Site-Specific Drug Delivery to the Gastrointestinal Tract
Received: 06-Jun-2013 / Accepted Date: 08-Jun-2013 / Published Date: 15-Jun-2013 DOI: 10.4172/2329-9053.1000e106
1523Editorial
There are many available routes for the administration of therapeutic agents, as denoted by the FDA’s recognition of over 100 different options for getting a drug into the body [1]. The oral (“per os” or PO) dosing of pharmaceutical products has a long and rich history in health care. This route of administering medications has several notable advantages, including the fact that it is relatively simple, it requires no additional equipment for patient administration (unlike injectable or inhaled agents), it is usually the least expensive option for both the manufacturer and the patient, and it is typically the safest route of getting a drug into the body (requiring no puncturing of body surfaces or membranes and the corresponding increased risk of disease transmission). Because of these and other advantages, the majority of currently used pharmaceutical agents are formulated and given as oral dosage forms. PO administration is also the preferred route for the treatment of diseases of the gastrointestinal tract, such as peptic/duodenal ulcer and inflammatory bowel diseases, because this technique puts the therapeutic agent at or near the desired site of action. In addition, the GI tract represents the major portal of entry of infectious agents into the body and the site at which most organisms exert their patho physiologic effects [2]. Due to this fact, one of the best ways to protect against infection would be the oral administration of vaccines to stimulate strong specific immunity in the gastrointestinal tract and other mucosal sites [3].
At the present time, however, oral dosing strategies are limited to those pharmaceutical agents that have acceptable oral bioavailability. This limitation has the effect of excluding many macromolecular agents from being given PO. Although a great deal of effort and resources have been expended to identify solutions to this issue, most peptide- and protein-based pharmaceuticals, including all insulins and most vaccines, are still currently given through other, non-oral routes of administration (e.g. subcutaneously or intramuscularly) [4]. Another issue that directly impacts oral bioavailability and, thus, the efficiency of the oral route of administration, is the residence time of the drug formulation at the desired site of action. The absorption of medicinal agents is often limited by the short contact time between the formulation and the absorption membrane and also by the fast washout of the agents from the body [5-7]. However, within the last decade, targeted delivery to increase the retention time of drugs has received a great deal of attention. The overall intent of these efforts has been to maximize the residence time of the dosage vehicles and to restrain and localize them to specific absorption sites in the GI tract [8,9]. Pharmaceutical advances in drug targeting thus far have mostly been limited to developments such as the use of enteric coatings to protect acid-sensitive agents from exposure to the harsh low pH gastric environment or to limit stomach irritation (e.g. enteric-coated aspirin), delayed-release or pH-sensitive formulations that enable drug delivery to the colon (e.g. numerous steroid formulations, such as Entocort® EC (budesonide), used in the treatment of inflammatory bowel diseases), and the use of prodrugs that rely on GI bacterial enzymes for intestinal release of the active form (e.g. the azo-based agents, such as sulfasalazine and olsalazine, used in the treatment of inflammatory bowel diseases). One notable recent development in the field of sitespecific GI drug delivery was the approval of an oral once-daily gastro retentive formulation of gabapentin for the treatment of postherpetic neuralgia [10]. In addition, gastric retentive delivery systems have been studied for the many drugs that are either less soluble or subject to degradation in the alkaline pH of the intestines but which can be absorbed in the stomach (e.g. captopril, furosemide, albuterol, amoxicillin and metoprolol). This type of delivery system is especially suitable for drugs that undergo significant metabolic degradation and have a high first pass effect [11,12].
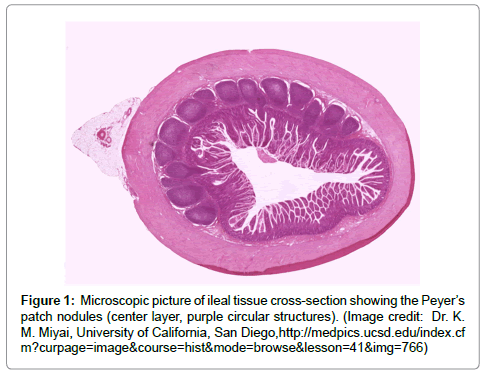
The successful targeting of therapeutic agents to any specific area of the GI tract requires both the exploitation of a unique feature of the site and also the protection of the active agent until it reaches the target site [13]. However, two scientific developments offer the potential promise of site-specific gastrointestinal drug delivery. The first is the discovery that E. coli microbes specifically adhere to the follicle-associated epithelium (FAE) that overlies the ileal Peyer’s patches (Figure 1) in the gastrointestinal tract [14,15]. Peyer’s patches are GI immune nodules, similar to lymph nodes, that form part of the gut-associated lymphoid tissue (GALT). Although the gastrointestinal tract may not typically be thought of as a component of the immune system, the GALT plays a key front-line role in the body’s defense mechanisms and contains more lymphocytes than all other secondary lymphoid organs combined [16]. As part of the immune surveillance role of these tissues, specialized microfold (or M) cells within the follicle-associated endothelium are actively involved in the endocytosis of macromolecules. M cells serve as a conduit for potential antigens from the gastrointestinal lumen into the Peyer’s patches. Endocytosed biomolecules are then processed by other immunocytes in the selymphoid tissues [17]. The combination of specific adherence (as exemplified by E. coli) along with endocytotic absorption suggests that, with the appropriate targeting or mimicry, it may be possible to formulate agents for site-specific GI drug delivery to the Peyer’s patches [18-20]. Initial animal studies suggest that this approach is feasible for vaccine delivery [21-23] and that, with an appropriate choice of formulation [24-26], it might provide an avenue for the delivery of other large biomolecules in humans.
The second development that may allow for this new type of specifically targeted pharmaceutical administration is related to the continuing advances made in bioadhesive drug formulation science. The process of bioadhesion involves the formation of a bond between two surfaces, where both surfaces are biological in nature or one is biological and the other is synthetic [27,28]. With regard to pharmaceutical agents, bioadhesion may be used to increase residence time and, thus, increase drug absorption, at the target site. Specific therapeutic formulations may be referred to as “mucoadhesive” [29] if the application area is a mucous membrane. However, because of the rapid turnover rate of mucus, product residence time for mucoadhesive agents at the site of action may be limited. For this reason, cytoadhesionbased approaches that target specific cell types may be preferred.
While it may not be widely known, bioadhesive drug delivery systems (BDDS) have a long history of use in medicine, dating back to the reported use of dental adhesive powder and tragacanth gum to deliver penicillin to the oral mucosal tissues in 1947 [30,31]. There are currently a small, but increasing, number of approved pharmaceutical products utilizing BDDS. For example, several buccal mucoadhesive products containing local anti-infectives (e.g. miconazole [32], acyclovir [33], and minocycline [34]), opioid analgesics [35], and agents with poor oral bioavailability (e.g. testosterone [36]) are now available. Oral mucoadhesive products on the market include agents for the treatment and prevention of aphthous ulcers (canker sores) [37]. Several ophthalmic agents are also available in gel-forming bioadhesive formulations [38,39]. There is also at least one example of an intranasal mucoadhesive product (CompleoTRT™, an bioadhesive intranasal gel testosterone product [40]) awaiting approval. In addition, some intra vaginal products (e.g. progesterone [41]) based on bioadhesion technology are now on the market. Because this type of pharmaceutical formulation allows for a longer residence time at the site of application [42], a bioadhesion-based oral product specifically targeted at the follicle-associated epithelial cells that cover the Peyer’s patches might thus allow for increased endocytosis and selective intestinal uptake of the active agent.
In summary, the ability to deliver pharmaceutical agents to the gastrointestinal tract in a site-specific manner has, to date, largely remained an elusive goal. However, the potential combination of selective molecular targeting to specific cells found in the gut-associated lymphoid tissues and the use of bioadhesive formulations offers a potential new route to the GI delivery of macromolecular therapeutic agents.
References
- Route of administration table. U. S. Food and Drug Administration. Accessed June 3, 2013.
- Sanderson IR, Walker WA (1993) Uptake and transport of macromolecules by the intestine: possible role of clinical disorders (an update). Gastroenterology 104: 622-639.
- Yeboah KG, D’Souza MJ (2009) Evaluation of albumin microspheres as oral delivery systems for Mycobacterium tuberculosis vaccines. J Microencapsul 26: 166-179.
- Hamman JH, Enslin GM, Kotze AF (2005) Oral delivery of peptide drugs: barriers and developments. Bio Drugs 19: 165-177.
- Coupe AJ, Davis SS, Wilding IR (1991) Variation in gastrointestinal transit of pharmaceutical dosage forms in healthy subjects. Pharm Res 8: 360-364.
- McConnell EL, Fadda HM, Basit AW (2008) Gut instincts: explorations in intestinal physiology and drug delivery. Int J Pharm 364: 213-226.
- Fadda HM, McConnell EL, Short MD, Basit AW (2009) Meal-induced acceleration of tablet transit through the human small intestine. Pharm Res 26: 356-360.
- Mathiowitz E, Chickering D, Jacob JS, Santos C (1999) Bioadhesive drug delivery systems in Encyclopedia of Controlled Drug Delivery (Vol. 1). E. Mathiowitz (Ed.), John Wiley & Sons: New York, pp. 9-44.
- Shah DN, Rectenwall-Work SM, Anseth KS (2008) The effect of bioactive hydrogels on the secretion of extracellular matrix molecules by valvular intestinal cells. Biomaterials 29: 2060-2072.
- Fell JT, Whitehead L, Collet H (2000) Prolonged gastric retention using floating dosage forms. Pharmaceutical Technology 24: 82-90.
- Matharu RS, Sanghavi NM (1992) Novel drug delivery system for captopril. Drug Develpoment and Industrial Pharmacy 18: 1567–1574.
- Fell JT (1996) Targeting of drugs and delivery systems to specific sites in the gastrointestinal tract. J Anat 189: 517-519.
- Inman LR, Cantey JR (1983) Specific adherence of Escherichia coli (strain RDEC-1) to membranous (M) cells of the Peyer’s patch in Escherichia coli diarrhea in the rabbit. J Clin Invest 71: 1-8.
- Inman LR, Cantey JR (1984) Peyer’s patch lymphoid follicle epithelial adherence of a rabbit enteropathogenic Escherichia coli (strain RDEC-1). Role of plasmid-mediated pili in initial adherence. J Clin Invest 74: 90-95.
- Nagler-Anderson C (2001) Man the barrier! Strategic defences in the intestinal mucosa. Nat Rev Immunol 1: 59-67.
- Dotan I, Mayer L (2010) Mucosal Immunity in Sleisenger and Fordtran’s Gastrointestinal and Liver Disease (9thedn), M. Feldman, L. S. Friedman & L. J. Brandt, Eds., Saunders Elsevier: Philadelphia, pp. 21-30.
- Lee VHL, Yang JJ (2001) Routes of Drug Delivery in Drug Delivery and Targeting: For Pharmacists and Pharmaceutical Scientists, Taylor & Francis: London, pp. 131-167.
- Clark MA, Jepson MA, Hirst BH (2001) Exploiting M cells for drug and vaccine delivery. Adv Drug Deliv Rev 50: 81-106.
- Shakweh M, Ponchel G, Fattal E (2004) Particle uptake by Peyer’s patches: a pathway for drug and vaccine delivery. Expert Opin Drug Deliv 1: 141-163.
- Eldridge JH, Hammond CJ, Meulbroek JA, Staas JK, Gilley RM, et al. (1990) Controlled vaccine release in the gut-associated lymphoid tissues. I. Orally administered biodegradable microspheres target the Peyer's patches. J Controlled Release 11: 205-214.
- Frey A, Neutra MR (1997) Targeting of mucosal vaccines to Peyer’s patch M cells. Behring Inst Mitt 98: 376-389.
- McKenzie BS, Corbett AJ, Brady JL, Boyle JS, Rockman SP, et al. (2005) Targeting lymphocyte Peyer’s patch adhesion molecule-1: a relay approach to gut immunization. Vaccine 23: 3668-3678.
- Jani PU, McCarthy DE, Florence AT (1992) Nanosphere and microsphere uptake via Peyer's patches: observation of the rate of uptake in the rat after a single oral dose. International Journal of Pharmaceutics 86: 239-246.
- Torche AM, Jouan H, Le Corre P, Albina E, Primault R, et al. (2000) Ex vivo and in situ PLGA microspheres uptake by pig ilealPeyer’s patch segment. Int J Pharm 201: 15-27.
- Ermak TH, Dougherty EP, Bhagat HR, Kabok Z, Pappo J (1995) Uptake and transport of copolymer biodegradable microspheres by rabbit Peyer’s patch M cells. Cell Tissue Res 279: 433-436.
- Lenaerts V, Gurny R (1989) Bioadhesive drug delivery systems. CRC Press: Boca Raton, FL.
- Thomas JB, Peppas NA (2008) Adhesives in Encyclopedia of Biomaterials and Biomedical Engineering, G. E. Wnek and G.L. Bowlin, Eds. ,Informa Healthcare: New York, pp. 1-7.
- Mathiowitz E, Chickering DE III, Lehr C-M (1999) Bioadhesive drug delivery systems: Fundamentals, novel approaches, and development. CRC Press: Boca Raton, FL.
- Scrivener C, Schantz C (1947) Penicillin. New methods for its use in dentistry. J Am Dent Assoc 35: 644–647.
- Vetter A, Bernkop-Schnurch A (2009) Bioadhesive delivery systems in Biodrug Delivery Systems: Fundamentals, Applications, and Clinical Development (1stedn), M. Morishita and K. Park, Eds.,Informa Healthcare: New York, pp. 218-233.
- Onsolis® fentanyl buccal soluble mucoadhesive film, Meda Pharmaceuticals, Inc.
- Striant® testosterone mucoadhesivebuccal system, Actient Pharmaceuticals.
- Pilopine HS® pilocarpine hydrochloride ophthalmic gel, Alcon Laboratories, Inc.
- Timoptic-XE® timolol maleate ophthalmic gel forming solution, Merck and Company, Inc.
- CompleoTRTâ„¢, bioadhesive intranasal gel testosterone, Trimel Pharmaceuticals Corp.
- Shaikh R, Singh TRR, Garland MJ, Woolfson AD, Donnelly RF (2011) Mucoadhesive drug delivery systems. J Pharm Bioallied Sci 3: 89-100.
Citation: Scott Weston G, Yeboah KG (2013) Site-Specific Drug Delivery to the Gastrointestinal Tract. J Mol Pharm Org Process Res 1: e106. DOI: 10.4172/2329-9053.1000e106
Copyright: ©2013 Scott Weston G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 18783
- [From(publication date): 10-2013 - Aug 24, 2025]
- Breakdown by view type
- HTML page views: 13808
- PDF downloads: 4975