SPHK1 Promotes the Migration and Invasion of Gastric Cancer Cells via NF-ΚB Signaling Pathway
Received: 12-Mar-2024 / Manuscript No. DPO-24-129080 / Editor assigned: 15-Mar-2024 / PreQC No. DPO-24-129080 (PQ) / Reviewed: 01-Apr-2024 / QC No. DPO-24-129080 / Revised: 16-Apr-2025 / Manuscript No. DPO-24-129080 (R) / Published Date: 23-Apr-2025
Abstract
Background: Gastric Cancer (GC) is one of the most common malignancy in digestive system. Sphingosine Kinase-1 (SPHK1) is a member of the SPHK family of proteins, which is involved in the pathogenesis of various cancers. This study aims to investigate SPHK1 expression in GC tissues and its effects on the migration and invasion of GC cells.
Methods: SPHK1 expression and prognosis were analyzed by bioinformatics and Immunohistochemistry (IHC). SPHK1mRNA expression was detected through by Real-Time PCR (qRT-PCR). The enrichment pathway of SPHK1 was predicted by public databases. Cell migration was detected by cell scratch methods. Cell migration and invasion were detected by Transwell assays. Protein expression was detected through Western blotting.
Results: High expression of SPHK1 was observed in GC tissues and correlated with poor prognosis. IHC and qRT-PCR analyses revealed upregulation of SPHK1 expression in GC tissues. Knockdown of SPHK1 reduced the migration and invasion of GC cells, while overexpression of SPHK1 has the opposite result. Mechanistically, SPHK1 positively regulated protein levels of phosphorylated P65 (p-P65), as well as protein expression of Vascular Endothelial Growth Factor A (VEGFA) and Interleukin 17 (IL-17) at transcriptional targets of the Nuclear Factor-κB (NF-κB/P65) signaling pathway. Blocking the NF-κB pathway with PDTC attenuated SPHK1-promoted migration and invasion of GC cells.
Conclusion: Our results elucidate the key role of SPHK1 in promoting migration and invasion of GC cells and suggest that SPHK1 may be a potential molecular target for preventing GC metastasis.
Keywords
Gastric cancer; SPHK1; NF-κB; Migration; Invasion
Introduction
Gastric Cancer (GC) is one of the most common malignant tumors in the digestive system worldwide and has been the second leading cause of cancer-related death in South America and East Asia [1]. According to the survey, the global incidence of GC is increasing every year. Patients with GC are usually treated with surgery, chemoradiotherapy and immunotherapy, but the clinical efficacy and prognosis are poor. High invasion and metastasis ability is an important feature of tumor malignancy. However, until now, the complex mechanisms that lead to cancer metastasis have been far from fully understood.
Sphingosine Kinase-1 (SPHK1) is a conserved lipid kinase that phosphorylates sphingosine to produce Sphingosin-1-Phosphate (S1P), which has been found to play various functions at different stages of tumor progression. SPHK1 exists in the cytoplasm, is a key signaling molecule mediating various aspects of cells and plays a role in a variety of carcinogenic processes, including cell proliferation, migration and invasion. According to studies, SPHK1 is an activator in colorectal cancer cells that promotes the development of cancer by regulating the expression of specific signaling pathways. In pancreatic cancer tissues, the expression of SPHK1 increased and the survival curve analysis of patients showed that low survival rate was closely related to high expression of SPHK1. SPHK1 is up-regulated in tissues and cell lines of Non-Small Cell Lung Cancer (NSCLC). Overexpression of SPHK1 increases proliferation and migration of NSCLC cells. Nuclear factor-κB (NF-κB/P65) is a member of the transcription factor family and is a key regulator of immunity, inflammation and cancer. Inhibition of NF-κB signal transduction in nasopharyngeal carcinoma cells can greatly reduce the proliferation and invasion ability of target genes on cancer cells. At the same time, activation of the NF-κB pathway increases TRIM52-mediated malignant behavior in ovarian cancer. However, it is unclear whether SPHK1 is involved in NF-κB signaling during GC progression. In this study, we analyzed the expression of SPHK1 in GC tissues and cells. The purpose of this study was to investigate the role of SPHK1 in GC tumorigenesis and its potential molecular mechanism [2].
Materials and Methods
This study was approved by the Ethics Committee of Bengbu Medical University (Bengbu, China; No. ((2023)311, (2023)405)). Forty patients with GC (28 males and 12 females, aged 35-75 years) who underwent surgical resection in the Department of Gastrointestinal Surgery of the First Affiliated Hospital of Bengbu Medical University (June-December 2023) were selected. GC and matched non-cancerous gastric tissue samples were obtained after the patients' written informed consent and included in this study. The specimens were stained with hematoxylin and eosin for Immunohistochemical (IHC) analysis. For specific staining procedures, refer to Deng et al. The scores were independently evaluated blind by 2 pathologists. Clinical characteristics of patients, including sex, age, tumor size and tumor stage, were retrieved from medical records (Table 1) [3].
| Characteristic | Cases | % |
| Gender | ||
| Male | 28 | 70 |
| Female | 12 | 30 |
| Age (years) | ||
| ≥ 60 | 24 | 60 |
| <60 | 16 | 40 |
| Tumor size (cm) | ||
| ≥ 5.0 | 22 | 55 |
| <5.0 | 18 | 45 |
| Clinical stage | ||
| I+II | 14 | 35 |
| III+IV | 26 | 65 |
| N stage | ||
| N0 | 10 | 25 |
| N1+N2+N3 | 30 | 75 |
Table 1: Clinicopathological characteristics (n=40).
Reagent consumables
PDTC (NF-κB pathway inhibitor, No: HY-18738) was purchased from MCE Corporation. RPMI1640 culture-medium, pancreatic enzyme and fetal bovine serum were purchased from Gibco Corporation. Mouse polyclonal antibodies SPHK1, P65, p-P65, PI3K, p-PI3K, MKI67 and monoclonal antibodies GAPDH, VEGFA, IL-7 was purchased from Wuhan Proteintech Corporation. Total RNA extraction kit, reverse transcription kit and quantitative PCR kit were purchased from Shanghai Novoprotein Technology Co., Ltd. The primer was synthesized in Shenggong Bioengineering (Shanghai) Co., Ltd. Lentivirus synthesis in Shanghai Gima Pharmaceutical Technology Co., Ltd.
Biological information retrieval
Using TIMER2.0 (http://timer.cistrome.org), HPA (https://www. prot -einatlas.org) database download GC with tissue adjacent to carcinoma. The expression and association of SPHK1-related genes were searched in GEPIA database (http://gepia. cancer-pku.cn). Kaplan-meier plotter database analyzed the correlation between SPHK1 and prognosis of GC patients. UALCAN(https://ualcan.path.uab.edu) database was used to search tumor stage and metastasis characteristics. The GO(https://geneontology.org) database was searched for biological enrichment pathways. The KEGG(https://www.kegg.jp) database was used to retrieve signaling pathways [4].
Cells culture
Human GC cell lines MGC-803, AGS, MKN-1, HGC-27 and normal gastric epithelial cells GES-1 were all purchased from Shanghai Culture Preservation Center, China and cultured in a fully prepared RPMI1640 culture medium in a constant temperature cell incubator at 37℃ and 5% CO2. The cells were digested and passed through when they grew to a suitable degree of fusion.
Western blotting analysis
The protein was extracted from the cells and the concentration was determined. The same amount of protein samples were taken for electrophoresis. After electrophoresis, the protein gel was transferred to 0.45 μm PVDF membrane for membrane transfer. After finishing, immerse it in the sealing liquid and seal it for 60 min. After washing the film for three times, the corresponding primary antibody was diluted and incubated in the refrigerator at 4℃ overnight. The film was washed the next day and incubated in a room temperature shaker with secondary antibody dilution for 90 min. ECL-plus detects protein signals on PVDF membranes [5].
Real-time PCR (qRT-PCR) analysis
Total RNA was extracted and the concentration and purity were determined, which was reversely transcribed into cDNA. Using GAPDH as the internal reference, qRT-PCR was performed according to the operation method of the kit to detect the expression of the target gene. The qRT-PCR reaction was performed using TBGreen®PremixExTaqTM and repeated three times. The mRNA expression level of each gene was calculated using a relatively quantitative method (2-ΔΔCt). The corresponding primers are shown in Table 2.
| Gene | Justice chain | Antisense chain |
| SPHK1 | 5´-TGGCATCTGCTGAACTCATTT-3´ | 5´-TGCAGCGAGGTCTAATTGTTT-3´ |
| GAPDH | 5´-GAGAAGTATGACACAGCCTCAA-3´ | 5´-GCCATCACGCCTGACAGTTT-3´ |
Table 2: Primer sequences.
RNA interference and overexpression of SPHK1
Human GC cell line HGC-27 and MGC-803 were planned and when the culture density reached 70%~80%, SPHK1 interference (carrier: GV382) and overexpression (carrier: GV492) and control lentivirus vector were knocked down and overexpressed into the above cells. After 6 h, the medium containing purinomycin was replaced. After 3 d, stable expression cell lines were screened with the medium containing 1 μg/mL purinomycin. Transfection sequences for SPHK1 lentivirus are shown in Table 3.
| Gene | 5'-3' |
| shSPHK1#1 | CTTCGTGTCAGATGTTGGATAT |
| shSPHK1#2 | GCTTTGCCCTCACCCTTACAT |
| shSPHK1#3 | AGCGGCCTACTTCTGCACTA |
| OE-SPHK1#1 | CTCGTGTCAGATATTGGTTAT |
| OE-SPHK1#2 | ATTGTGTGAGACATCCGTAAG |
| OE-SPHK1#3 | TTGAGTCCTGCTTCTTCATTG |
Table 3: Sequences.
Cell scratch assays
After the cell density was adjusted to 2.0 × 106 cells/well, the plate was placed in the 6-well plate. When the cells were observed to fuse into a single-layer state under the microscope, the sterile 200 μl gun tip was vertically and evenly marked in the hole, and the culture was continued in the incubator. At 0 h and 24 h, the inverted microscope (×40) was used to observe and take pictures at random in 5 areas. The obtained images were processed by Image J software and the scratch mobility was calculated [6].
Trans well migration and invasion assays
Trans well plate with 8 μm hole filter. Stable transmissible cells suspended in 0.2 ml serum-free medium were placed in the upper hole of the trans well plate (1 × 104/ hole), and complete medium containing 0.6 ml was placed in the lower hole. After culture at 37℃ for 24 h, the cells in the upper cavity were completely removed with cotton swabs and the cells in the lower cavity were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Then the inverted microscope (×200) was randomly divided into 5 areas to take pictures and count and the average value was obtained. To hit test, the experiment process is similar to the migration test. Just before the cell load, the upper pore of trans well was added with Matrigel (50 μl) and incubated for 1 h.
Statistical analysis
All statistical data were analyzed by Prism9.0 (Graph pad Software) and Image J software. Data are expressed as mean ± standard deviation (SD). Analysis of variance (ANOVA) test and two-sample t test were used for the significance of statistical analysis. P<0.05 was considered statistically significant. All experiments were repeated three times [7].
Results
Based on bioinformatics: SPHK1 is highly expressed in GC and associated with poor prognosis
The expression of SPHK1 at pan-cancer level was searched using TIMER2.0 database. The results showed that SPHK1 expression was significantly up-regulated in 15 cancer types and down-regulated in 2 cancer types, among which it was significantly expressed in GC (Figure 1A). Based on the GEPIA database, the expression levels of SPHK1 and MKI67 in GC tissues (n=408) were higher than those in normal tissues (n=211) (Figure 1B). In addition, the expression levels of the two were positively correlated (Figure 1C). Analysis of the Kaplan-Meier Plotter database showed that high expression of SPHK1 in GC patients predicted poorer overall survival (OS) (Figure 1D) and poorer survival after progression (PPS) (Figure 1E). The UALCAN database showed that the expression level of SPHK1 was positively correlated with the tumor stage of GC patients (Figure 1F). At the same time, the expression of SPHK1 was also closely related to the malignant degree of tumor metastasis (Figure 1).
Figure 1: Based on gene database: SPHK1 is highly expressed in GC and associated with poor prognosis. (A). TIMER2.0 database was used to search the expression of SPHK1 in pancarcinoma. (B). GEPIA database was used to search the expression of SPHK1 and MKI67 in GC. (C). GEPIA database analyzed the correlation between the SPHK1 and MKI67. (D, E). Kaplan-Meier plotter database analyzed the overall survival and survival after progression of GC patients. (F, G). UALCAN database was used to search the effect of SPHK1 on tumor stage and malignant metastasis in GC patients. Data represent mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
Up-regulation of SPHK1 expression in GC
The expression of SPHK1 in GC tissues was predicted using HPA database. IHC results showed that the degree of positive staining of SPHK1 in tumor tissues was stronger than that in normal tissues (Figure 2A). At the same time, we detected the expression of SPHK1 and MKI67 in GC and matched non-cancerous gastric tissues in 40 cases of our hospital by IHC. The results showed that the positive staining intensity of SPHK1 and MKI67 in GC tissues was higher than that in non-cancerous gastric tissues (Figure 2B). The comprehensive staining scores of SPHK1 and MKI67 proteins in GC tissue sections were also significantly higher than those in normal tissue sections (Figure 2C, 2D). At the same time, the relative expression levels of the two proteins showed a significant positive correlation (Figure 2E). In addition, the expression of SPHK1 in GC cell lines was detected by Western Blotting and qRT-PCR and SPHK1 was highly expressed in HGC-27 cells and low expressed in MGC-803 cells (Figure 2) [8].
Figure 2: Up-regulation of SPHK1 expression in GC. (A). The expression of SPHK1 in GC tissues was searched by HPA database (×200). (B). The expression of SPHK1 and MKI67 was detected by IHC (×200). (C). IHC score of SPHK1. (D). IHC score of MKI67. (E). Correlation between SPHK1 and MKI67 expression. (F). The expression of SPHK1 in GC cell lines was detected by Western blotting. (G). The level of SPHK1mRNA in GC cell lines was detected by qRT-PCR. Data represent mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
SPHK1 promotes GC cell migration and invasion
To investigate whether SPHK1 expression affects the migration and invasion of GC cells. We constructed HGC-27 and MGC-803 cell stable strains from lentiviruses and used them in subsequent experiments (shSPHK1#1 and OE-SPHK1#2). As shown in Figure 3A, shSPHK1 significantly down-regulated SPHK1 expression in HGC-27 cells, while OE-SPHK1 significantly up-regulated SPHK1 expression in MGC-803 cells. In addition, Western blotting results showed that the expression of MKI67 protein in shSPHK1 group was inhibited, while that in OE-SPHK1 group was up-regulated (Figure 3B). In addition, as shown in Figure 3C and 3E, the migration ability of silenced SPHK1 expression in HGC-27 cells was inhibited, while the result of overexpression of SPHK1 was the opposite. At the same time, the invasion effect of SPHK1 on GC cells was also the same (Figure 3) [9].
Figure 3: SPHK1 promotes GC cell migration and invasion. (A). The efficiency of lentivirus cells stable transmutation was detected by Western blotting. (B). The effect of SPHK1 on cell proliferation was detected by Western blotting. (C, E). Cell scratch assay was used to detect the migration ability of SPHK1 to cells (×40). (D, F). The invasion ability of SPHK1 to cells was detected by Transwell assays (×200). Data represent mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
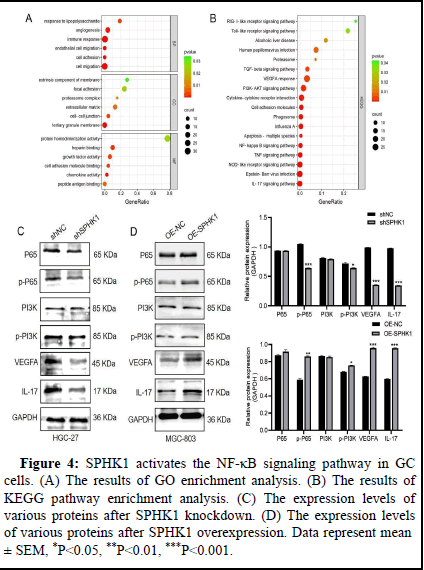
SPHK1 activates the NF-κB signaling pathway in GC cells
A recent study showed that targeting the NF-κB/p65 pathway mediated Epithelial-Mesenchymal Transformation (EMT) promotes colorectal cancer metastasis and tumor growth in vivo. NF-κB signaling also plays a crucial role in GC metastasis and angiogenesis. Therefore, we investigated whether SPHK1 affects NF-κB/p65 signaling in GC cells. Firstly, based on GO enrichment analysis, SPHK1-related genes were involved in cell adhesion, migration and angiogenesis in Biological Processes (BP), Cell Composition (CC) and Molecular Function (MF) (Figure 4A). The KEGG pathway showed NF-κB and PI3K enrichment in it (Figure 4). In addition, as shown in Figure 4C and 4D, knocked down SPHK1 inhibited the levels of phosphorylated P65(p-P65) and PI3K(p-PI3K) (this experiment mainly studied the changes of p-P65 levels), while the total protein P65 and PI3K had no significant changes. VEGFA and IL-17 expression levels are also inhibited, which may be transcriptional targets of the NF-κB signaling pathway. In contrast, overexpression of SPHK1 enhanced this performance. Taken together, these data suggest that SPHK1 can activate NF-κB/P65 signaling in GC cells.
Figure 4: SPHK1 activates the NF-κB signaling pathway in GC cells. (A). The results of GO enrichment analysis. (B). The results of KEGG pathway enrichment analysis. (C). The expression levels of various proteins after SPHK1 knockdown. (D). The expression levels of various proteins after SPHK1 overexpression. Data represent mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
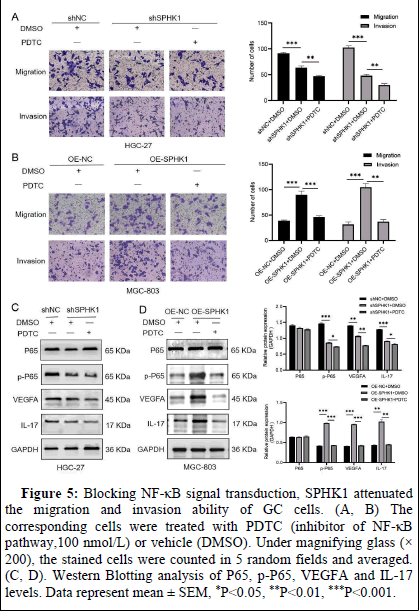
Blocking NF-κB signal transduction, SPHK1 attenuated the migration and invasion ability of GC cells
To evaluate whether the effect of SPHK1 on GC cells is mediated by the NF-κB signaling pathway, we treated pre-constructed viral stable transmutation cells with PDTC (NF-κB signaling inhibitor) for 24 h. As expected, inhibition of NF-κB signaling decreased SPHK1's ability to migrate and invade GC cells (Figures 5) and p-P65, VEGFA and IL-17 levels (Figures 5C, 5D).
Figure 5: Blocking NF-κB signal transduction, SPHK1 attenuated the migration and invasion ability of GC cells. (A, B). The corresponding cells were treated with PDTC (inhibitor of NF-κB pathway,100 nmol/L) or vehicle (DMSO). Under magnifying glass (×200), the stained cells were counted in 5 random fields and averaged. (C, D). Western Blotting analysis of P65, p-P65, VEGFA and IL-17 levels. Data represent mean ± SEM, *P<0.05, **P<0.01, ***P<0.001.
Discussion
There is evidence that Sphingosine Kinase (SPHK) family proteins are involved in various cancer progression processes. For example, S1PR1 signals promote GC metastasis by enhancing the expression of chemokines in tumor cells. SPHK1 can inhibit the apoptosis of GC cells by stimulating Akt/FoxO3a signaling to down-regulate the expression of apoptotic protein. SPHK2 is up-regulated in GC tissues and plays a carcinogenic role in GC. In this study, based on bioinformatics analysis, the expression of SPHK1 in GC tissues was higher than that in normal gastric tissues and high expression of SPHK1 predicted worse overall survival and survival after progression. At the same time, the higher the SPHK1 expression level, the higher the tumor stage and metastasis degree (Figure 1). This is consistent with our findings that SPHK1 is positively correlated with MKI67 (Figure 2). MKI67 is a malignant proliferation factor of tumor cells. With the establishment of MKI67 index, MKI67 has developed into a standard for diagnosis and prognosis evaluation of cancer patients.
Distant metastasis is one of the reasons for the high mortality of GC patients. Tumor metastasis is a multi-stage process. The migration and invasion of tumor cells are the key links in the metastasis process. SPHK1 has demonstrated tumor cell migration and invasion functions in breast cancer and prostate cancer. Consistent with the above findings, our data showed that SPHK1 knockdown inhibited GC cells migration and invasion, while overexpression of SPHK1 did the opposite (Figure 3). Our data further confirm the important role of SPHK1 in tumor progression.
SPHK1 has been reported to activate several cancer-related signaling pathways, such as the TRAF6/ULK1 pathway in colorectal cancer, the NONO/STAT3 pathway in bladder cancer and the JAK/mTOR pathway in NSCLC. This study focuses on the NF-κB pathway, and the effects of SPHK1 on other pathways will be further explored in our future studies. The NF-κB signaling pathway plays an important role in tumor-related activities such as cell proliferation, migration and invasion. Studies have shown that NF-κB is closely related to GC transfer. In this study, SPHK1 activated the NF-κB signaling pathway in GC cells (Figure 4). PDTC (inhibitor of NF-κB pathway) was used to interfere with SPHK1 knockdown and overexpression cell lines and the results showed that SPHK1 weakened the migration and invasion of GC cells, suggesting that NF-κB was involved in SPHK1-mediated migration and invasion of GC cells. VEGFA is an important angiogenic factor associated with GC invasion and metastasis. IL-17 works with immune cells to promote tumor metastasis. VEGFA and IL-17 may be transcriptional targets of NF-κB signaling pathway. In this study, knocking down SPHK1 down-regulates the expression of p-P65, VEGFA and IL-17, while overexpression of SPHK1 has the opposite effect (Figure 5).
Therefore, we speculate that NF-κB is involved in GC progression. However, the mechanism by which SPHK1 activates NF-κB pathway and other interacting proteins jointly regulate and promote GC transfer needs further study. In addition, mouse tumorigenic experiments will be conducted in future studies to verify the cancer-promoting effect of SPHK1 on regulating NF-κB pathway.
Conclusion
This study revealed the increased expression of SPHK1 in GC samples and the important role of SPHK1 in the migration and invasion of GC cells through the NF-κB signaling pathway. SPHK1 may be a potential molecular target to prevent GC transfer.
Statements and Declarations Competing Interests
The authors declare that they have no known competing financial interests or personal relationships that could affect the work reported herein.
Acknowledgement
Thanks to the members of the research team for their help in the paper. This work was supported by Scientific research project of Anhui Provincial Education Department(KJ2021ZD0090) and Bengbu Medical University Graduate Research Innovation Project (Byycx22090).
Ethical Approval and Participative Consent
Work involving human subjects has been conducted in accordance with the World Medical Association Code of Ethics (Declaration of Helsinki). This study was approved by the Ethics Committee of Bengbu Medical University (Bengbu, China; No. ((2023)311, (2023)405)).
Author Contribution Information
Qianlong Ling: Data management, formal analysis, methodology, writing-original manuscript, funding acquisition.
Kai Ji: Data management, formal analysis, methodology formal analysis, methodology.
Jiajia Guan: Formal analysis, methodology, writing.
Ruipeng Wang: Formal analysis, methodology.
Bing Zhu: Conceptualization, data management, supervision, writing-review and editing, funding acquisition.
References
- Qinghai Z, Yanying W, Yunfang C, Xukui Z, Xiaoqiao Z (2014) Effect of interleukin-17a and interleukin-17f gene polymorphisms on the risk of gastric cancer in a Chinese population. Gene 537: 328-332.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Wu WK, Gao J, Li Z, Dong B, et al. (2019) Autophagy inhibition enhances PD-L1 expression in gastric cancer. J Exp Clin Cancer Res 38: 1-14.
[Crossref] [Google Scholar] [PubMed]
- Yeong J, Lum HYJ, Teo CB, Tan BKJ, Chan YH, et al. (2022) Choice of pd-l1 immunohistochemistry assay influences clinical eligibility for gastric cancer immunotherapy. Gastric Cancer 25: 741-750.
[Crossref] [Google Scholar] [PubMed]
- Tang L, Guo C, Li X, Zhang B, Huang L (2023) TAF15 promotes cell proliferation, migration and invasion of gastric cancer via activation of the RAF1/MEK/ERK signalling pathway. Sci Rep 13: 5846.
[Crossref] [Google Scholar] [PubMed]
- Qin Z, Tong H, Li T, Cao H, Zhu J, et al. (2021). Sphk1 contributes to cisplatin resistance in bladder cancer cells via the nono/stat3 axis. Int J Mol Med 48: 204.
[Crossref] [Google Scholar] [PubMed]
- Bernacchioni C, Squecco R, Gamberi T, Ghini V, Schumacher F, et al. (2022) S1P signalling axis is necessary for adiponectin-directed regulation of electrophysiological properties and oxidative metabolism in C2C12 myotubes. Cells 11: 713.
[Crossref] [Google Scholar] [PubMed]
- Zhang L, Dong Y, Wang Y, Hu W, Dong S, et al. (2020) Sphingosine‐1‐phosphate (s1p) receptors: Promising drug targets for treating bone‐related diseases. J Cell Mol Med 24: 4389-4401.
[Crossref] [Google Scholar] [PubMed]
- Long J, Yao Z, Sui Y, Fang S (2022) Sphk1 promotes cancer progression through activating jak/stat pathway and up-regulating S1PR1 expression in colon cancer cells. Anticancer Agents Med Chem 22: 254-260.
[Crossref] [Google Scholar] [PubMed]
- Wu JN, Lin L, Luo SB, Qiu XZ, Zhu LY, et al. (2021) Sphk1‐driven autophagy potentiates focal adhesion paxillin‐mediated metastasis in colorectal cancer. Cancer Med 10: 6010-6021.
[Crossref] [Google Scholar] [PubMed]
Citation: Zhu B, Ling Q, Ji K, Guan J, Wang R (2025) SPHK1 Promotes the Migration and Invasion of Gastric Cancer Cells via Nf-Îb Signaling Pathway. Diagnos Pathol Open 10: 250.
Copyright: © 2025 Zhu B, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 311
- [From(publication date): 0-0 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 227
- PDF downloads: 84