Study of Pesticide Contamination in Soil, Water and Produce Using Gas Chromatography Mass Spectrometry
Received: 18-May-2018 / Accepted Date: 04-Jun-2018 / Published Date: 07-Jun-2018 DOI: 10.4172/2155-9872.1000409
Keywords: Pesticides; Tomato; Soil; Water; Speciation; Contamination
Introduction
Pest is defined as any living organisms interfering with the agricultural activity in a negative way. The major pests were observed that hamper the growth of agricultural crops are insects, fungi, and weeds. Pesticides are the chemicals used against pests. Many precautions are taken to avoid these pests. But, there should be a strategy for use of the chemicals for the sake of food safety. Which means many different products should be under routine control for pesticides residue in a specific zone [1]. Governments in many countries have established new institutions, standards, and methods for regulating food safety and have increased investments in hazard control. United Nations Conference on Environment and Development recognized food as major vehicles for trade commodity and environmental contamination. Sustainable agricultural practices that are promoted for mitigating climate change have the potential to also improve pest management [2]. The over use of synthetic chemicals to control pests and diseases has become widespread in the 20th century especially after the Second World War. An increase in food production has been observed with the increasing World population. However, the quality of food came as a big questions over time. It has been observed that farmers aim to get more and more yield lead to apply more synthetic pesticides. The pesticides were identified as persistent organic pollutants (POPs) by the Stockholm Convention in 2001. Chemical substances and their persistance in the environment, bio-accumulation through the food web, pose a risk of causing adverse effects to human health and the environment. Pesticides are grouped in many classes among them Organochlorine Pesticides (OCPs) played an important role at the beginning.
Dichlorodiphenyltrichloroethane (DDT) is the most well-known member of the chlorinated pesticides. Which was developed as an insecticide in the 1940's. Organochlorine pesticides were banned from begining of 1960s but still used fugitivly in some countries. Initially it was used to combat malaria, typhus, and the other insect-borne diseases [3]. DDT is biodegraded to Dichlorodiphenyldichloroethylene (DDE) under aerobic conditions and to Dichlorodiphenyldichloroethane (DDD) under anaerobic conditions [4,5]. DDT is commonly considered to be carcinogenic by the California Environmental protection Agency [6] and International Agency for Research on Cancer [7]. Observed effect on human and environment leads to implement limitation for their use. But, developing countries still use these pesticides, especially organochlorine pesticides since they are cheap and easy to access [8]. Therefore, organochlorine pesticides are constant contaminants in the environment in developing countries. The key requirements for assessing the toxicity of pesticides in the environment is speciation [9]. Organochlorine pesticides (OCPs) are known as hydrophobic compounds mostly solid with low aqueous solubility [10]. This causes their adsorption or sorption on the surface of dissolved organic carbon (DOC) and suspended particulate matter (SPM). The World Health Organization [11] still believes that the benefits of indoor DDT use in case of malaria problem may outweight its health and environmental risks.
Turkey is main exporter of fresh fruits and vegetables to Russia and Europe. About 40% of Europe fresh fruit and vegetables demand is met by Turkey [12]. Among all vegetables tomato is the primary export produce. It is produced almost everywhere in central and western Turkey. This study is about the validation of method in the aspect of extraction and analyses and environmental impact evaluation of pesticides in soil, water and tomato samples. The aim was also to determine the biosecurity of agricultural product in Central Turkey by investigation of chlorinated pesticide distribution from water-soiltomato.
Materials and Methods
Sampling and study area
Samples were collected from Ayaş region nearby capital city Ankara, Turkey. The land area of Ayaş region is about 1,112 km2 (429 sq. mi) and region is famous for agricultural activities especially for tomatoes. Total sixteen soil samples, sixteen tomato samples and four water samples were collected from two different tomato fields for this study. The district is also well - known for its tasty tomatoes, mulberry trees, and its healing mineral water spas, both for drinking and bathing. The image of study area is given in Figure 1.
Reagents and materials
The organochlorine pesticide standards (EPA Method 508-Chlorinated Pesticide Mix 1, 1000 ng/μl), Internal standards (Pentachloronitrobenzene (PCNB), 1.0 mg/ml) and Surrogate standards (2, 4, 5, 6-Tetrachloro-m-xylene (TCMX), 200 ng/ml and Decachlorobiphenyl (DCP), 200 ng/ml) were purchased from Dr. Ehrenstorfer (Augsburg, Germany). The intermediate standard solutions were prepared from the stock solutions with appropriate dilutions with acetone, hexane and cyclohexane. All the stocks, intermediates and standard solutions were stored in refrigerator. Hamilton gas tight glass syringes (500, 100, 50 and 10 μl) were used for the preparation of the standards into 1.5 ml amber vials (Supelco). Ultrasonic bath extractions were performed by Branson ultrasonic bath and rotary evaporator (Laborota 4000) was used to evaporate the solvents of both standards and samples. The extracted samples were transferred to 1.5 ml amber glass vials for further reduction of the volume.
Extraction methodologies
The extraction of organochlorine pesticides in tomato, soil and waters samples can be done with the following extractions methodologies; Ultrasonic bath extraction, Solid phase micro extraction, Soxhlet extraction [13], Accelerated solvent extraction [14,15], Supercritical fluid extraction and Liquid-liquid extraction [16- 19]. The most popular extraction techniques used in tomato and soil was Ultrasonic Bath Extraction (UBE) [19] and for water, Solid Phase Extraction (SPE) [20-22]. These techniques were used in this study for extraction of pesticides.
A blend of 15 g tomato samples was weighed and mixed with 30 ml of dichloromethane with addition of 1.0 ml of 1.0 μg/ml surrogate standards TCMX and DCP. All the ingredients were mixed for 2-3 min with a glass budged, then 30 g anhydrous sodium sulfate were added and allowed to rest for 2 min in an Ultrasonic bath at 40°C [23]. Samples were filtered with filter paper followed by anhydrous Na2SO4 filled column. The final solution was pre-concentrated to 1 ml by evaporating under pure nitrogen gas and addition of internal standard.
Two grams of soil samples were weighed in an amber glass bottle with Teflon cap and 1.0 ml of 1.0 μg/ml surrogate standards (TCMX and DCP) were added. Then 60 ml of hexane: acetone mixture (3:1) was added to the bottle and closed for ultrasonic bath extraction for two hours at room temperature. The samples were filtered with filter paper followed by anhydrous Na2SO4 filled column. The final solution was pre-concentrated to 1 ml by evaporating under pure nitrogen gas and addition of internal standard.
Water samples were filtered with filter paper. About 200 ml water was taken into a beaker and the surrogate’s standards TCMX and DCP were added. Water was extracted with solid phase extraction disk. The SPE disk was conditioned before use. The final solution was pre concentrated to 1 ml by evaporating under pure nitrogen gas and addition of internal standard.
Tomato and soil and water samples were taken into a 1.5 ml amber glass vial and kept in refrigerator at 4°C before the analysis with Gas chromatography- Mass spectrometer (GC-MS) [24].
Solid phase extraction disk was conditioned by sequential addition of 10.0 ml DCM, 10.0 ml methanol and 10.0 ml deionized water. The analytes trapped on the disk were eluted by passing 20 ml DCM inside the Erlenmeyer flask with the help of vacuum pump. The solvent was added by 10+5+5 mL portions with a total contact time of 5 minutes. The extract was removed and dried by passing through a drying column of anhydrous Na2SO4. The anhydrous Na2SO4 column bed was wetted by 6.0 ml DCM before use. After passing the extract, the drying column was rinsed with 5.0 ml of the same solvents and this portion was collected with sample extract.
Gas chromatographic analysis
A HP (Hewlett Packard) 6890 series Gas Chromatography (GC) coupled with HP 5973 Mass Spectrometer (MS) was used for the analysis. A 30 m, 0.25 mm id, 0.25 mm film thickness, cross -linked 5% Phenyl methyl siloxane HP 5MS capillary column (Agilent Tech.) was used for the separation of OCPs in the study. The operating parameters of GC-MS was splitless, inlet temperature was 250°C, oven program was followed by 80-150°C at 10°C/minutes wait 5 minutes 150-275°C at 5°C/minutes wait 3 minutes. MS source temperature was 290°C and Injection volume was 1 μl and carrier gas Ultra purified Helium, 99.999% at a rate of 1 ml/min [25].
Results and Discussion
Percent extraction recoveries for all type of sample matrix; soil, tomato and water were calculated as:
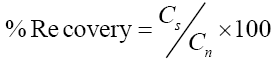
Where, Cs: Measured concentration of the spiked sample aliquot, Cn: Nominal (theoretical) concentration of the spiked aliquot [26].
The mean recoveries for the residues relative standard deviation (RSD), limit of detection (LOD) and limit of quantification (LOQ) were listed in Table 1. Percent recoveries of standard reference materials are changing from 44.5-125. These recoveries were for blank samples. Detection limits were in the range from 0. 330-25.7 ng/mg in soil, 0.840-43.1 ng/mg in tomato and 0.680-23.7 ng/ml in water respectively. The standard deviations indicate good repeatability. The LOD was calculated as S/N ratio of 3:1 and LOQ as of 10:1. For different matrix minimum LOD were different for each pesticide, for soil, tomato, and water a-HCH, b-HCH and Endosulfan I were maximum LOD respectively. Endosulfan I was detected with minimum LOD in tomato but maximum in water samples.
| Soil | Tomato | Water | ||||||
|---|---|---|---|---|---|---|---|---|
| Name of Pesticide | % Recoveries | RSD (%) | LOD (ng/mg) | LOQ (ng/mg) | LOD (ng/mg) | LOQ (ng/mg) | LOD (ng/ml) | LOQ (ng/ml) |
| alpha-HCH | 87.4 | 5.22 | 25.7 | 76.9 | 10.2 | 30.5 | - | - |
| beta-HCH | 88.7 | 5.82 | 2.60 | 6.17 | 43.1 | 129 | - | - |
| gamma-HCH | 112 | 4.93 | 0.330 | 1.00 | 10.7 | 32.2 | - | - |
| delta-HCH | 90.2 | 7.22 | 1.75 | 5.25 | - | - | - | - |
| Heptachlor | 88.3 | 7.46 | 4.70 | 14.1 | 26.3 | 78.9 | ||
| Aldrin | 125 | 5.54 | 1.89 | 5.68 | - | - | 1.91 | 5.72 |
| Heptachlroepoxide | 107 | 4.84 | 0.500 | 1.51 | - | - | - | - |
| Endosulfan I | 93.1 | 6.26 | 0.840 | 2.53 | 16.2 | 48.7 | ||
| Endosulfan II | 82.5 | 5.47 | 5.65 | 16.9 | 7.29 | 21.9 | 2.76 | 8.28 |
| p.p’-DDE | 107 | 11.9 | 3.35 | 10.1 | 7.39 | 22.2 | 4.39 | 13.2 |
| Endrin | 106 | 5.16 | 0.370 | 1.11 | 8.71 | 26.1 | 4.55 | 13.6 |
| p.p’-DDD | 44.5 | 10.2 | 8.78 | 26.4 | 13.1 | 39.3 | 0.680 | 2.04 |
| Endrin aldehyde | 104 | 5.40 | 8.39 | 25.2 | 7.93 | 23.8 | 5.18 | 15.6 |
| p.p’-DDT | 90.6 | 10.6 | 0.800 | 2.39 | 13.3 | 39.8 | 23.7 | 71.1 |
Table 1: Mean recovery of blank sample added with various standard reference materials examined in this study (n=3).
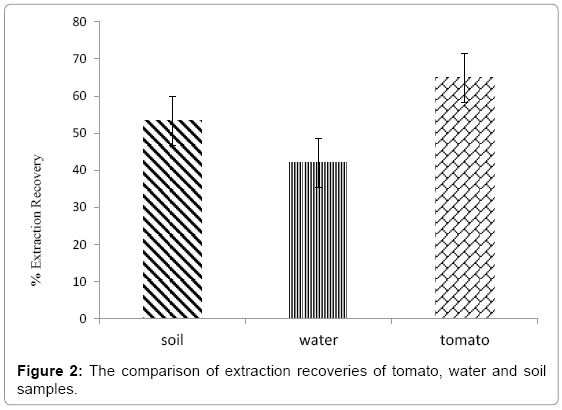
The percentage extraction recoveries of pesticides calculated in soil, water and tomato samples are given in Figure 2. Data given is the averages of all extractable pesticides for each sample matrix. The extraction recoveries of tomato were the highest (64.9%) and lowest was observed for water (42.0%). This was an unexpected results as the water is simpler matrix as compared to tomato and soil.
Occurrences of pesticides
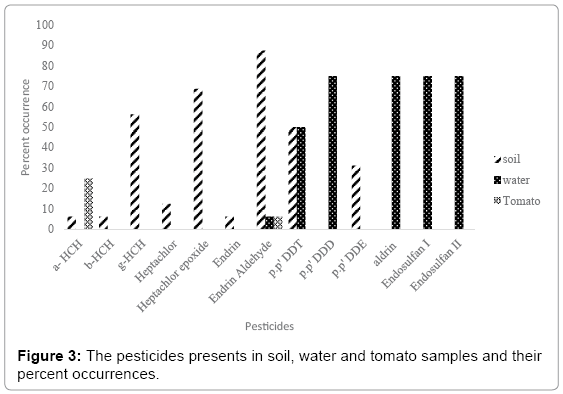
Thirty-six samples were analyzed and two third of the samples were observed with the contamination of different pesticides. The Figure 3 shows the occurrences of pesticides in soil (n=16), water (n=4) and tomato (n=16) samples. In total thirteen pesticides were detected. In soil samples a-HCH, b- HCH, and g-HCH, heptachlor, heptachlor epoxide, Endrin, Endrin aldehyde, p.p’ DDT and p.p’ DDE were observed. A comparison study was done and similar pesticides were obtained in Egypt [27], Germany [28] and China [29]. In water samples six pesticides were observed Aldrin, Endosulfan I, Endosulfan II, Endrin aldehyde p.p’ DDT and p.p’ DDD. The contamination of water samples with different pesticides were calculated as 75 percent and compared with observed pesticides in Portugal [30] Egypt [31], China [32] and Brazil [33]. Aldrin, Endosulfan I, Endosulfan II were only observed in water matrix. The contamination of water in this study could be explained by the runoff of irrigated water. The solubility of these pesticides is very low and their contribution is low. Only two pesticides were observed in tomato samples a-HCH and Endrin aldehyde, a-HCH was observed in 12.5 percent samples and Endrin aldehyde was observed in half of the samples in Field 1. Tomato obtained from Field 2, none of the pesticides residue were observed. A comparison is done with a study in Egypt and it also indicated similar pesticides present in tomato samples [34,35]. Endrin aldehyde was the only pesticide which has been detected in all three types of samples. This is probably due to abundant use of pesticides in Turkey, in a study in Tokat and Canakkale, Turkey, the average usage of different pesticides per hectare land concerning their active ingredients were observed as follows: 7,760 g (insecticides) and 1,200 g (fungicides), 2,640 g (herbicides) and 450 g (acaricides), respectively [36,37]. Another study done in Izmir, Turkey, the average usage per hectare land concerning the active ingredients of pesticides were 228 g (insecticides), 1,367 g (fungicides), 9 g (acaricides), and 1,007 (herbicides) g, respectively [38]. These values are higher than some western countries. As a matter of fact, that there is no strict control of pesticide consumption in the country.
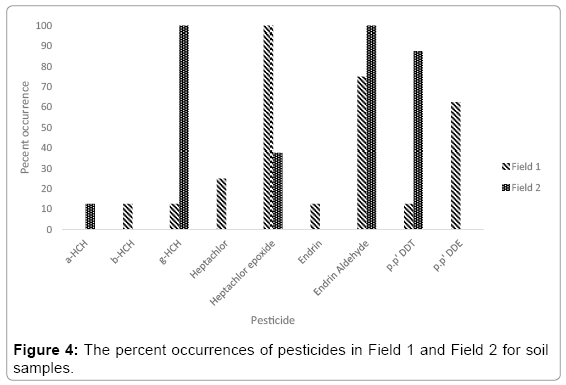
Since the sampling was conducted in two different fields owned by different people, the percent occurrences for each field were calculated separately. Figure 4 shows the observed pesticides as percent occurrences in Field 1 and Field 2. As it can be seen from the Figure 4, all samples contain Heptachlor epoxide and 75 percent samples have Endrin Aldehyde. The p.p’ DDE was detected more than half of the soil samples in Field 1. All the samples in Field 2 contain Endrin aldehydes and g-HCH. The pesticides observed in both fields are Endrin aldehydes, heptachlor epoxide and g-HCH. Though p.p’ DDT was banned since from 1960, still its derivatives are detected. The pesticides spraying was done in different periods and the DDT degraded to DDE in Field 1 but it was seen as DDT in Field 2. This result clearly indicates different amount of pesticides used at different times by different owners. Similar results were obtained elsewhere. For example; in a study in Egypt in soil samples a-HCH, Heptachlor, heptachlroepoxide and p.p’ DDT pesticides were detected [27]. A similar study conducted in Germany, a-HCH, b-HCH, p.p’ DDE and p.p’ DDT pesticides were detected [28]. In China a study conducted a-HCH, b-HCH, Heptachlor, p.p’ DDE, p.p’ DDT, Endrin and Endrin aldehyde pesticides were observed [29].
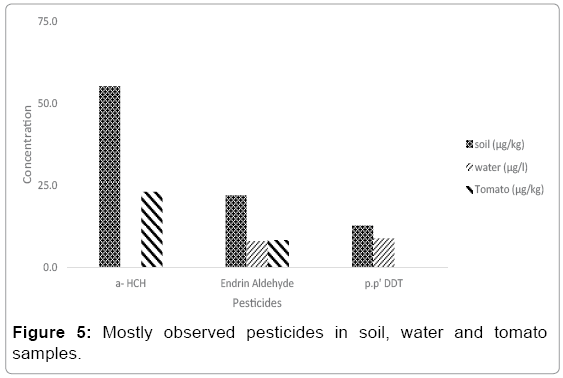
Figure 5 shows pesticide concentrations; which were observed at least in two matrices. Endrin aldehydes was observed in all different samples matrices and p.p’ DDT was seen in water and soil. However, a- HCH was seen in Tomato and Soil samples. The concentration in water for p.p’ DDT was multiplied by 10. The concentration in soil for Endrin aldehyde was divided 10. If speciation ratio was calculated for Endrin aldehyde; soil/water was 27.4, Soil/Tomato was 26.4 and Tomato/ water was 1.04. For a-HCH the ratio of Soil/ Tomato was 2.40 and P.P’ DDT Soil/ Water was 14.4. Soil/Tomato ratio shows almost the same variation for Endrin aldehyde and a- HCH. In Turkey Endrin aldehyde is used widely. In different studies Endrin aldehyde was observed in air [39], water [40], soil and vegetables.
Individual pesticides concentrations with their standard deviations in soil, water and tomato samples are listed in Table 2. Endrin aldehyde is usually high in soil and tomato samples 219 μg/kg and 8.30 μg/kg respectively. A study undertaken in Ghana reports that the concentration of endrin aldehyde as 10.0 μg/kg which is higher than the values observed in this study [35], as it was mentioned above only two pesticides were detected in tomato samples. Among these two a-HCH (23.0 μg/kg) was not seen in water samples and seen almost half of the soil concentrations. This indicate the transfer from soil not from water to the produce.
| Soil (n=16) | Water (n=4) | Tomato (n=16) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| MRL (WHO) μg/l |
MRL (CA) μg /Kg |
Mean μg/kg |
SD | Range μg /Kg |
Mean μg /L |
SD | Range μg /L |
Mean μg/ kg |
SD | Range μg/Kg |
|
| alpha-HCH | 2.0 | 10.0 | 55.2 | - | 0-55.2 | - | - | - | 23.0 | 26.7 | 14.2-69.3 |
| beta-HCH | 2.0 | 10.0 | 3.80 | - | 0-3.80 | - | - | - | - | - | - |
| gamma-HCH | 2.0 | 10.0 | 41.2 | 29.6 | 12.1-99.2 | - | - | - | - | - | - |
| Heptachlor | 0.03 | 10.0 | 10.7 | 5.31 | 6.98-14.5 | - | - | - | - | - | - |
| Aldrin | 0.01 | 100 | - | - | - | 0.257 | 0.0182 | 0.237-0.273 | - | - | - |
| Heptachlroepoxide | 0.03 | 0.600 | 10.3 | 5.91 | 5.34-21.9 | - | - | - | - | - | - |
| Endosulfan I | 0.01 | 500 | - | - | - | 2.88 | 2.42 | 1.45-5.68 | - | - | - |
| Endosulfan II | 0.01 | 500 | - | - | - | 1.83 | 0.777 | 1.09-2.68 | - | - | - |
| p.p’-DDE | 2.0 | 50.0 | 15.0 | 7.49 | 5.69-25.4 | - | - | - | - | - | - |
| Endrin | 0.002 | 50.0 | 0.815 | - | 0-0.815 | - | - | - | - | - | - |
| p.p’-DDD | 2.0 | 50.0 | - | - | - | 0.188 | 0.0812 | 0.103-0.370 | - | - | - |
| Endrin aldehyde | 0.002 | 0.300 | 219 | 117 | 81.4-511 | 8.01 | 3.81 | 5.34-12.4 | 8.30 | 31.2 | 0-8.30 |
| p.p’-DDT | 2.0 | 50.0 | 12.7 | 26.6 | 1.22-10.2 | 0.879 | 0.177 | 0.753-1.01 | - | - | - |
Table 2: Organochlorine pesticides (OCPs) detected from soil, water and tomato samples.
The new standards for pesticides are emerging almost every year. The main two organizations World Health organization (WHO) and Codex Alimentarius (CA) have different set of Standard for Maximum Residue Level (MRL), which can be seen from Table 2. The pesticides mean concentrations and ranges of this study were also given for soil, water and tomato samples. Standard deviation was calculated and the numbers of samples are given in parentheses. According to the Table 2, order of pesticides contamination was soil>water>tomato except Endrin aldehyde. For Endrin aldehyde order changed as soil>tomato>water.
Statistical analysis
ANOVA test was applied to understand if there were differences in concentration of pesticides measured in Turkey and other countries. Pesticides observed in all compared countries are used. In this case Heptachlor epoxide, p.p’ DDT and HCH were chosen for soil, water and tomato respectively.
ANOVA analysis decomposes the variance of the data into two components: 1- between- the groups and 2- within- the groups. The F-ratio was calculated as ratio of the between-group to within-group of estimate. The P-value of the F-test was calculated and if P-value is less than 0.05 then, there is a statistically significant difference between the means of the all variables at the 95.0% confidence level [41].
ANOVA analysis was performed by using stat graphic package program [42]. Results are listed in Table 3. This table was prepared with the available data in the literature. That is why compared countries for each matrix are different.
| Countries | Pesticide | F- ratio | P-value |
|---|---|---|---|
| *Soil | Heptachlor epoxide (ug/kg) | ||
| Turkey, | 10.4 | 6.96 | 0.0128 |
| China, | 0.200 | ||
| Czech Republic | 0.720 | ||
| **Water | P.P' DDT (ug/l) | ||
| Turkey | 0.879 | 4.21 | 0.0719 |
| Portugal | 0.00100 | ||
| Egypt | 2.30 | ||
| ***Tomato | HCH (ug/kg) | ||
| Turkey | 23.0 | 36.0 | 0.0005 |
| Ghana | 0.00000300 | ||
| Egypt | 40.0 |
Where, *Czech Republic: from Ref [42], China: from Ref [29], ** Portugal: from Ref [30], Egypt: from Ref [31], *** Egypt: from Ref [34], Ghana: from Ref [35].
Table 3: Literature comparison by Anova analysis of soil, water and tomato samples.
Heptachlor epoxide was the analyte compared for soil among the countries; Turkey, China, and Czech Republic. Turkey has the highest concentration and China has the lowest concentration. The F-ratio was 6.96 and P-value was 0.0128. Hence, there is no statistically significant difference between the other countries but, there is a statistically significant difference between Turkey - China and Turkey-Czech Republic at the 95.0% confidence level.
For water samples, P.P’DDT was compared in between these countries; Turkey, Portugal and Egypt. The F-ratio was 4.21 and P-value was 0.0719. According to test results, there is a statistically significant difference between Portugal and Egypt at 95% confidence level. Turkey is between these countries. Water in Egypt was more polluted, and Portugal was less polluted.
Turkey, Ghana and Egypt data were compared for tomato samples and the analyte was HCH. The F-ratio was 36 and P-value was 0.0005. According to test results there was a statistically significant difference between these three countries at 95.0% confidence level. Turkey is between these countries. Tomatoes in Ghana have the highest amount of residue and Egypt has the least.
Health risk estimation
The study of fresh fruits and vegetables always leads to a question; is it safe? To answer this question health risk (HR) estimation were done. Hazard risk can be calculated by using the reference dose (RD) [35,36] and acceptable daily intake (ADI) [43]. Health guidelines assumes that hypothetical body weight of 10 kg for children and 70 kg for adults; and maximum absorption rate of 100% and bioavailability rate of 100%. Food consumption rate for fruits in Turkey was estimated to be 0.250 kg/person/day [44]. The exposure dose (ED) and Estimated daily intake (EDI) were calculated as follows:
Exposure dose (ED and EDI)=(Conc. Pesticide (mg/kg)) × Food consumption (kg/day)/ Body weight (kg)
Exposure dose can be calculated either using RD or ADI values. Calculations were done based on reference dose (RD) and acceptable daily intake (ADI) values and listed in Table 4. The hazard index (HI) calculation can be formulated as follows: HI=ED/RD and HI=EDI/ ADI. Hazard Index (HI) provides safe levels of exposure over the life time. The reference value for Health risk (HR) was taken as one [35]. HI value is greater than one mean there is a health risk of consumption of that product less than one which in turn means no risk. The health risk for tomato consumption were calculated for Endrin Aldehyde and a-HCH, which are the two pesticides observed in tomato and results are given in Table 3. HI’s based on either ED or EDI indicated that it is harmful for children but not for adult. In a similar studies in Ghana HI were calculated based on RD and it was found harmful for children and toxic for adult [35,45].
| Calculated based on RD | Calculated based on ADI | |||||||
|---|---|---|---|---|---|---|---|---|
| Pesticide | Mean mg/kg | *RD mg/kg/day | ED mg/kg/day | HI | **ADI mg/Kg/day | EDI mg/Kg/day | HI | HR |
| a-HCH | 0.023 | 0.0030 | 0.0000823 (Adult) 0.000576 (Children) |
0.0274 0.192 | 0.005 | 0.0000823 (Adult) 0.000576 (Children) |
0.0165 0.115 | No No |
| Endrin Aldehyde | 0.0083 | 0.0002 | 0.297 (adult) 0.000207 (children) |
0.148 1.04 | 0.0002 | 0.0000297 (Adult) 0.000208 (Children) |
0.148 1.04 | No Yes |
Table 4: Health Risk for Tomato Consumption calculated by RD and ADI.
Conclusion
Based on study performed with GC-MS all three matrices are contaminated with Pesticides. The order of contamination as follows Soil>Water>Tomatoes. A statistical SPSS package programs used to calculate ANOVA, according to results, Turkey was in middle among Ghana and Egypt for tomato contaminations at 95% confidence level. Speciation ratio was calculated for Endrin aldehyde, a-HCH and DDT; for Endrin aldehyde soil/water was 27.4, Soil/Tomato was 26.4 and Tomato/water was 1.04. For a-HCH the ratio of Soil/ Tomato was 2.40 and DDT then Soil/ Water was 14.4. Soil/Tomato ratio shows almost the same variation for Endrin aldehyde and a- HCH. Health risk assessment was done with reference dose (RD) and acceptable daily intake (ADI) values and the results was almost the same. It indicated that the consumption of tomatoes was harmful for children not for adult.
Acknowledgments
This study is funded by METU research Grant No: BAP-07-02-2012-007-184.
References
- Şik B (2013) Pesticides and food safety in the era of global warming. Heinrich BöllStiftung, pp: 30-32
- Murrell EG (2017) Can agricultural practices that mitigate or improve crop resilience to climate change also manage crop pests? Current Opinion in Insect Science 23: 81-88.
- Jennings AA, Li Z (2015) Residential surface soil guidance applied worldwide to the pesticides added to the Stockholm Convention in 2009 and 2011. Journal of Environmental Management 160: 226e240
- Hong Z, Yonglong L, Dawson RW, Yajuan S, Tieyu W (2005) Classification and ordination of DDT and HCH in soil samples from the Guanting Reservoir. China Chemosphere 60: 762-769.
- Zhou R, Zhu L, Yang K, Chen Y (2006) Distribution of organochlorine pesticides in surface water and sediments from Qiantang River, East China. J Hazard Mater 137: 68-75.
- California Environmental Protection Agency (CEPA) (2014) Chemicals Known to the State to Cause cancer or Reproductive Toxicity.
- International Agency for Research on Cancer (IARC) (2011) Agents Classified by the IARC Monographs.
- Latif Y, Sherazi STH, Bhanger MI (2011) Assessment of pesticide residues in commonly used vegetables in Hyderabad. Pakistan Ecotoxicology and Environmental Safety 74: 2299-2303.
- Shah J, Jan MR, Saeed K (1998) Separation and speciation of organophosphorus pesticides based on hydrolysis using reverse phase HPLC. Jour Chem Soc Pak 20: 264-267.
- Connell DW (2005) Basic Concepts of Environmental Chemistry, pp: 168-175
- World Health Organization (WHO) (2006) WHO Gives Indoor Use of DDT a Clean Bill of Health for Controlling Malaria.
- http://www.yms.org.tr/files/downloads/istatistikler/2016/ymsdegerlendirme-raporu-ocak-haziran-2016.pdf
- Yinhai L, Zhengmei C, Xinhua N (2005) Extraction of Organochlorine Pesticides in Sediments Using Soxhlet, Ultrasonic and Accelerated Solvent Extraction Techniques. J Ocean 4: 173-176.
- Bidari A, Ganjali MR, Norouzi P, Hosseini MRM, Assadi Y (2011) Sample preparation method for the analysis of someorganophosphorus pesticides residues in tomato by ultrasoundassisted solvent extraction followed by dispersive liquid–liquid microextraction. Food Chem 126: 1840-1844.
- Wu G, Bao X, Zhou S, Wu J, Han A, et al. (2011) Analysis of multi pesticide residues in the foods of animal origin by GC–MS coupled with accelerated solvent extraction and gel permeation chromatography clean-up. Food Chem 126: 646-654.
- Fenoll J, Hellin P, Martinez CM, Miguel M, Flores P (2007) Multiresidue method for analysis of pesticides in pepper and tomato by gas chromatography with nitrogen-phosphorus detection. Food Chem 105: 711-719.
- Wang X, Zhao X, Liu X, Li Y, Fu L, et al. (2008) Homogenous liquid–liquid extraction combined with gas chromatography electron capture detector for the determination of three pesticide residues in soils. Anal Chim Acta 620: 162-169.
- Pirard C, Widart J, Nguyen BK, Deleuze C, Heudt L, et al. (2007) Development and validation of a multi-residue method for pesticide determination in honey using on-column liquid–liquid extraction and liquid chromatography– tandem mass spectrometry. J Chromatogr A 1152: 116-123.
- Ozcan S, Tor A, Aydin ME (2009) Application of miniaturised ultrasonic extraction to the analysis of organochlorine pesticides in soil. Analytica Chimica Acta 640: 52-57.
- Doong R, Liao PL (2001) Determination of organochlorine pesticides and their metabolites in soil samples using headspace solid-phase microextraction. J Chromato A 918: 177-188.
- Albero B, Sanchez-Brunete C, Tadeo JL (2005) Multiresidue determination of pesticides in juice by solid-phase extraction and gas chromatography–mass spectrometry. Talanta 66: 917-924.
- Wang S, Yang S, Ren L, Qian C, Liu F, et al. (2009) Determination of organophosphorus pesticides in leeks (Allium porrum L.) by GC–FPD. Chromatographia 69: 79-84.
- Arrebola FJ, Vidal JLM, Rodriguez MJG, Frenich AG, Morito NS (2003) Reduction of analysis time in gas chromatography: Application of low-pressure gas chromatography–tandem mass spectrometry to the determination of pesticide residues in vegetables. Journal of Chromatography A 1005: 131-141.
- Manirakiza P, Covacia A, Nizigiymana L, Ntakimazi G, Schepens P (2002) Persistent chlorinated pesticides and polychlorinated biphenyls in selected fish species from Lake Tanganyika, Burundi, Africa. Environmental Pollution 117: 447-455.
- Tuncel SG, Oztas NB, Erduran MS (2008) Air and groundwater pollution in an agricultural region of the Turkish Mediterranean coast. J Air Waste Manag Assoc 58: 1240-1249.
- United States Environmental Protection Agency (USEPA) (2003) Determinative chromatographic separations method 8000C.
- Ahmad MT, Ismail SMM, Mabrouk SS (1998) Residues of some chlorinated hydrocarbon pesticides in rain water, soil and ground water, and their influence on some soil microorganisms.
- Kiersch K, Jandl G, Meissner R, Leinweber P (2010) Small scale variability of chlorinated POPs in the river Elbe floodplain soils (Germany). Chemosphere 79: 745-753.
- Zhou Q, Wang J, Meng B, Cheng J, Lin G, et al. (2013) Distribution and sources of organochlorine pesticides in agricultural soils from central China. Huazhong University of Science and Technology 93: 163-170.
- Pinto MI, Sontag G, Bernardino RJ, Noronha JP (2010) Pesticides in water and the performance of the liquid-phase microextraction based techniques. A review. Microchemical Journal, pp: 282-516.
- El-Kabbany S, Rashed MM, Zayed MA (2011) Monitoring of the pesticide levels in some water supplies and agricultural land 72: 11-21.
- Zhang C, Liao X, Li J, Xu L, Liu M, et al. (2013) Influence of long-term sewage irrigation on the distribution of organochlorine pesticides in soil–groundwater systems. Chemosphere 92: 337-343.
- Rissato SR, Galhiane MS, Ximenes VF, de Andrade RMB, Talamoni JLB, et al. (2006) Organochlorine pesticides and polychlorinated biphenyls in soil and water samples in the North eastern part of Sao Paulo State, Brazil. 65: 1949-1958
- Abou-Arab AAK (1999) Behavior of pesticides in tomatoes during commercial and home preparation. Food Technology Department, National Research Center, Dokki, Cairo, Egypt.
- Bempah CK, Donkor A, Yeboah PO, Dubey B, Osei-Fosu P (2011) A preliminary assessment of consumer’s exposure to organochlorine pesticides in fruits and vegetables and the potential health risk in Accra Metropolis, Ghana. Food Chemistry 128: 1058-1065.
- Esengun K, Erdal G, Gunduz O, Erdal H (2007) An economic analysis and energy use in stake-tomato production in ToÂkat province of Turkey. Renewable Energy 32: 1873-1881.
- Turhan S, Özbag BC, Rehber E (2008) A comparison of energy use in organic and conventional tomato production. J Food Agric Environ 6: 318-321.
- Engindeniz S (2006) Economic analysis of pesticide use on proÂcessing tomato growing: a case study for Turkey. Crop Prot 25: 534-541.
- Ozcan S, Aydin ME (2009) Polycyclic aromatic hydrocarbons, polychlorinated biphenyls and organochlorine pesticides in urban air of Konya, Turkey. Atmospheric Research 93: 715-722.
- Turgut C (2003) The contamination with organochlorine pesticides and heavy metals in surface water in Kucuk Menderes River in Turkey. Turkey Environment International 29: 29-32.
- Shegunova P, Klanova J, Holoubek I (2007) Residues of organochlorinated pesticides in soils from the Czech Republic. Environmental Pollution 146: 257-261.
- Akbay C, Boz I, Chern WS (2007) Household food consumption in Turkey. European Review of Agricultural Economics 34: 209-231.
- Augustine D, Crentsil KB, Brajesh KD, Nelson A (2015) Chlorinated Pesticide Residues in Selected Fruits from Some Ghanaian Markets and the Possible Impact on Children’s Health. Res J Chem Env Sci 3: 1-9.
Citation: Sifatullah KM, Tuncel GS (2018) Study of Pesticide Contamination in Soil, Water and Produce Using Gas Chromatography Mass Spectrometry. J Anal Bioanal Tech 9:409. DOI: 10.4172/2155-9872.1000409
Copyright: © 2018 Sifatullah KM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 7298
- [From(publication date): 0-2018 - Dec 09, 2025]
- Breakdown by view type
- HTML page views: 6255
- PDF downloads: 1043