gastric wall smooth muscle cells demonstrates the key role of serotonin in gastrointestinal motility.
Conclusion: In order to obtain a better understanding of the functional role of serotonin, we investigate the localization of serotonin-producing EC cells, their ultrastructural characteristics and the presence and distribution of serotonin receptor 5HTR3 in the stomach. Enzymes needed for serotonin secretion and degradation, its transporters, as it great numbers of receptors are the base new drugs to be synthesized aiding clinical practice in gastroenterology.
Keywords: Gastric mucosa secretion; Gastrointestinal motility; Enterochromaffin cells; Serotonin; Serotonin receptors
Introduction
The holistic vision on examining the human body in contemporary medicine is supported by regulatory factors with multidirectional action in different organs and systems. Serotonin is such a physiologically active substance. Serotonin, described as the hormone of happiness, is synthesized by the amino acid tryptophan via hydroxylation and decarboxylation. The diet is of major significance for the body serotonin level. Banana, kiwi, pineapple, plums, tomatoes, hazelnuts contain serotonin, while rich in tryptophan are almonds, banana, bean plants, cheese, chicken and duck meat, eggs, fish, milk, peanuts, soya products [1,2].
Serotonin synthesis is assisted by food rich in calcium, magnesium and vitamin B, omega-3, omega-6 and gamma linoleic acids [3]. The total amount of serotonin in the human body is about 10 μg. Only 5% of serotonin in human body is found in platelets and in the central nervous system (CNS). Serotonin plays the role of neurotransmitter in reticular formation nuclei, hypothalamus and limbic system [4,5]. The level of serotonin in CNS affects higher nerve functions as mood, sleep, memory, cognitive processes, sexual and behavioral reactions [6,7]. The sunlight triggers serotonin synthesis by epiphyseal pinealocytes [8]. Almost 95% of it is found in the gastrointestinal tract (GIT), mainly in epithelial and glandular enterochromaffin (EC) cells.
Minimal serotonin is found in myenteric and submucosal plexus nerve fibers [9]. Gastrointestinal wall mast cells contain 10% of serotonin within the tube. As a gastrointestinal hormone, serotonin takes place in the regulation of smooth muscle motor activity [10,11] and glandular secretion [12]. It affects the gastrointestinal sensation of pain, nausea and vomiting [13]. Being part of platelets and mast cells specific granules, serotonin is a potent vasoconstrictor, an active factor in the processes of hemostasis, inflammation and allergic reactions [14,15]. Serotonin plays its role via specific receptors. They are identified and cloned in seven types and subtypes [16]. A great number of serotonin receptors is related to the long evolution history of serotonin intercellular signaling based on gene repetitions followed by mutations, coding different receptor subtypes [17].
Aim
The aim of our study is to determine the presence and localization of serotonin-producing EC cells and distribution of serotonin receptor 5-HTR3 in the human stomach, being the morphologic substrate of physiologically active serotonin in the gastro intestinal tract.
Materials and Methods
The study is carried out on biopsy material from stomach-body and antrum of 25 patients aged 40-70 years from Clinic of Gastroenterology, UMHAT “St. George”, Plovdiv after written informed consent from each of the patient. Our study was conducted over a period of six months in 2016. Biopsy specimens were collected from the 25 healthy subjects. In our study were included only cases with a typical characteristic of the gastric wall without endoscopic and histological data for a pathological process. Immunohistochemical (IHC) methods (IHC reactions for serotonin and serotonin receptor 5- HTR3) and transmission electron microscopic study (TEM) are used.
IHC reactions are based on ABC method via rabbit ABC Staining System (Santa Cruz Biotechnology, USA). Biopsy material for the IHC study are fixated in Buen solution for 24 hours and it included paraffin. Paraffin cuts with thickness of 5 μm are deparaffinated and incubated for 30 min. in 2% H2O2 methanol for inactivation of the endogenous peroxidase. The primary antibody for serotonin (rabbit polyclonal antibody, MAB352 serotonin- Chemicon USA is diluted in PBS in ratio 1:200. The primary antibody for serotonin receptor (goat polyclonal antibody, SR-3A: sc-19150, Santa Cruz Biotechnology USA is diluted in PBS in ratio 1:100.
The incubation of the cuts with the corresponding antibody is performed at 4°C for 12 hours in damp camera. What follows is an incubation with the biotinylated secondary antibody for 30 min. in ABC complex for 15 min. and visualization with DAB chromogen. Deparaffinated and included in Vecta mount cuts are observed under microscope and photographed. Semi-qualitative method is used for received results assessment. The specificity of IHC reactions is confirmed via negative controls with a buffer (PBS) or normal nonimmune serum. Observation and photo documentation of the microscopic preparations are performed with digital photo microscopic camera of a light microscope “Olympus BX51”.
The biopsy material for TEM is fixed in 4% glutaric aldehyde, NaPO4 buffer 0.1 M, with followed post-fixation in 4% OsO4 and 0.2 M S’collin buffer. It is dehydrated in ascending alcohol series and was included in durcopan. It is warmed in gelatin capsules at 56°C for 48 hour. Gelatin capsules are removed with warm water and the material is cut into thick slices 0.5 mm. Cuts are mounted on glass slides, stained with methylene blue, covered and observed with a light microscope to determine the area of cutting.
What follows is the cutting of ultrathin slices with thickness of 300 nm and their mounting on platinum nets. The mounted cuts are contrasted with 5% uranyl acetate, diluted 1:1 with 100% alcohol for 80 min. in dark. They are washed with 50% alcohol and Reinold’s solution (water solution of Pb(NO3)2, Na(CoH5O7)2H2O for 20 min. and bid H2O. Observation and microphotography we performed on ??M Philips CM 12.
Results
IHC study for serotonin
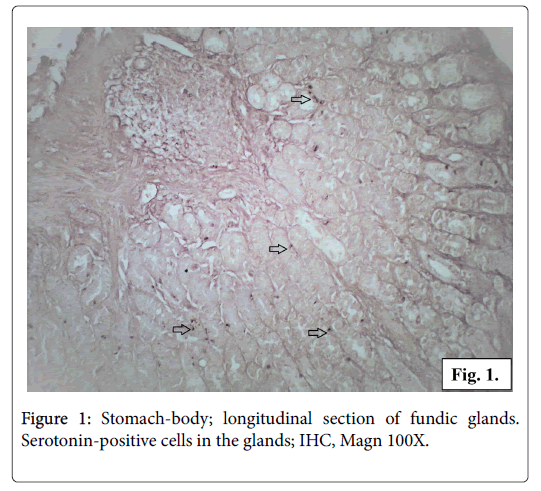
The biopsy material from the body and antrum of the gastric wall showed positive reaction for serotonin. Serotonin-positive cells are found to be located either in groups or as single cells in the fundic and in the pyloric glands. Some of the serotonin-positive cells are distributed between columnar cells of gastric epithelium. Serotonin positive cells are found in the base of the glands, rarely in the neck region (Figure 1).
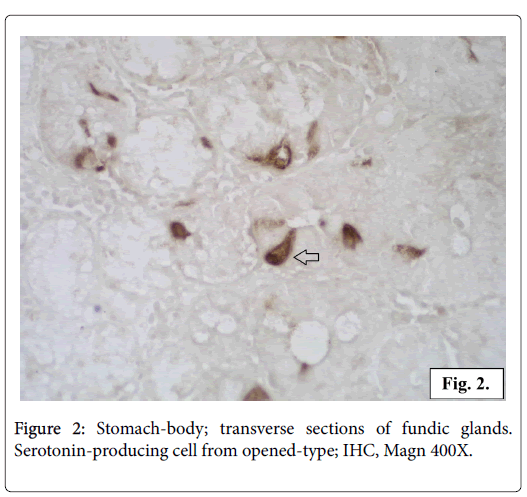
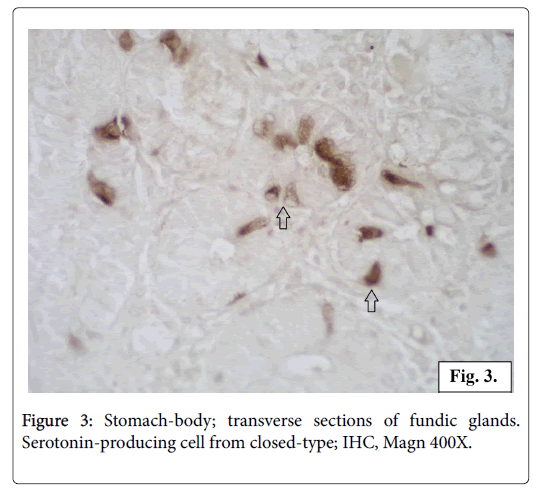
All the serotonin-positive cells have a dark brown colour in the cell body and cytoplasmic processes. The nuclei with a spherical shape are located in the middle part of the cells. The intracellular localization of the serotonin production is observed in transverse sections of the glands. In some of the endocrine cells it is concentrated in the apical part while in others cells in the basal part. The most of the serotoninpositive cells appear as closed type, without a lumen contact, but some of the cells seem to reach the lumen opened type (Figures 2 and 3).
IHC study for serotonin receptor 5-HTR3
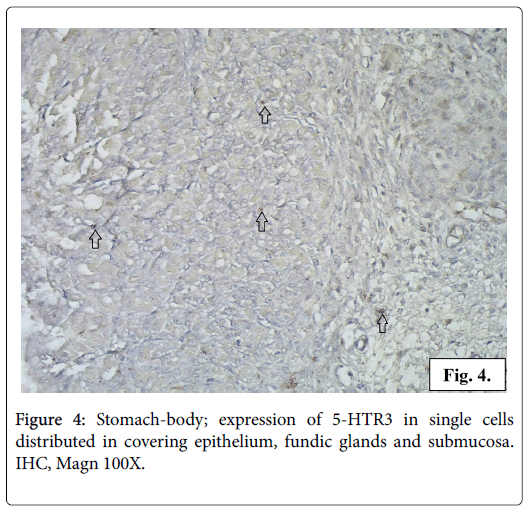
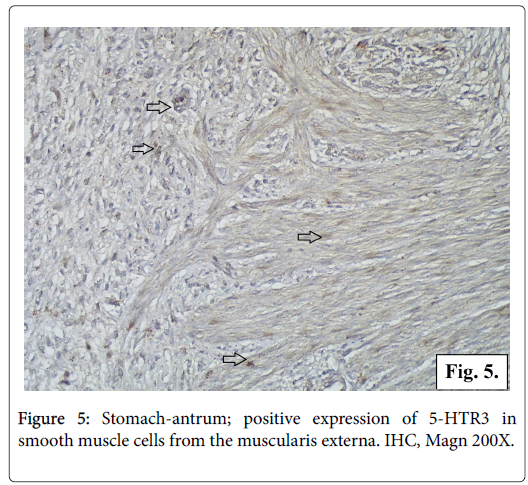
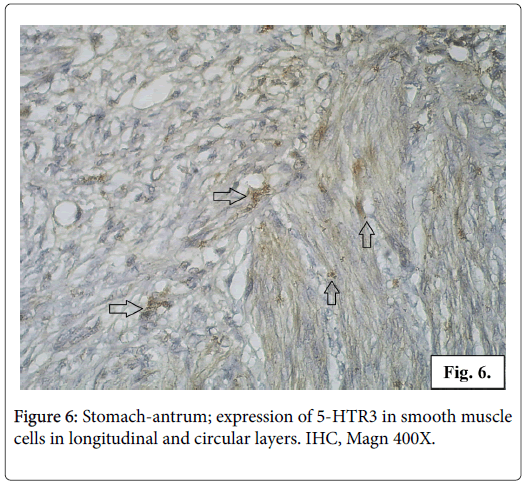
The IHC reaction for serotonin receptor 5-HTR3 is positive in the gastric mucosa and in the smooth muscle fibers from the muscularis externa of gastric wall. In the gastric mucosa 5-HTR3 is expressed in small number of columnar epithelial cells, fundic and pyloric glandular cells, interstitial cells from the loose connective tissue of lamina propria and submucosa (Figure 4). The reaction for serotonin receptor 5-HTR3 is positive in a great number of smooth muscle cells in stomach wall of different layers longitudinal, circular and oblique. The cytoplasm of the leiomyocytes is full of fine brown granulation visualizing the serotonin receptor 5-HTR3 (Figures 5 and 6).
Electron microscopic study
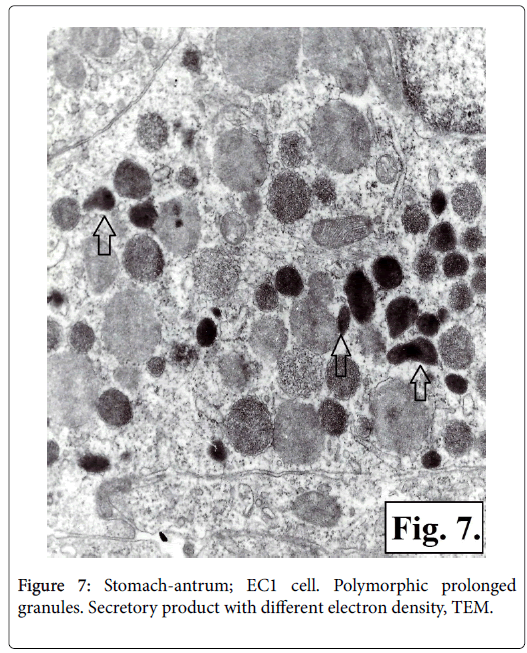
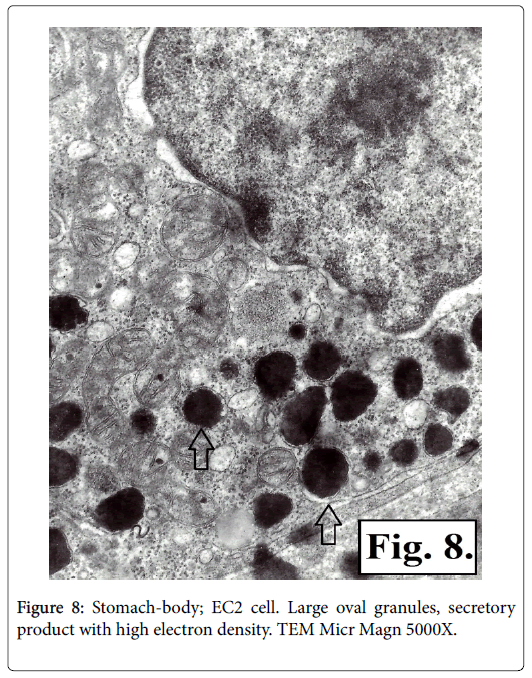
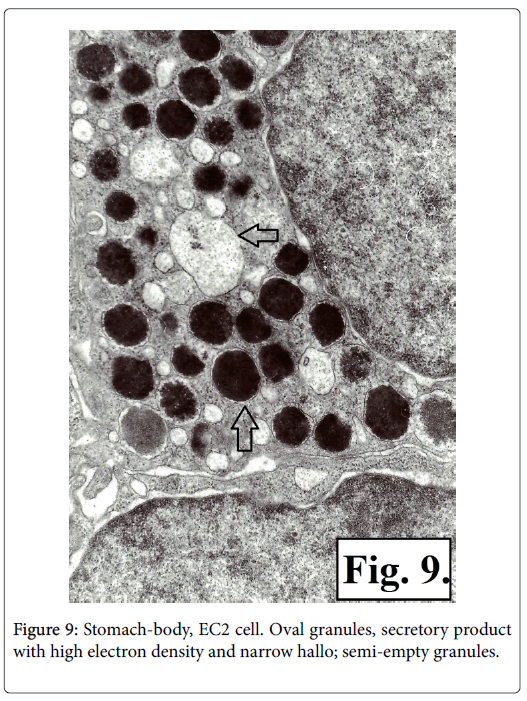
It was established that there are two types of serotonin-producing enterochromaffin (EC) cells EC1 and EC2 based on TEM of corporal and antral gastric biopsy specimens. The ultrastructural characteristics of the two types of serotonin-producing EC cells granules showed presence of membrane and narrow hallo. The EC1 cells granules are with lower dimensions, polymorphic, some being prolonged or rodshaped and containing secretor product with different electronic density-low, medium or high. It is homogenic with low electron density in the greater number of the secretory granuls, while other granuls contain material either with fine grit, or with high electron density (Figure 7). The granules of EC2 type cells are with bigger dimensions, mainly oval in shape and with high electron density (Figures 8 and 9).
Discussion
In our IHC study of human stomach we registered serotoninproducing cells, scattered in the covering epithelium, fungic and antral glands. The granules electronic microscopic characteristics show two types of serotonin-secreting enterohromaffin cells EC1 and EC2. The enterochromaffin EC cells secreting serotonin are found in all GIT sections, being the most rich population [18]. In the stomach they are located mainly in the antral and not so often in the corporal mucosa [19,20]. On the base of their granular ultrastructural morphology enterohromaffin EC cells are divided into EC1, EC2 and ECn type [21]. EC1-type cells are mainly found in the small and large intestine. EC2-type are greatest in number in the duodenum. ECn are mostly in the stomach. Enteroendocrine cells of EC type are differentiated not only in terms of their granular morphology but according to the secreted hormones, too. Immunohistochemical studies show that all EC cells produce serotonin [22]. EC1 cells secrete serotonin and substance P [23], while EC2 serotonin and motilin [24,25]. Up to now only serotonin is known as secretory product of ECn cells.
The serotonin-producing cells we registered in the gastric mucosa are of two structural types: opened and closed. The opened-type cells show serotonin expression concentrated in the apical part. These cells possess an apical surface with microvilli reaching the lumen and have the ability to analyze chemically the food. The serotonin-producing cells of the so called closed-type do not reach the lumen. Their serotonin secretion is concentrated in the basal part of the cell. These cells are triggered by mechanical distension and stimuli coming from blood. Both types of serotonin secreting cells respond to different irritants by hormones synthesis, storage and release [26].
As biogenic amine serotonin is synthesized in EC cells cytosol. The serotonin molecules are packed as secretory product in EC granules via vesicular monoamine transporter VMAT1. Proton gradient, generated by ATPase is needed for this process [27]. Secreted by EC cells serotonin is an electrically loaded molecule. As its action is intracellular, it has to be transported through the target cell membrane. Highly effective transport molecule is needed. Such transporter, 5- H?? or SERT in CNS is produced by serotonergic neurons, not available in GIT mucosa. As far as GIT is concerned, this molecule is secreted by enterocytes [28]. SERT is localized on the intestinal epithelial cells apical and basal membrane [29]. Some scientists determine serotonin-producing EC cells as sensors reacting to mechanical tension and substances like glucose short- and long chained fatty acids, peptides [30].
The strategic localization of EC cells within the gastrointestinal covering epithelium gives them a chance to serve as visceral stimuli sensory transducers [31]. One of the first steps in this process is the release of serotonin from EC cells [32]. Serotonin, secreted by EC cells and serotoninergic neurons within myenteric and submucosal plexus is the main molecule transmitter synchronizing gastrointestinal reflexes and transmitting information to CNS [33]. Under physiological conditions the serotonin release leads to reflex coordination responsible for the transition of food with velocity suitable for digestion and absorption [34].
Serotonin plays role in motor, secretory and sensory functions of GIT acting via specific receptors. On the base of their pharmaceutical profile and DNA sequence the serotonin receptors are identified and cloned in seven groups [35]. According to NC-IUPHAR they are: 5- H?1, 5-HT2, 5-HT3, 5-HT4, 5-HT5, 5-HT6 and 5-HT7. All the seven main types serotonin receptors are identified in different cell populations in GIT. Glandular and resorptive cells, smooth muscle cells, enteroendocrine cells and enteric neurons express receptors for serotonin [36-38]. Not like all other receptors for serotonin coupled to G-protein activating adenylate cyclase or phospholipase, 5-HTR3 receptors are ion channels.
In our IHC study we establish the presence and distribution of serotonin receptor 5-HTR3. Its expression is positive in the covering epithelium and glands and in some mucosal interstitial cells, but is mainly localized in gastric smooth muscle cells. In regard to motor function serotonin has precise effects on each GIT segment. Some authors report on the specific role of the serotonin-producing cells in the base of the foveolae of the gastric cardia mucosa – this narrow band of tissue between the oesophagus and the stomach. The authors suggest that serotonin secreted in this region is involved in the regulation of the lower esophageal sphincter [39].
Various types and subtypes of serotonin receptors in the smooth muscle cells of the circular and longitudinal layers of the wall of the small and large intestine are found [40,41]. The fine coordination of their contractions and relaxation determines the normal peristalsis of the GIT. 5-HTR3 receptor mediates ascending and descending peristalsis reflex components thus determining the transit velocity in GIT [42,43]. Unfortunately the great expectations for serotonin receptor agonists and antagonists in the treatment of diarrhea and constipation are not confirmed. The reason probably is the great number of types and sub-types serotonin receptors and lack of ligands for studies in vivo [33].
The secretory effect of serotonin is mediated by epithelial 5-HT2 and neuronal 5-HT1?, 5-HT3 ? 5-HT4 receptors [12,44]. Serotonin increases the serosal secretion of salivary glands, activates the gastric principal cells directly or indirectly via gastrin but inhibits the secretion of HCl by wall cells [45]. In the small intestine it stimulates the secretion of mucus and fluid as mucosa protector reaction or in pathologic reactions like carcinoid syndrome. Serotonin activates the secretion of bile contracting the smooth muscle cells within the extra hepatic bile pathways and gall bladder. The secretion of pancreatic juice is not affected significantly [46].
In regard to the sensory role of serotonin within the GIT, it is studied in terms of inflammatory and functional diseases as duodenal ulcer, irritated bowel syndrome (IBS), gastrointestinal reflux disease, conditions after radiotherapy [47]. Symptoms like abdominal pain, nausea and vomiting in IBS are related to the increased secretion of serotonin by mast cells and EC cells within GIT [48]. It is established that in mucosal biopsy there is correlation between the expression of IBS, EC cell number in the large intestine mucosa and the level of serotonin [49,50].
5-HT3 receptors take part in the complicated vomit reflexes during functional gastrointestinal disorders as well as in therapy of cancer diseases. The blocking of these receptors by 5-HT3 antagonists interrupts the afferent impulses to the vestibular system, the cerebral cortex and the chemoreceptor trigger zone located in floor of the fourth ventricle that regulates the physiological emetic centre. 5-HT3 and 5-HT4 receptors take part in the gastrointestinal sensitivity. They are located in vagal and visceral nociceptive neurons that activate different pain systems. The fibers of those neurons transmit signal from the viscera to the specific lamine of the dorsal horn, nuclei of the thalamus and cerebral cortex [51].
Serotonin plays the role of critical signal molecule in great number of physiologic processes and pathologic symptoms of GIT [11,52,53]. Enzymes needed for serotonin secretion and degradation: tryptophan– 5-hydroxylase (TpH1) and monoaminoxidase (M?O); its transporters: VMAT1 and SERT; as its great number of receptors is going to be the morphologic substrate determining the physiologic functions of serotonin in GIT. They are the base new drugs to be synthesized aiding clinical practice in gastroenterology.
Conclusion
In order to obtain a better understanding of the functional role of serotonin, we investigate the serotonin-producing EC cells in human stomach. In our study the serotonin-producing cells localized in the covering epithelium, fundic and pyloric glands are found in the gastric mucosa of adult individuals. Their ultrastructural characteristics show the presence of EC1 and EC2 types. The expression of 5-HTR3 serotonin receptor in epithelial, glandular and interstitial cells shows the possibility serotonin to affect gastric mucosa secretion. The intensive expression of 5-HTR3 receptors in gastric wall smooth muscle cells demonstrates the key role of serotonin in GIT motility.
References
- Shively CA, Mirkes SJ, Lu NZ, Henderson JA, Bethea CL (2003) Soy and social stress affect serotonin neurotransmission in primates. Pharmacogenomics J 3: 114-121.
- Islam J, Shirakawa H, Nguyen T, Aso H, Komai M (2016) Simultaneous analysis of serotonin, tryptophan and tryptamine levels in common fresh fruits and vegetables in Japan using fluorescence HPLC. Food Biosci 13: 56-59.
- Guillén-Casla V, Rosales-Conrado N, León-González ME, Pérez-Arribas LV, Polo-DÃez LM (2012) Determination of serotonin and its precursors in chocolate samples by capillary liquid chromatography with mass spectrometry detection. J Chromatogr A 1232: 158-165.
- Hornung JP (2003) The human raphe nuclei and the serotonergic system. J Chem Neuroanat 26: 331-343.
- Li Y, Zhong W, Wang D, Feng Q, Liu Z (2016) Serotonin neurons in the dorsal raphe nucleus encode reward signals. Nat Commun 7: 10503.
- Davis JM, Alderson N, Welsh RS (2000) Serotonin and central nervous system fatigue: Nutritional considerations. Am J Clin Nutr 72: 573-578.
- Jenkins TA, Nguyen JC, Polglaze K, Bertran PP (2016) Influence of tryptophan and serotonin on mood and cognition with a possible role of the gut-brain axis. Nutrients 8: 56.
- Simon YN (2007) How to increase serotonin in the human brain without drugs. J Psychiatry Neurosci 32: 394-399.
- Glatzle J, Sternini C, Robin C, Zittel, T, Wong H, et al (2002) Expression of 5-HT3 receptors in the rat gastrointestinal tract. Gastroenterol 123: 217-226.
- Niesler B, Frank B, Kapeller J, Rappold GA (2003) Cloning, physical mapping and expression analysis of the human 5-HT3 serotonin receptor-like genes HTR3C, HTR3D and HTR3E. Gene 310: 101-111.
- Manabe N, Wong BS, Camilleri M (2010) New-generation 5-HT4 receptor agonists: Potential for treatment of gastrointestinal motility disorders. Expert Opin Investig Drugs 19: 765-775.
- Hansen MB, Witte AB (2008) The role of serotonin in intestinal luminal sensing and secretion. Acta Physiol (Oxf) 193: 311-323.
- Smith HS, Cox LR, Smith EJ (2012) 5-HT3 receptor antagonists for the treatment of nausea/vomiting. Ann Palliat Med 1: 115-120.
- Ringvall M, Rönnberg E, Wernersson S, Duelli A, Henningsson F, et al. (2008) Serotonin and histamine storage in mast cell secretory granules is dependent on serglycin proteoglycan. J Allergy Clin Immunol 121: 1020-1026.
- Duerschmied D, Bode C (2009) The role of serotonin in haemostasis. Hamostaseologie 29: 356-359.
- Berger M, Gray JA, Roth BL (2009) The expanded biology of serotonin. Annu Rev Med 60: 355-366.
- Gillette R (2006) Evolution and Function in Serotonergic Systems. Int Comp Biol 46: 838-846.
- Gunawardene AR, Corfe BM, Staton CA (2011) Classification and functions of enteroendocrine cells of the lower gastrointestinal tract. Int J Exp Pathol 92: 219-231.
- Yacoub W, Thompson A, Hooper P, Jevell L (1996) Immuno-cytochemical and morphometric studies of gastrin-, somatostatin-, and serotonin cells in the stomach and duodenum of patients with acid peptic disorders. Canadian J gastroenterol 10: 396-400.
- Takahashi Y, Imanaka T, Takano T (1998) Spatial pattern of smooth muscle differentiation is specified by the epithelium in the stomach of mouse embryo. Dev Dyn 212: 448-460.
- Takahara E, Yamamoto M, Ito H, Shimamoto F, Sumii K (1981) The effects of gastrin on the ultrastructure of rat stomach endocrine cells. Exp Molec Path 35: 211-218.
- Modlin I, Kidd M, Pfragner R, Eick GN, Champaneria MC (2006) The functional characterization of normal and neoplastic human enterochromaffin cells. J Clin Endocrinol Metab 91: 2340-2348.
- Heitz P, Polak JM, Timson DM, Pearse AG (1976) Enterochromaffin cells as the endocrine source of gastrointestinal substance P. Histochemistry 49: 343-347.
- Kobayashi S, Iwanaga T, Fujita T, Yanaihara N (1980) Do enterochromaffin (EC) cells contain motilin? Arch Histol Jpn 43: 85-98.
- Kamerling IM, Burggraaf J, Van Haarst AD, Oppenhuizen-Duinker MF, Schoemaker HC, et al. (2003) The effect of motilin on the rectum in healthy volunteers. Br J Clin Pharmacol 55: 538-543.
- Kuramoto H, Kadowaki M, Sacamoto H, Yuasa K, Todo A, et al. (2007) Distinct morphology of serotonin-containing enterochromaffin (EC) cells in the rat distal colon. Arch of Hyst and Cyt 70: 235-241.
- Anlauf M, Eissele R, Schäfer MK, Eiden LE, Arnold R, et al. (2003) Expression of the two isoforms of the vesicular monoamine transporter (VMAT1 and VMAT2) in the endocrine pancreas and pancreatic endocrine tumors. J Histochem Cytochem 51: 1027-1040.
- Gershon M (2003) Plasticity in serotonin control mechanisms in the gut. Curr Opin Pharmacol 3: 600-607.
- Gill RK, Pant N, Saksena S, Singla A, Nazir TM, et al (2008) Function, expression, and characterization of the serotonin transporter in the native human intestine. Am J Physiol Gastrointest Liver Physiol 294: 254-262.
- Zelkas L, Raghupathi R, Lumsden A, Martin A, Sun E, et al (2015) Serotonin-secreting enteroendocrine cells respond via diverse mechanisms to acute and chronic changes in glucose availability. Nutr Metab (Lond) 12: 55.
- Blackshaw LA, Brookes SJ, Grundy D, Schemann M (2007) Sensory transmission in the gastrointestinal tract. Neurogastroenterol Motil 19: 1-19.
- Bertrand PP, Bertrand RL (2010) Serotonin release and uptake in the gastrointestinal tract. Auton Neurosci 153: 47-57.
- Gershon MD, Tack J (2007) The serotonin signaling system: From basic understanding to drug development for functional GI disorders. Gastroenterol 132: 397-414.
- Grundy D (2008) 5-HT system in the gut: Roles in the regulation of visceral sensitivity and motor functions. Eur Rev Med Pharmacol Sci 12: 63-67.
- Nichols DE, Nichols CD (2008) Serotonin receptors. Chem Rev 108: 1614-1641.
- Tonini M (2005) 5-Hydroxytryptamine effect in the gut: The 3,4 and 7 receptors. Neurogastroenterol Motil 17: 637-642.
- Van Lelyveld N, Ter Linde J, Schipper ME, Samsom M (2007) Regional differences in expression of TPH-1, SERT, 5-HT3, and 5-HT4 receptors in the human stomach and duodenum. Neurogastroenterol Motil 19: 342-348.
- Holbrook JD, Gill CH, Zebda N, Spencer JP, Leyland R, et al. (2009) Characterisation of 5-HTc, 5-HT3d and 5-HT3e receptor subunits: Evolution, distribution and function. J Neurochem 108: 384-396.
- Voutilainen M, Juhova M, Pijkanen R, Farkkila M, Sipponen P (2002) Immunohistochemical study of neuroendocrine cells at the gastric cardia mucosa. J Clin Pathol 55: 767-769.
- Borman RA, Tilford NS, Harmer DW, Day N, Ellis ES, et al. (2002) 5-HT(2B) receptors play a key role in mediating the excitatory effects of 5-HT in human colon in vitro. Br J Pharmacol 135: 1144-1151.
- Fujitsuka N, Asakawa A, Hayashi M, Sameshima M, Amitani H, et al. (2009) Selective serotonin reuptake inhibitors modify physiological gastrointestinal motor activities via 5-HT2c receptor and acyl ghrelin. Biol Psychiatry 65: 748-759.
- Bjornssen ES, ChenWD, Hooper F, Woods ML, Owyang, C, et al. (2002) Impaired gastrocolonic response and peristaltic reflex in slow transit constipation: Role of 5-HT3 pathways. Am J Physiol 283: 400-407.
- Michel K, Zeller F, Langer R, Nekarda H, Kruger D, et al. (2005) Serotonin excites neurons in the human submucous plexus via 5-HT3 receptors. Gastroenterol 128: 1317-1326.
- Volpi-Abadie J, Kaye AM, Kaye AD (2013) Serotonin Syndrome. Ochsner J 13: 533-540.
- Lai YC, Ho Y, Huang KH, Tsai LH (2009) Effects of serotonin on acid secretion in isolated rat stomach: the role of 5-HT3 receptors. Chin J Physiol 52: 395-405.
- Camilleri M (2009) Serotonin in the Gastrointestinal Tract. Curr Opin Endocrinol Diabetes Obes 16: 53-59.
- Manocha M, Khan W (2012) Serotonin and GI disorders: An update on clinical and experimental studies. Clin Transl Gastroenterol 3: e13.
- Cremon C, Carini G, Wang B, VasinaV, Cogliandro RF, et al. (2011) Intestinal serotonin release, sensory neuron activation, and abdominal pain in irritable bowel syndrome. Am J Gastroenterol 106: 1290-1298.
- Miwa J, Echizen H, Matsueda K, Umeda N (2001) Patients with constipation-predominant irritable bowel syndrome (IBS) may have elevated serotonin concentrations in colonic mucosa as compared with diarrhea-predominant patients and subjects with normal bowel habits. Digestion 63: 188-194.
- Faure C, Patey N, Gauthier C, Brooks EM, Mawe GM (2010) Serotonin signaling is altered in irritable bowel syndrome with diarrhea but not in functional dyspepsia in pediatric age patients. Gastroenterol 139: 249-258.
- Crowell M (2004) Role of serotonin in the pathophysiology of the irritable bowel syndrome. British J Pharmacol 141: 1285-1293.
- Neal KB, Bornstein JC (2006) Serotonergic receptors in therapeutic approaches to gastrointestinal disorders. Curr opin Pharmacol 6: 547-552.
- Kindt S, Tack J (2007) Mechanisms of serotonergic agents for treatment of gastrointestinal motility and functional bowel disorders. Neurogastroenterol Motil 19: 32-39.
Citation: Penkova NI, Nikolova JG (2017) Serotonin and its Functions as Gastrointestinal Hormone. J Gastrointest Dig Syst 7: 537. DOI: 10.4172/2161-069X.1000537
Copyright: © 2017 Penkova NI, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 12431
- [From(publication date): 0-2017 - Dec 14, 2025]
- Breakdown by view type
- HTML page views: 11178
- PDF downloads: 1253