Standardization of Marketed Churna
Received: 01-Feb-2023 / Manuscript No. ijrdpl-22-84365 / Editor assigned: 03-Feb-2023 / PreQC No. ijrdpl-22-84365 / Reviewed: 17-Feb-2023 / QC No. ijrdpl-22-84365 / Revised: 21-Feb-2023 / Manuscript No. ijrdpl-22-84365 / Accepted Date: 27-Feb-2023 / Published Date: 28-Feb-2023 QI No. / ijrdpl-22-84365
Abstract
The therapeutic potential of ethno medicinal plants has been studied for several years. Over the years, many ailments have been cured with herbal remedies. Due to the enormous range of therapeutically important secondary metabolites in plants, which are natural products, they are also important sources of novel medications. At specific concentrations of their chemical components, drugs or formulations are anticipated to exert the intended biological action. Standardization is a crucial stage in formulation since it affects the product's quality. Drugs or formulations are expected to exert a desired biological activity at particular concentrations of their chemical constituents. The overall aim of drug standardization is to ensure the quality, efficacy, and uniformity of the products, in terms of their chemical and biological properties, across the manufacturers. It is important to create a methodology for standardizing every product sold on the market to prevent variance from occurring from batch to batch. Plant materials differ from synthetic medications in numerous ways, including their chemical composition, depending on the time, and year they are collected, where they are grown, and other factors. The goal of the present effort is to offer standardization of marketed polyherbal churna Pet saffa. The churn was purchased and processed to standardization for factors including organoleptic characteristics, physical characteristics, physiochemical properties, and heavy metal detection.According to the findings, they complied with the standard readings.
Keywords
Polyherbal; Standardization; Churna; Physicochemical parameters
Introduction
India has a diverse range of medicinal plant life. These plants are used to treat numerous diseases directly and also through the preparation of a formulation. People seem to think that natural remedies are safer than synthetic ones. The development of these traditional systems of medicine leads to the proper quality of the product. [1] Due to their increased diversity and shifting chemical makeup or characteristics, crude medications require a substantial amount of identification and examination during the herbal formulation process. All pharmacopoeias conform to a set of standards to minimize this issue [2]. The polyherbal formulation includes two or more herbs with distinct phytoconstituents that have either similar or differing therapeutic potential and have been working together to provide potent therapeutic effect when treating human illnesses. The acceptance and development of polyherbal formulations are at their peak because of their many benefits, like being effective at a low dose and safe at a high dose, with a low risk of adverse effects if misused [3]. Standardization is an essential step in the formulation of finished products for the determination of quality and purity. Standardization of all commercially available products is mandatory to prevent variation from batch to batch. Plant materials differ from synthetic medications in numerous ways, including their chemical composition, depending on the time of year they are collected, where they are grown, and other factors [4, 5]. The World Health Organization (WHO) has appreciated the importance of medicinal plants for public health care in developing nations and has evolved guidelines to support the member states in their efforts to formulate national policies on traditional medicine and to study their potential usefulness including evaluation, safety and efficacy. The increasing demand of the population and the chronic shortage of authentic raw materials have made it incumbent, so there should be some sort of uniformity in the manufacture of Ayurvedic medicines so as to ensure quality control and quality assurance [5].
Material and Methods
Pet saffa churna (granules) of Divisa Herbals Private Limited was chosen because no specific scientific work had previously been reported.
Pet saffa churna is used in constipation and acidity. The objective of the ongoing work is to standardise the marketed churna. It was purchased from an ayurvedic pharmacy from Amravati's local market. Table no. 01 shows the composition of polyherbal churna.
Ayurvedic proprietary medicine
Organoleptic evaluation
Evaluation of a formulation using its color, odor, taste and other morphological attributes is known as organoleptic evaluation. It is carried out on the basis of method specified in Indian Pharmacopoeia. [5]
Physico-Chemical Parameters
pH, moisture content, ash value, total ash value, acid insoluble ash, water soluble ash value, extractive value ,alcohol soluble extractive value, water soluble extractive value was carried out for the physicochemical parameters. [4]
PH
An electrical pH metre was used to determine pH along with different buffer tablets. A 1% solution of churn in distilled water was prepared, and pH was determined with the help of buffer tablets that have pH 7 and pH 11 for calibration. [3]
Determination of moisture content
The moisture content of churna was determined by loss on drying method with the help of hot air oven. Weigh about 02 gm. of the powdered drug into a preweighed porcelain dish. Dry in the oven at 1050C for 2 hours, and at every 1-hour interval, weigh the sample until two consecutive weighing do not differ by more than 0.5 mg. Allow it to cool before weighing it. The loss in weight is usually recorded as moisture. [1] Calculate % of loss on drying by using following formula.
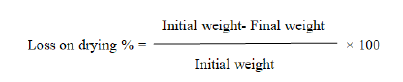
Ash value [1]
Total Ash Value
Weigh about 2 gm. of churn into preweighed crucible and incinerated at a temperature not exceeding 450°C in muffle furnace, cooled and weighed. The difference between initial and final gives the total ash value [1].

z = weight of the crucible + ash (after complete incineration).
x = weight of the empty crucible.
y = weight of the drug taken (2 gm.).
Acid Insoluble Ash
The residue of ash obtained in total ash was added with 25 ml of dilute hydrochloric acid and boiled for 05 minutes. The insoluble matter was filtered using ashless filter paper, washed the residue twice with hot water and ignited for 15 minutes at a temperature not exceeding 450˚C. Cool it and then weight of the insoluble matter was subtracted from the weight of the total ash.
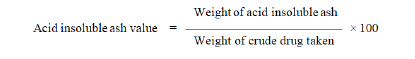
Water soluble Ash Value
The residue of the total ash was boiled for 5 minutes with 25 ml of water. Water soluble ash is determined in the similar way to acid insoluble ash.
Extractive Value [1]
Alcohol Soluble Extractive Value
Weigh about 5 gm. of churn and macerate with 100 ml alcohol (90%) into stoppered flask for 24 hrs, with frequent shaking. Filter the menstrum and transfer 25 ml of the filtrate to a weighed, thin porcelain dish. Evaporate to dryness on water bath and complete the drying in an oven at 1050C for 6 hrs. Cool in a desiccator for 30 minutes and weigh immediately. Calculate the % w/w of extractive with reference to air dried drug.
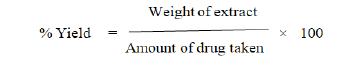
Water Soluble Extractive Value
5 g of churna was macerated in 100 ml of chloroform water and kept for 24 hours, and the same procedure was followed as for the alcohol-soluble extractive value. Calculate the water-soluble extractive value by using the above-mentioned formula.
Physical Evaluation [1]
Bulk Density
10 gm of churna was weighed and transfer in a graduated cylinder with the help of a funnel. The initial volume was noted down and then the ratio of the occupied weight of volume was calculated.
Bulk density is calculated by using the formula.
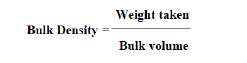
Tap Density
Tapped density was determined by transferring the known weight of powder into a graduated tap density apparatus and tapping was done for 50 times. The initial volume before tapping was noted and then after tapping the final volume was again noted. The density was calculated by the ratio of the mass of the powder to the tapped volume. The following formula can be used for the determination of tap density.
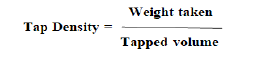
Angle of Repose
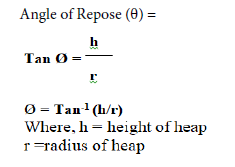
Angle of repose was determined by using funnel method. The powder was allowed to flow through a funnel fixed on a stand at a height of 4 cm to form a heap. The height and the radius of the pile were measured. Angle of repose of the powder was calculated using the following formula.
Compressibility / Carr’s Index
It is yet another method for determining powder flow from bulk density. This is calculated using the formula:

Hausner’s Ratio
The Hausner's ratio is used to determine the flow characteristics of powder. The ratio of tapped density to bulk density of powder is known as the Hausner's ratio.
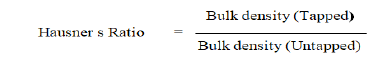
Detection of heavy metal test in churna[1] Instrumentation
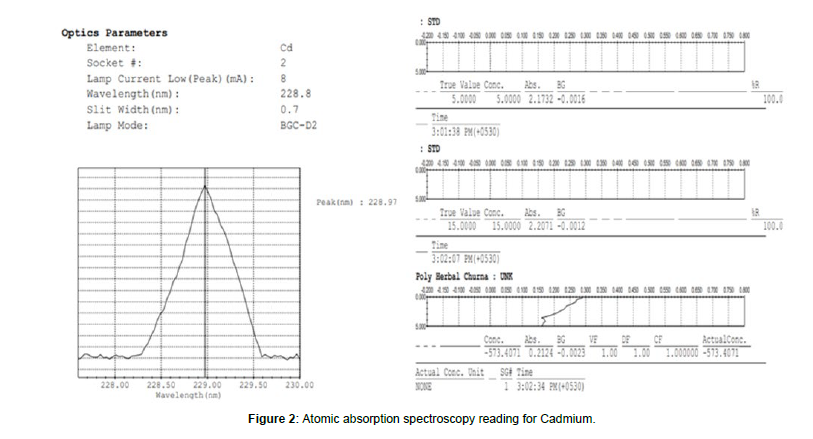
Heavy metals determination was done using atomic absorption spectroscopy (Figure 1) (Shimadzu). Standard operating parameters were set and given in table. The hollow cathode lamps for Cadmium (Cd), Lead (Pb), (Figure 2) were used as radiation source and fuel was air acetylene. All the samples and standard were run in duplicate. The wet digestion method is used to determine the presence of heavy metals in churna. In a 100-mL volumetric flask, 0.2 g of sample was placed, and 4 mL of nitric acid was added. The solution was allowed to stand for a few hours before being carefully heated over a water bath until red fumes stopped completely. The flask was allowed to cool at room temperature, and then about 4 mL of hydrochloric acid was added. The flask was then heated over the water bath again until only a small portion remained, which was then filtered through Whattman filter paper no. 42. The sample that remained on filter paper was further dissolved in distilled water, and the volume was made up to 100 ml.
Discussion
In the present study, the marketed polyherbal churna was evaluated on the basis of organoleptic characteristics, physical, physicochemical parameters, and heavy metal presence. It was concluded that the marketed polyherbal churna evaluation parameters were within the official limits. The angle of repose, Carr's index, and Hausner's ratio results all indicated that the churn has good flow qualities. The presence of heavy metals in herbal medicines was below the WHO permissible limit. The results obtained from the study could be utilized as a reference for evaluating the quality and purity of the prepared or marketed powder formulations.
Results
The organoleptic properties of polyherbal formulations are shown in Table 1. The mean percentage of physiochemical parameters like pH, moisture content, ash value, extractive value are shown in. The physical characteristics like bulk density, tapped density, Carr’s index, Hausner ratio and angle of repose are shown in. The heavy metals presence is shown in Table 1.
| Sr. No | Ingredients | Common Name | Each 100 gram Contains |
|---|---|---|---|
| Cassia angustifolia | Senna leaf | 50.60 | |
| Trachyspermum ammi | Ajwain fruit | 10.08 | |
| Operculina tupethum | Nisot root | 5.05 | |
| Foenicuiulm vulgare | Saunf fruit | 1.80 | |
| Terminalia chebula | Haritaki fruit | 10.08 | |
| Ricinus communis | Castor oil seed | 0.33 | |
| Himalayan black salt | Kala namak powder | 13.50 | |
| Sodium bicarbonate | Svarjiksara powder | 4.85 | |
| Rock salt | Saindhava lavana powder | 3.84 | |
Table1: Composition of polyherbal Pet saffa churna.
References
- Anderson SE, Meade BJ (2014) Potential health effects associated with dermal exposure to occupational chemicals. Environ Health Insights 8: 51–62.
- Azandjeme CS, Bouchard M, Fayomi B, Djrolo F, Houinato D (2013) Growing of diabetes in sub-saharan Africa: contribution of pesticides? Curr Diabetes Rev 9: 437–449.
- Beard JD, Umbach DM, Hoppin JA (2014) Pesticide exposure and depressionamong male private pesticide applicators in the agricultural health study. EHP 122: 984–991.
- Bulut S, Erdogus SF, Konuk M, Cemek M (2010) The organochlorine pesticide residues in the drinking waters of Afyonkarahisar, Turkey. Ekoloji Dergisi 19: 24– 31.
- Covaci A, Tutudaki M, Tsatsakis AM, Schepens P (2002) Hair analysis: another approach for the assessment of human exposure to selected persistent organochlorine pollutants. Chemosphere 46: 413–418.
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Indexed at, Google Scholar, Crossref
Citation: Manekar SS, Muley DP, Patode AP (2023) Standardization of MarketedChurna. Int J Res Dev Pharm L Sci, 9: 146.
Copyright: © 2023 Manekar SS, et al. This is an open-access article distributedunder the terms of the Creative Commons Attribution License, which permitsunrestricted use, distribution, and reproduction in any medium, provided theoriginal author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 1958
- [From(publication date): 0-2023 - Dec 19, 2025]
- Breakdown by view type
- HTML page views: 1564
- PDF downloads: 394