Small Molecule Inhibitors of PFKFB3 as Therapeutic Targets for Atherosclerosis in ApoE-/-mice
Received: 10-Mar-2025 / Manuscript No. DPO-25-162220 / Editor assigned: 12-Mar-2025 / PreQC No. DPO-25-162220 / Reviewed: 26-Mar-2025 / QC No. DPO-25-162220 / Revised: 02-Apr-2025 / Manuscript No. DPO-25-162220 / Accepted Date: 09-Apr-2025 / Published Date: 09-Apr-2025
Abstract
Atherosclerotic cardiovascular disease, a leading cause of global mortality and morbidity, is driven by chronic inflammation and metabolic dysregulation. The inhibition of monocyte-macrophage inflammatory responses is a critical therapeutic strategy, as these cells play a central role in atherosclerosis progression. Glycolysis, a universal metabolic pathway, is regulated by the bifunctional enzyme PFKFB3, which modulates intracellular levels of fructose‑ 2,6‑bisphosphate (Fru‑2,6‑BP). Despite the potential of PFKFB3 inhibitors like 3PO in atherosclerosis treatment, their mechanisms and therapeutic efficacy remain incompletely understood. This study investigates the role of PFKFB3 inhibition in atherosclerosis using ApoE^‑/‑^ mice, highlighting its impact on monocyte‑macrophage responses and plaque formation. Our findings suggest that 3PO-mediated PFKFB3 inhibition attenuates atherosclerosis by reducing inflammatory markers and vascular remodeling, offering a promising therapeutic avenue.
Keywords: PFKFB3; 3PO; Coronary atherosclerosis; Glycolysis; Monocyte-macrophage; Inflammatory response
Introduction
Atherosclerosis, a major contributor to global cardiovascular morbidity and mortality, is characterized by chronic inflammation and lipid accumulation within arterial walls [1]. Monocyte-derived macrophages are pivotal in this process, driving plaque formation through inflammatory cytokine release and foam cell generation [2-4]. All organisms must go through the identical stage of glycolysis. Macrophages are packed in atherosclerotic arteries and perform an essential function in glycolysis. Glycolysis, a key metabolic pathway, is upregulated in atherosclerotic lesions, with PFKFB3 emerging as a critical regulator of glycolytic flux [5]. PFKFB3, a member of the PFKFB family, enhances glycolysis by increasing intracellular Fru-2,6-BP levels, thereby promoting cellular proliferation and survival [6]. Smallmolecule inhibitors of PFKFB3, such as 3PO, have shown promise in preclinical models of cancer and cardiovascular diseases [7,15,16]. However, their role in atherosclerosis remains underexplored. This study aims to elucidate the therapeutic potential of 3PO in atherosclerosis by examining its effects on monocyte-macrophage responses, plaque formation and vascular remodelling in ApoE^-/-^ mice.
Materials and Methods
Mice
Male ApoE^-/-^ mice (6-8 weeks old) were obtained from Collective Pharmacoko, China. Mice were randomized into three groups: 1) 3POtreated; 2) high-cholesterol diet (HCD) model and 3) control (normal diet). A blank control group of C57BL/6 mice was also included. The 3PO group received intravenous injections of 3PO (35 mg/kg, twice weekly) for 16 weeks. Mice were housed in a Specific Pathogen-Free (SPF) facility and all procedures were approved by the Institutional Animal Care and Use Committee of Lanzhou University. During this duration, the bedding was changed and the feed and drinking water were replenished every 4-5 days. At the end of the 16-week period, the mice were anesthetized with tribromoethanol, which is tertiary amyl alcohol in 100% master mix which was diluted with salinel 1.25%. Mice were anesthetized at 16 weeks by Tribromoethanol (tertiary amyl alcohol 100% mother solution, Salinel dilution 1.25%) was applied to produce deep sensory unresponsiveness. The mice's aorta was removed from the carotid to the iliac arteries under an electron microscope for the purpose to collect heart tissue, which then was preserved in 4% PFA fixative for further investigation. The eyeballs were removed to obtain plasma and centrifuged to obtain the upper layer of serum and stored at -80°C.
Creations
Aortic tissues were harvested for histological staining (Oil Red O, H&E and Masson’s trichrome) and Immunohistochemistry (IHC). Serum lipid profiles (LDL-C, HDL-C, TG and TC) and inflammatory markers (MCP-1, FKN and oxLDL) were quantified using ELISA. Western blotting was performed to assess PFKFB3 expression.
Mice echocardiography
ApoE-/-Mice were anesthetized with the drug before the examination; the anesthetic flow rate was adjusted to 2-3L/min based on the mice's weight. Placed on a test plate, were given inhalation anesthesia raised and then their pulses (between 300 and 370 bpm) noted. The mice were mounted with their limbs pointing upward on the the belly. The front of the mouse's chest and neck were hairless. Then, the 30MHZ probe was picked, coated with the correct amount of couplant and placed on the left side of the mouse's chest. The aortic and descending aortic blood flow was tracked as the probe's position and angle were changed. The chosen 30 MHZ probe was positioned with the correct quantity of coupler on the left side of the mouse chest. The probe's position and angle were then altered to observe the aorta, head and arm trunks, internal and external neck diameters of the left and right, the aortic thickness and blood circulation in the ascending and descending aorta.
Analysis of serum lipides
The eyeballs of the mice were removed when they showed no reaction to deep sensory stimulation after being with tribromoethanol. After extracting approximately 0.75 ml of whole blood and keeping at room temperature for night, the serum was separated by sedimentation for 5 minutes at 4°C 3000 × rpm. Applying a kit from Enzyme-Linked Bio, the amounts of LDL-C, HDL-C, TG and TC were calculated.
Enzyme-Linked Immunosorbent Assay (ELISA)
Mouse serum has been examined for levels of Fractalkine (FKN), Monocyte Chemokine (MCP-1) and mouse oxLDL through enzymelinked immunosorbent assay (ELISA). The ELISA kits, self-prepared distilled water, spiking apparatus (5ul, 10ul, 50ul, 100 ul, 200ul, 500ul, 1000 ul), an oscillator and a magnetic stirrer were amongst the kits which were deployed. They were obtained from Enzyme-Linked Bio (China). As it is stated in the usage procedure of the kit.
Coloring with Hematoxylin-Eosin (H&E)
Frozen sections were removed from the -20°C refrigerator and brought to room temperature to prepare for staining. After 15 minutes for tissue fixative the application, they were rinsed under running water. Following soaking for three to five minutes in hematoxylin staining solution, the sections were rinsed with running water, divided using differentiation solution and then returned to blue utilizing return to blue solution. Sections were dehydrated in 85% and 95% alcohol for five minutes each, followed by five minutes of staining with eosin. They were then sequentially put into anhydrous ethanol I, II and III for five minutes each, xylene I and II for five minutes each, and stored with labels written on the neutral gum sealer. Image acquisition was analyzed by microscopic examination under a microscope.
Red O coloring in oil
The sections were dipped in an oil-red staining solution and stained with an oil-red O staining solution. The slices spent eight to ten minutes submerged in the oil-red dye solution. Remove the slices, stay for 3 seconds and then soak them in two tanks of 60% isopropyl alcohol each for 3 and 5 seconds, then into two tanks of pure water for 10 seconds each. The slices are removed and left for three seconds and finally submerged in hematoxylin for a further three to five minutes. subsequently that, they are placed in each of three tanks of pure water for washing for five, ten and thirty seconds each and then in two tanks of differentiation solution for two to eight seconds, then in two tanks of distilled water for 10 seconds, in two tanks of blue solution for a second and lastly, gently, in two tanks of tap water for washing. Container of tap water.
Masson
Sections fixed with tissue fixative for 15 minutes and washed with running water. After spending the night submerged in Masson A, the sections were washed under flowing water. After submerging sections for a minute in an equal mixture of Masson B and Masson C staining solutions, they were rinsed with tap water, allowed to differentiate for a few seconds in the differentiation solution and then again with tap water. Sections were dyed for six minutes in Masson D, rinsed with tap water and then colored for one minute in Masson E. After that, they were dyed directly in Masson F for two to thirty seconds without being rinsed or drained, rinsed in 1% acetic acid for differentiation and dried in two anhydrous ethanol tanks. Sections were placed in the third vat of anhydrous ethanol for 5 minutes, xylene for 5 minutes and sealed with clear neutral gum. Images were acquired and analyzed under microscopic examination.
Immunohistochemistry (IHC)
Frozen sections were air-dried at room temperature, baked at 37℃ for 10-20 minutes, fixed with alcohol for 20 minutes and washing in PBS (PH7.4) on a decolorizing shaker for 3 times, each taking 5 minutes. After antigen repair, slides were let gradually cool before being placed in PBS (PH7.4) on a decolorizing shaker and shaken three times for five minutes each. Sections were placed in a solution comprising 3% hydrogen peroxide, then left at room temperature for 25 minutes and sheltered from light. Incubate the sections in a 3% hydrogen peroxide solution at room temperature for 25 minutes, then wash with PBS (PH7.4) and shake on a decolorizing shaking table three times, each for 5 minutes. After the sections' drying out, using a histochemistry pen to draw a circle around the tissues. Then, carefully cover the tissues with a drop of 3% BSA inside the circle and leave them remain locked for 30 minutes at room temperature. Remove the sealing solution, put the sections flat on the damp box, and incubate them at 4°C for the whole night. Drops of freshly prepared DAB color development solution were added and the color development time was recorded under a microscope. Rinsing that area with tap water prevented the color development for the positive shade, which was brownish yellow. Then re-staining for approximately three minutes, washing with tap water, separating the hematoxylin solution for a brief period of time, cleaning with tap water, mixing the hematoxylin back to the blue solution and finally washing with faucet water. For dehydration and transparency, the sections were continually placed in xylene for 15 minutes, 85% alcohol for 5 minutes, anhydrous ethanol for 15 minutes, anhydrous ethanol II for 5 minutes, n-butanol for 5 minutes and 75% alcohol for 15 minutes. After the sections had been in xylene for a while, they were removed to enable it to dry barely.
Oil red
The aorta was obtained with forceps in 4% PFA fixative to remove the fatty tissue around the vessels and the vessels had been submerged in the fixative for more than 24 hours. After taking the tissues out of the fixative and giving them two PBS immersion washes, the vessels were gently split longitudinally along their walls with dissecting scissors. For five seconds, the split blood arteries were gently washed with tap water. The blood vessels were extracted using forceps and immersed in 60% isopropyl alcohol for differentiation. The differentiation process took one minute to reach the point where the fatty plaques in the lumen were bright red or orange and the other parts of the lumen were almost colorless. The differentiation process was then stopped by washing with distilled water. The blood vessels were first immersed in 60% isopropyl alcohol 3S and then in oil red O staining solution for 60 minutes at 37°C protected from light. After removing the blood vessels and blotting the extra water with filter paper, a slide was taken and put on a backdrop plate that was either black or white with a scale; the blood vessels were spread out on the slide; a well-lit place was chosen; and the focus and exposure of the camera were adjusted to take pictures.
Western blot protein immunoblotting
tube, putting ten times the volume of tissue lysate and cutting the temperature to -8°C for more than ten minutes before removing it. Finally, place the crushed tissue in an ultrasonic cell crusher and set a homogenization program. Once the centrifugal tubes have been removed and the homogenized tissue has been homogenized, place the tissue on ice lysate for thirty minutes. Following the homogenization procedure, the centrifuge tube was taken out of the machine and left on ice for 30 minutes, shaking every five minutes to guarantee that all of the tissue had been lysed. After calculating the protein concentration, add 5* Reduced Protein Sampling Buffer to the protein solution at a 4:1 ratio, denature in a boiling water bath for 15 minutes and store in the refrigerator at -20°C for later use. SDS-PAGE In electrophoresis, the glass plate are cleaned and then dried before creating a pair with a flat glass plate and a piece of flat glass into a glue maker. The glass plate should then be fixed with a slanting insert plate; ensure that the bottom of the plate is aligned to prevent leaks; and formulate the gel in accordance with the instructions. Prevent glue leaks by making concentrated glue per the instructions in the handbook and promptly filled the container. Fill the remaining gap with concentrated glue, then add a 1.0-mm comb. Look intently at the comb underneath; air bubbles should not be visible.
Following the concentrated gel becomes solid for 15 minutes, bring out the glue maker and carefully remove the comb. Add enough electrophoresis liquid to the electrophoresis sample after placing the glue maker into the electrophoresis tank. When the bromophenol blue was about 1 cm from the bottom, the electrophoresis could be paused and the membrane transfer could begin. The electrophoresis was run for about 30 minutes at a constant pressure of 80 volts. To transfer the membrane, prepare 4 pieces of 7 × 9cm filter paper and a mediumsized PVDF (0.45um) membrane. Activate the PVDF membrane with methanol for 2 minutes before use. Put the clip for transferring the membrane, two sponges, two layers of filter paper and the activated PVDF membrane into the container with the transferring liquid. Open the clip with the left side in red and the right side in black and add a piece of sponge and two layers of filter paper on each. Place two layers of filter paper and a sponge on each side; the right side is black. Peel off the adhesive between the air bubbles, the adhesive on the filter paper and the PVDF membrane on the adhesive using caution.
Cover the membrane with a pair of filter paper and remove any air bubbles. At last, add another sponge layer; transfer film situations: The transfer process will be bathed in ice water to cool down in the transfer equipment for 90 minutes at a continuous current of 300 mA. Place the transferred membrane into the TBST-equipped incubation tank, shabu-shabu once quickly, add the milk sealing solution, placed it on the decolorizing shaker and let it sit for 60 minutes at room temperature. After that, dilution of the primary antibody should be done in compliance with the antibody's instructions and then the antibody should be established. Remove the sealing solution from the incubation tank, shake the shaking bed gently for one minute, add the configured primary antibody and then incubate at 4°C for an entire night.
After that, recycle the primary antibody. Add TBST and quickly elute three times, each for five minutes, on the decolorizing shaker. After dilution to a ratio of 1:5000 with TBST, add the secondary antibody to the incubation tank, place it on a shaker for gentle shaking and allow it to sit at room temperature for half an hour. Shabu-shabu the membrane three times quickly with TBST, place it on the decolorizing shaker for quick elution for five minutes each time and then wash it three times. Chemiluminescence: Combine ECLA with liquid B at a 1:1 ratio. Get rid of the eluted PVDF membrane on absorbent paper; Put the membrane on the chemiluminescence instrument's shelf, slightly absorb the liquid on top of it, add the mixed ECL glowing liquid, allow the liquid to completely submerge the membrane, then wait a minute for the reaction to begin. Use paper that absorbed; Put the additional liquid into the chemiluminescence devices after soaking it from the membrane's surface. Begin the chemiluminescence in line with the specified schedule; save the original image in TIF format after the lighting process is finished. Save the original image in TIF type after the exposure.
Analysis data
Data are presented as mean ± standard deviation. One-way ANOVA followed by post-hoc tests was used for group comparisons. Statistical significance was set at *p<0.05.
Results
A diet that contains cholesterol enhances lipid metabolism
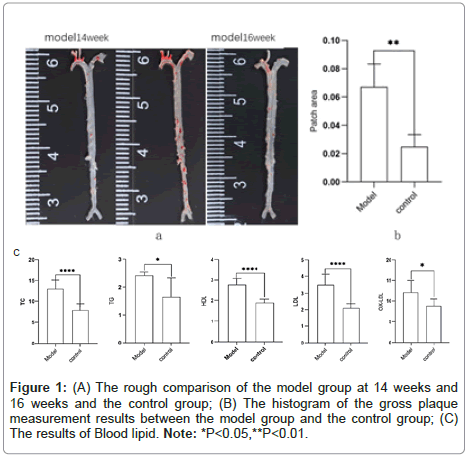
Apo-E, known as Apolipoprotein E, is a protein related with lipid particles that transfers chyme particles. It exists in very Low-Density Lipoproteins Cholesterol (LDL-C) and High-Density Lipoproteins Cholesterol (HDL-C) and plays a role in cholesterol transport. The binding of hepatic and peripheral cell receptors accounts for its selectivity [8]. According to studies, lipogranules start to form after 14 weeks of feeding and ApoE-/-mice spontaneously acquire hypercholesterolemia. Under normal dietary conditions, substantial atherosclerotic plaque lesions take 20–24 weeks to develop [9]. Increased plasma cholesterol levels and faster vascular lesion process were the results of a high-fat/ high-cholesterol diet. The aortic root, aortic arch, innominate arteries, aortic branches and renal artery bifurcations are the primary areas affected in these animals. Studies on human atherosclerosis frequently use ApoE-/-mice [10]. After 16 weeks of feeding, ApoE-/-mice compared with the model which on a high-cholesterol diet showed significantly greater blood atherosclerosis-related lipid indicators of triglycerides, cholesterol, LDL, and oxidized LDL, and the opposite levels of HDL than the control group (Figure 1).
The consequence of glycolysis through PFBFB3 on monocyte macrophage responses
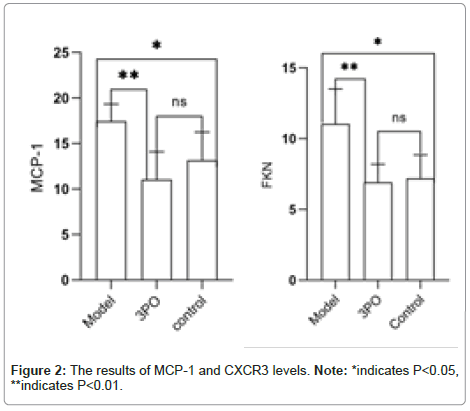
Under normal applications glycolysis is the main method of energy acquisition for endothelial cells and also the main supply of energy for their movement and proliferation. Even while at rest, normal endothelium cells are extremely glycolytic. Lipoprotein A is exposed to high-fat and inflammatory stimuli and endothelial cells limit the amounts of glycolysis by stimulating PFKFB3, which accelerates cellular metabolism and increases the beginning of inflammation [11]. PFKFB3, an essential glycolysis enzyme, is involved in the development of an array of vascular diseases, such pulmonary artery hypertension and atherosclerosis [12-15]. Monocytes undergo differentiation into macrophages that are traditionally activated in responses to inflammatory stimuli and these types of cells display increased amounts of glycolysis caused by PFKFB3. The first human C-family chemokine to be discovered is CCL2, frequently referred to as Monocyte Chemoattractant Protein-1 (MCP-1) [16]. The main sources of CCL2 are macrophages and the monocytes [17]. FKN, known as the chemokine receptor CXCR3, is a marker for activation of helper T-lymphocyte type 1 lymphocytes, which are the main T-lymphocyte type detected in atherosclerosis [19] and are related to inflammatory responses. MCP- 1 plays an intermediary role in macrophage-induced inflammation [18]. 3PO treatment significantly reduced serum levels of MCP-1 and FKN, markers of monocyte-macrophage activation, compared to the HCD model group, this suggests that PFKFB3 inhibition suppresses inflammatory responses in atherosclerosis (Figure 2) (Supplementary Figure 1 and Table 1).
Reduction of vessel thickness and inhibition of arterial plaque formation by 3PO-induced PFKFB3 expression
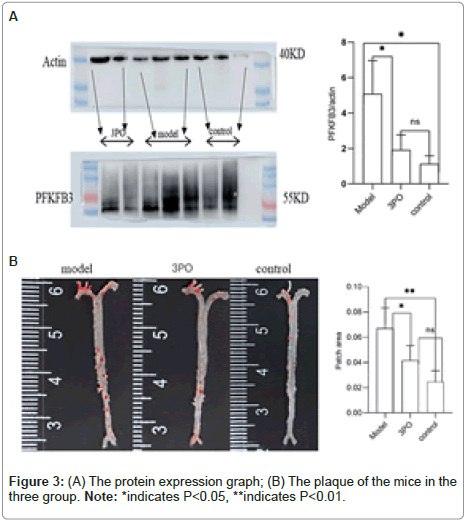
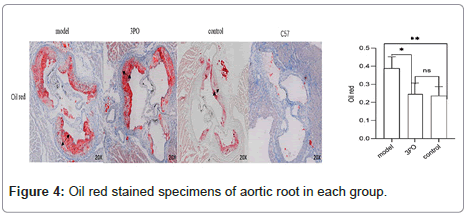
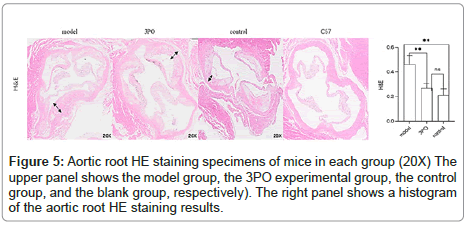
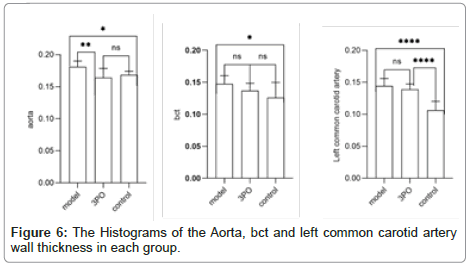
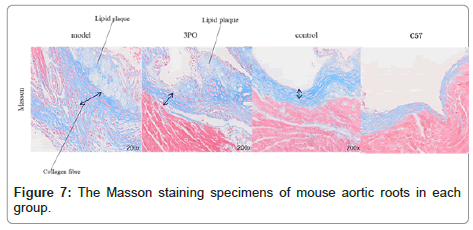
In order to clarify the inhibitory effect of 3PO on PFKFB3, ApoE-/-mice received a week of adaptation to a high-cholesterol diet. Continuing that, the 3PO group got subcutaneous injections of 3PO three times per day. After 14 weeks, PFKFB3 expression levels in the tissues of ApoE-/-mice were assessed, as it was found that 3PO repressed PFKFB3 expression; the experimental group's PFKFB3 expression was even lower than the control group's (Figure 3a). Meantime, PFKFB3 repression by 3PO extensively lowered the degree of arterial plaque formation, due to an analysis of the oil-red staining results of mouse macrosomes (Figures 3A and 3B). While comparing the aortic root's oil red plaque area and HE staining, the experimental group showed a statistically important reduction in comparison with the model group (Figure 4) Histological analysis revealed that 3PO treatment decreased plaque area and vascular collagen deposition. Aggravation of atherosclerotic lesions thicken the arterial wall, which causes regional oxygen exchange limitation. Glycolysis levels increase in hypoxic environments and trigger an inflammatory response, which exacerbates atherosclerosis [20]. Using cardiac ultrasound in mice, ascending aortic blood flow and vascular thickness of the ascending aorta, head and arm trunks, left common carotid artery and right common carotid artery were assessed (Figure 5). The amount of vascular collagen deposition was assessed using Masson staining and there were significant differences between the experimental, model and control groups (Figure 6). The results demonstrated that 3PO reduced intravascular collagen deposition levels and vascular thickness in atherosclerotic arteries while enhancing heart function.
3PO stimulates the production of associated chemokine and monocytes
We confirmed in the previously mentioned trials that PFKFB3 promotes monocyte-macrophage responses and enhances the inflammatory response that impacts atherosclerosis. Furthermore, we assessed the expression of CD68 in the aortic root to further confirm that 3PO declines this process. The expression level of CD68, a highly glycosylated glycoprotein that only exists in monocytes and macrophages, can be utilized to measure the extent of the change from monocyte to macrophage. The degree of monocyte-macrophage transition is confirmed by the expression of CD68, which is an instance of macrophage expression. CD68 is highly increased in responses to oxLDL-C stimulation [21]. High levels of plaque have been correlated with high levels of CD68 expression in mice, while there was a lack of statistical significance in the significant association among CD68 and oxidized low-density lipoprotein expression (Figure 7). In addition, we evaluated the levels of FKN and MCP-1 in the control group with the experimental group that received 3PO (Figure 2 shows the results of MCP-1 and CXCR3 levels). It is evident that 3PO significantly decreased the inflammatory response attributed to monocyte macrophages.
Discussion
The accumulation of minimally oxLDL is the initial event of atherosclerosis, which causes the production of multiple inflammatory chemicals by the overlying endocrine secretions and the products of minimally oxLDL also hinders nitric oxide the development [22]. Chemical mediator nitric oxide has several anti-atherosclerotic effects. Because of their higher blood pressure, mice lacking endothelial NO synthase show increased atherosclerosis [23]. We observed that in mice, the lipid metabolism connected with atherosclerosis and hypercholesterolemia were associated with each other. After 16 weeks of high-cholesterol diet, the impact of 3PO on atherosclerosis in mice was investigated. Over all, the findings shown that 3PO's inhibition of PFKFB3 decreased monocyte-transformed macrophage reflection and associated inflammatory markers; however, it had different impacts on the arterial vascular wall and circulating lipids and eventually inhibited atherosclerosis from spreading.
Monocyte macrophages plays a crucial role in the genesis and progression of atherosclerosis [24-26]. Hypercholesterolemic stimulation causes atherosclerosis-associated monocytes to differentiate into classically activated macrophages. Macrophages are the main leukocytes during plaque inflammation and development; macrophages phagocytose lipids within the plaque while further producing cytokines and chemokines, transforming into foam cells to form a large number of lipid accumulations that necrose to form fatty streaks. In the later stage, foam cells accumulate in the blood vessel sub endothelium, causing an immense amount of lipid buildup and necrosis to form fat lines. Under an electron microscope, atheromatous plaques are noticeable caused by the proliferation and migration of smooth muscle cells and the synthesis of fiber-encapsulated lipid nuclei by extracellular matrix proliferation. Similar to human Th1 and Th2 CD4T cells, Mills (2000) identified the M1 and M2 kinds of macrophages in Balb/c and C57BL/6 mice [27] and called the macrophages M1 and M2 phenotypes. According to Italiani's research, M2 macrophages stimulate tissue regeneration and cell proliferation while M1 macrophages have the proinflammatory capacity to eliminate infections [28]. Widely expressed in human tissues, PFKFB3 is a critically essential glycolysis-regulating enzyme that has been shown to impact the pathogenic activity of a range of tissues. Recent studies have shown that PFKFB3 expression is significantly enhanced in animal models of several diseases, such as acute kidney injury, acute lung injury and sepsis and the underlying mechanisms have been investigated [29- 32]. Furthermore, PFKFB3 demonstrated significant results in healing a variety of human carcinomas, rheumatoid arthritis, pulmonary fibrosis and fibrosis after liver injury [34-39]. Additionally, in corresponding breakthrough examinations [24,25,33,34,40], PFKFB3 has also been toured in the prevention of long-term heart failure during myocardial infarction, improvement of cardiac function following acute myocardial infarction and suppression of the progression of atherosclerosis. In the study by Tillie et al., the effect of plaque size and composition on the total plaque burden in mice in both advanced and early lesions [34]. In this work, we demonstrate that PFKFB3 expression influences the degree of plaque formation and increases dramatically during the development of atherosclerosis. Research indicates that PFKFB3 plays an essential part in the initial phases of atherosclerosis development. Likewise, it impacts the inflammatory response of atherosclerosisassociated monocyte macrophages, which might persist into the later stages of plaque formation.
3PO, an effective inhibitor of PFKFB3, has been identified in a wide range of PFKFB3-related cancer research [34,35]. Furthermore, chemotherapy treating primary and metastatic tumors is enhanced when PFKFB3 is blocked by 3PO. When combined with existing anticancer medications, the administration of 3PO and other PFKFB3 inhibitors has proven synergistic benefits [36,37]. Mejias, et al., discovered that 3PO markedly reduced the degree of fibrosis following hepatic damage and the activation of hepatic stellate cells [38]; Zuo et al. noted that PFKFB is a possible target for the clinical treatment of RA. The safe inhibition or overexpression of glycolysis rate-limiting enzymes has the potential to lead to the development of new antirheumatic therapies [39]. Yang et al. claimed that 3PO treatment may prevent long-term heart failure following myocardial infarction [40]. Han noticed that 3PO-induced PFKFB3 expression inhibition improves cardiac function and the degree of fibrosis in mice adhering to acute myocardial infarction [24]. Paola P, et al., proposed that 3PO inhibits coronary artery plaque formation, resulting in an overall improvement in cardiac function [25]. However, there areStill up for debate, though, is whether 3PO actually slows down the progression of plaque and atherosclerosis [15,25,33]. Thereby, ApoE-/-mice were acclimated to a high-cholesterol diet for one week in order to better understand the impact of 3PO inhibition of PFKFB3 on the development of atherosclerosis and plaque formation. The experimental group then received intraperitoneal injections of 3PO at three-day intervals. After 16 weeks, the mice in the 3PO and HTD groups had serum levels of triglycerides, cholesterol, LDL-C and oxLDL which were significantly lower in the 3PO group than in the model group, with the opposite levels of HDL-C. There were significant differences between the two groups in the oil-red staining of macros and the oil-red and him staining of plaque areas in the aortic root. Thoracic aortic tissues have been utilized to extract proteins and PFKFB3 expression levels were established. The level of expression of PFKFB3, determined using protein obtained from thoracic aortic tissue, was shown to be considerably lower in the 3PO group comparing to the model group. Based to the previous findings, 3PO reduced the production of linked inflammatory indicators and the inflammatory response of monocyte macrophages, hence slowing the progression of atherosclerosis and the growth of plaque. Hypoxia improves the glycolytic travel in macrophages, as demonstrated by Tawakol A, et al., [46]. Its pro-inflammatory component is upregulated by PFKFB3. oxLDL stimulation dramatically boosts the expression of CD68, a highly glycosylated glycoprotein that is exclusively expressed in monocytes and macrophages [47]. For the purpose of immunostaining monocytes and macrophages in inflammatory tissues, tumor tissues and other immunohistopathological applications, CD68 has been extensively employed as a useful cytochemical marker [48]. As a myeloid-specific surface marker, CD68 is widely expressed, especially in macrophages, as reported by Betjes, et al., [50]. Chemokines and their receptors play an important role in the development of atherosclerotic lesions and drive proatherogenic leukocyte responses. Chemokines and their receptors play a critical role in the formation of atherosclerotic lesions, driving proatherogenic leukocyte reactions. Chemokines are proteins that mediate angiogenesis, hematopoiesis and leukocyte degranulation and cell motility. Their molecular weights are between 8 to 12 kDa, Specifically, PFKFB3 promotes monocyte-macrophage activation by enhancing glycolytic flux, which fuels inflammatory cytokine production and foam cell formation [16]. The main sources of CCL2 are macrophages and monocytes [17]. MCP-1 facilitates the response of inflammation triggered by macrophages [18], Monocyte chemoattractant protein-1 (MCP-1) is one of the secreted factors that draws immune cells to adipose tissue; studies have demonstrated that MCP-1 is necessary for macrophage infiltration of adipose tissue. In addition, irregular chemokines are independent factors that have a negative correlation with the left ventricle ejection fraction (LVEF) in hypertensive patients. A negative connection was discovered. A study performed in 2003 clearly showed that transferring bone marrow cells lacking in CCL2 significantly decreased atherosclerotic lesions [19].
The majority of acute cardiovascular events have been brought on by occlusive thrombosis, which can be brought on by atherosclerotic plaque destruction or rupture. Localized restrictions on oxygen exchange result from the thickness of the arterial wall resulting from the development of atherosclerotic plaques. More severe vascular lesions are caused by vascular cells in response to hypoxic situations [20]. The frequency of acute coronary events caused by thrombus is primarily determined by the composition and sensitivity of the plaque, rather by the degree of stenosis. Neovascularization and calcification, which are common features of advanced lesions, could potentially have an effect on the durability of atherosclerotic lesions. However, several limitations should be noted. First, the long-term effects of 3PO on plaque stability and cardiovascular outcomes remain to be investigated. Second, the translational potential of PFKFB3 inhibitors in human atherosclerosis requires further validation. Future studies should explore the efficacy of 3PO in combination with existing therapies, such as statins, to determine synergistic effects [33].
Conclusion
Overall, this study underscores the potential of targeting PFKFB3 as a therapeutic strategy to manage atherosclerosis and other inflammatory vascular diseases. By reducing monocyte-macrophage responses and limiting plaque formation, PFKFB3 inhibition may offer a promising approach to mitigate the progression of atherosclerosis and improve vascular health.
Conflict of Interests
The authors declare that they have no conflict of interest. All data and materials available and original source
Ethics Disclaimer
This animal study was reviewed and approved by the regulatory body of the Animal Center of the First Clinical Medical College of Lanzhou University.
Funding
The research fund was provided by Gansu Provincial Health Research Program and Lan Yu personally.
References
- Summary of the China Cardiovascular Health and Disease Report (2021). Cardiovascular Disease Prevention and Control 22:20-36.
- Xu S, Kamato D, Little PJ, Nakagawa S, Pelisek J, et al. (2019) Targeting epigenetics and non-coding RNAs in atherosclerosis: From mechanisms to therapeutics. Pharmacol Ther 196:15-43.
[Crossref] [Google Scholar] [PubMed]
- Hongtao W, Liuyi MA, Hongli LIU, Hongrong LI (2015) Monocytes and macrophages undergo behavioral alterations during the atherosclerotic process. J Atherosclerosis 23:1291-1296.
- Blagov AV, Markin AM, Bogatyreva AI, Tolstik TV, Sukhorukov VN, et al. (2023) The role of macrophages in the pathogenesis of atherosclerosis. Cells 12:522-522.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, Qu C, Liu T, Wang C (2020) PFKFB3 inhibitors as potential anticancer agents: mechanisms of action, current developments, and structure-activity relationships. Eur J Med Chem 203:112612.
[Crossref] [Google Scholar] [PubMed]
- Zaiyong Z, Jun C, Weilan W (2021) Impact of 3PO, a small molecule inhibitor of PFKFB3, on neovascularization in HUVEC stimulated by inflammation. J Card Rehab Med 30:12-28.
- Guo S, Li A, Fu X, Li Z, Cao K, et al. (2022) Gene-dosage effect of PFKFB3 on monocyte/macrophage biology in atherosclerosis. Br J Pharmacol 179:4974-4991.
[Crossref] [Google Scholar] [PubMed]
- Liu K, Chen B, Zeng F, Wang G, Wu X, et al. (2022) ApoE/NOS3 knockout mice as a novel cardiovascular disease model of hypertension and atherosclerosis. Genes (Basel) 13:1998.
[Crossref] [Google Scholar] [PubMed]
- Huanhuan W, Lishi Z, Xiaoran Z, Song J, Guo Q, et al. (2022) Prediction model for different progressions of Atherosclerosis in ApoE-/- mice based on lipidomics. J Pharm Biomed Anal 214:114734.
[Crossref] [Google Scholar] [PubMed]
- Hui C, Le-Min Z, Yansong G, Xinchun Y, Xinli L, et al. (2022) Expert consensus on common experimental models of cardiovascular illness in rats and mice. Chinese J Hypertens 30: 710-719.
[Crossref]
- Bierhansl L, Conradi LC, Treps L, Dewerchin M, Carmeliet P (2017) Central role of metabolism in endothelial cell function and vascular disease. Physiology 32:126-140.
[Crossref] [Google Scholar] [PubMed]
- Yapeng C, Xiaoyu Z, Lina W, Yang Q, Ma Q, et al. (2019) PFKFB3-mediated endothelial glycolysis promot es pulmonary hypertension. Proc Natl Acad Sci U S A 116:13394-13403.
[Crossref] [Google Scholar] [PubMed]
- Shuai G, Anqi L, Xiaodong F, Li Z, Cao K, et al. (2022) Gene-dosage effect of Pfkfb3 on monocyte/mac rophage biology in atherosclerosis. Br J Pharmacol 179:4974-4991.
[Crossref] [Google Scholar] [PubMed]
- Biruk K, Rahul K, Claudia M, Sandres L, Vohwinkel C, et al. (2021) Endothelial cell PHD2-HIF1α-PFKFB3 contributes to right ventricle vascular adaptation in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol 321: L675-L685.
[Crossref] [Google Scholar] [PubMed]
- Perrotta P, de Vires MR, Peeters B, Guns PJ, De Meyer GRY, et al. (2021) PFKFB3 gene deletion in endothelial cells inhibits intraplaque angiogenesis and lesion formation in a murine model of venous bypass grafting. Angiogenesis 25:1-15.
[Crossref] [Google Scholar] [PubMed]
- Cochran BH, Reffel AC, Stiles CD (1983) Molecular cloning of gene sequences regulated by platelet-derived growth factor. Cell 33:939-947.
[Crossref] [Google Scholar] [PubMed]
- Yoshimura T, Yuhki N, Moore SK, Appella E, Lerman MI, et al. (1989) Human monocyte chemoattractant protein-1 (MCP-1) full-length cDNA cloning, expression in mitogen-stimulated blood mononuclear leukocytes, and sequence similarity to mouse competence gene JE. FEBS Lett 244:487-493.
[Crossref] [Google Scholar] [PubMed]
- Gálvez I, Hinchado MD, Martín-Cordero L, Morán-Plata FJ, Graham G, et al. (2023) The anti-inflammatory and bioregulatory effects of habitual exercise in high-fat diet-induced obesity involve crown-like structures and MCP-1 in white adipose tissue. Exerc Immunol Rev 29:111-120.
[Google Scholar] [PubMed]
- Guo J, Van Eck M, Twisk J, Maeda N, Benson GM, et al. (2003) Transplantation of monocyte CC-chemokine receptor 2-deficient bone marrow into ApoE3-Leiden mice inhibits atherogenesis. Arterioscler Thromb Vasc Biol 23:447-453.
[Crossref] [Google Scholar] [PubMed]
- Jain RK (2014) Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell 26:605-622.
[Crossref] [Google Scholar] [PubMed]
- Duan L, Zhao Y, Jia J, Chao T, Wang H, et al. (2023) Myeloid-restricted CD68 deficiency attenuates atherosclerosis via inhibition of ROS- MAPK-apoptosis axis. Biochim Biophys Acta Mol Basis Dis 1869:166698.
[Crossref] [Google Scholar] [PubMed]
- Knowles JW, Reddick RL, Jennette JC, Shesely EG, Smithies O, et al. (2000) Enhanced atherosclerosis and kidney dysfunction in eNOS(-/-) Apoe(-/-) mice are ameliorated by enalapril treatment. J Clin Invest 105:451-458.
[Crossref] [Google Scholar] [PubMed]
- Han B (2022) Myocardial fibrosis: The function and mechanism of PFKFB3-mediated glycolysis. University of Lanzhou.
- Paola P, Bieke VDV, Pieter VDV, et al. (2020) Partial inhibition of glycolysis reduces atherogenesis independent of intraplaque neovascularization in mice. Arterioscler Thromb Vasc Biol 40:1168-1181.
[Crossref] [Google Scholar] [PubMed]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM (2000) M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 164:6166-6173.
[Crossref] [Google Scholar] [PubMed]
- Lusis AJ (2000) Atherosclerosis. Nature 407:233-241.
- Italiani P, Boraschi D (2014) From monocytes to M1/M2 macrophages: Phenotypical vs. Functional differentiation. Front Immunol 5:514.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, Liu Y, Xie Z, Liu Q, Zhuang Y, et al. (2022) Inhibition of PFKFB preserves intestinal barrier function in sepsis by inhibiting NLRP3/GSDMD. Oxid Med Cell Longev 2022:8704016.
- Sun D, Chen S, Li S, Wang N, Zhang S, et al. (2023) Enhancement of glycolysis-dependent DNA repair regulated by FOXO1 knockdown via PFKFB3 attenuates hyperglycemia-induced endothelial oxidative stress injury. Redox Biol 59:102589.
[Crossref] [Google Scholar] [PubMed]
- Wen L, Wei Q, Livingston MJ, Dong G, Li S, et al. (2023) PFKFB3 mediates tubular cell death in cisplatin nephrotoxicity by activating CDK4. Transl Res 253:31-40.
[Crossref] [Google Scholar] [PubMed]
- Vohwinkel CU, Burns N, Coit E, Yuan X, Vladar EK, et al. (2022) HIF1A-dependent induction of alveolar epithelial PFKFB3 dampens acute lung injury. JCI Insight 7:e157855.
[Crossref] [Google Scholar] [PubMed]
- Poels K, Schnitzler JG, Waissi F, Levels JHM, Stroes ESG, et al. (2020) Inhibition of PFKFB3 Hampers the Progression of Atherosclerosis and Promotes Plaque Stability. Front Cell Dev Biol 8:581641.
[Crossref] [Google Scholar] [PubMed]
- Tillie RJHA, De Bruijn J, Perales-Patón J, Temmerman L, Ghosheh Y, et al. (2021) Partial inhibition of the 6-Phosphofructo-2-Kinase/Fructose-2,6-Bisphosphatase-3 (PFKFB3) enzyme in myeloid cells does not affect atherosclerosis. Front Cell Dev Biol 9:695684.
[Crossref] [Google Scholar] [PubMed]
- Cantelmo AR, Conradi LC, Brajic A, Goveia J, Kalucka J, et al. (2016) Inhibition of the glycolytic activator PFKFB3 in endothelium induces tumor vessel normalization, impairs metastasis, and improves chemotherapy. Cancer Cell 30:968-985.
[Crossref] [Google Scholar] [PubMed]
- Krzysztof K, Rosik J, Machaj F, Supplitt S, Wiczew D, et al. (2021) Role of PFKFB3 and PFKFB4 in cancer: Genetic basis, impact on disease development/progression, and potential as therapeutic targets. Cancers 13:909-909.
[Crossref] [Google Scholar] [PubMed]
- Xiaoting H, Qiaoyi X, Hanxi W, et al. (2020) PI3K-Akt-mTOR/PFKFB3 pathway mediated lung fibroblast aerobic glycolysis and collagen synthesis in lipopolysaccharide-induced pulmonary fibrosis. Lab Invest 100:801-811 .
[Crossref] [Google Scholar] [PubMed]
- Mejias M, Gallego J, Naranjo-Suarez S, et al. (2020) CPEB4 increases expression of PFKFB3 to induce glycolysis and activate mouse and human hepatic stellate cells, promoting liver fibrosis. Gastroenterology 159:273-288.
[Crossref] [Google Scholar] [PubMed]
- Zuo J, Tang J, Lu M, Zhou Z, Li Y, et al. (2021) Glycolysis rate-limiting enzymes: Novel potential regulators of rheumatoid arthritis pathogenesis. Front Immunol 12:779787.
[Crossref] [Google Scholar] [PubMed]
- Qian Y, Zong X, Zhuang L, Pan R, Tudi X, et al. (2023) PFKFB3 inhibitor 3PO reduces cardiac remodeling after myocardial infarction by regulating the TGF-β1/SMAD2/3 pathway. Biomolecules 13:1072.
[Crossref] [Google Scholar] [PubMed]
- Dommel S, Blüher M (2021) Does C-C Motif Chemokine Ligand 2 (CCL2) link obesity to a pro-inflammatory state? Int J Mol Sci 22:1500.
[Crossref] [Google Scholar] [PubMed]
- Qiao P, Ren L, Hou X (2021) Serum irregular chemokine and endothelial cell-specific molecule 1 levels correlate with hypertension patients' left ventricular systolic function. J Hypertens 29:762-765.
- Veillard NR, Steffens S, Pelli G, Lu B, Kwak BR, et al. (2005) Differential influence of chemokine receptors CCR2 and CXCR3 in development of atherosclerosis in vivo. Circulation 112:870-878.
[Crossref] [Google Scholar] [PubMed]
- Tanaka Y, Hoshino-Negishi K, Kuboi Y, Tago F, Yasuda N, et al. (2020) Emerging role of fractalkine in the treatment of rheumatic diseases. Immunotargets Ther 9:241-253.
[Crossref] [Google Scholar] [PubMed]
- Sans M, Danese S, de la Motte C, de Souza HS, Rivera-Reyes BM, et al. (2007) Enhanced recruitment of CX3CR1+ T cells by mucosal endothelial cell-derived fractalkine in inflammatory bowel disease. Gastroenterology 132:139-153.
[Crossref] [Google Scholar] [PubMed]
- Tawakol A, Singh P, Mojena M, Pimentel-Santillana M, Emami H, et al. (2015) HIF-1α and PFKFB3 mediate a tight relationship between proinflammatory activation and anerobic metabolism in atherosclerotic macrophages. Arterioscler Thromb Vasc Biol 35:1463-1471.
[Crossref] [Google Scholar] [PubMed]
- Duan L, Zhao Y, Jia J, Chao T, Wang H, et al. (2023) Myeloid-restricted CD68 deficiency attenuates atherosclerosis via inhibition of ROS- MAPK-apoptosis axis. Biochim Biophys Acta Mol Basis Dis 1869:166698.
[Crossref] [Google Scholar] [PubMed]
- Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV (2017) CD68/macrosialin: Not just a histochemical marker. Lab Invest 97:4-13.
[Crossref] [Google Scholar] [PubMed]
- Gencer S, Evans BR, van der vorst EPC, Döring Y, Weber C (2021) Inflammatory Chemokines in Atherosclerosis. Cells 10:226-226.
[Crossref] [Google Scholar] [PubMed]
- Miller MC, Mayo KH (2017) Chemokines from a structural perspective. Int J Mol Sci 18:2088.
[Crossref] [Google Scholar] [PubMed]
- Betjes MG, Haks MC, Tuk CW, Beelen RHJ (1991) Monoclonal antibody EBM11 (anti-CD68) discriminates between dendritic cells and macrophages after short-termculture. Immunobiology 183:79-87. [Crossref] [Google Scholar] [PubMed]
Citation: Yu L, Runqing W, Jin Z (2025) Small Molecule Inhibitors of PFKFB3 as Therapeutic Targets for Atherosclerosis in ApoE‑/‑mice. Diagnos Pathol Open 10:246.
Copyright: © 2025 Yu L, et al. This is an open‑access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 619
- [From(publication date): 0-0 - Nov 13, 2025]
- Breakdown by view type
- HTML page views: 493
- PDF downloads: 126