Case Report Open Access
Successful Antibiotic Treatment of Mycobacterium abscessus Pulmonary Disease in an Immunocompetent Individual
| Anna Beltrame1* Giovanni Cattani1 Federica Brillo1 Maria Merelli1 Claudio Scarparo2 Giuseppe Como3 Maria Consuelo Screm2 Tortoli4 Alberto Matteell5 | ||
| 1Clinic of Infectious Diseases, S.M. Misericordia University Hospital, P.le S.M. Misericordia 15, 33100 Udine, Italy | ||
| 2Microbiology Department, S.M. Misericordia University Hospital, Udine, Italy | ||
| 3Institute of Diagnostic Radiology, S.M. Misericordia University Hospital, Udine, Italy | ||
| 4Regional Reference Center for Mycobacteria, Microbiology and Virology Laboratory, Careggi University Hospital, Firenze, Italy | ||
| 5Institute of Infectious and Tropical Diseases, Spedali Civili University Hospital, Brescia, Italy | ||
| Corresponding Author : | Anna Beltrame Clinic of Infectious Diseases S.M. Misericordia University Hospital P.le S.M. Misericordia 15 33100 Udine, Italy Tel: +39.0432 559354 Fax: +39.0432 559360 E-mail: beltrame.anna@aoud.sanita.fvg.it |
|
| Received March 18, 2013; Accepted April 08, 2013; Published April 13, 2013 | ||
| Citation: Beltrame A, Cattani G, Screm MC, Brillo F, Merelli M, et al. (2013) Successful Antibiotic Treatment of Mycobacterium abscessus Pulmonary Disease in an Immunocompetent Individual. J Infect Dis Ther 1:103. doi:10.4172/2332-0877.1000103 | ||
| Copyright: © 2013 Beltrame A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | ||
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
||
Visit for more related articles at Journal of Infectious Diseases & Therapy
Abstract
The number of subjects with Mycobacterium abscessus lung disease is increasing. The optimal treatment to prevent clinical relapse in these patients has not been well established. At times, antibiotic regimens have also been shown to produce long-term sputum conversion. We describe a case of M. abscessus lung disease in an immunocompetent individual with successful outcome at 24 months, following antibiotic therapy. Initially, an empirical treatment was started intravenously with amikacin (700 mg once-daily), cefoxitin (4 g every 8 h), and oral clarithromycin (500 mg twice daily). Although, the symptoms resolved rapidly, the anti-mycobacterial drugs were discontinued three weeks later, following the onset of hepatotoxicity. Two weeks after discontinuation of therapy, the liver enzymes were returned to normal levels, and amikacin and clarithromycin were reintroduced and continued for another 8 months, when the individual developed hearing loss, which led to further discontinuation of antibiotic therapy. During the subsequent 24 months, broncoscopy, sputum AFB examination and mycobacterial culture were negative. Our management suggests that prompt, individualized and protracted treatment can be curative, in case of mild disease.
| Keywords | |
| Mycobacterium abscessus; Rapidly growing mycobacteria; Nontuberculous mycobacteria; Antibiotic therapy; Adverse events | |
| Introduction | |
| Pulmonary disease caused by Mycobacterium abscessus (M. abscessus) is usually described in individuals with underlying chronic lung disease [1-3]. Long-term sputum conversion is uncommon, following antibiotic treatment [4-5]. Therefore, the American Thoracic Society (ATS)/Infectious Diseases Society of America (IDSA) suggest a combination of long-term treatment with multiple antibiotics, and sometimes surgical resection of the lung sites involved in cases of refractory disease [4]. In recent years, the number of subjects developing lung disease due to M. abscessus without underlying disorders, has been increasing [2,6]. Mechanisms responsible of invasive disease in this population are unknown [1]. Furthermore, optimal treatment strategies to prevent the onset of relapse in these patients have not been well established. | |
| Case Report | |
| On December 6, 2007 a 75-year-old male was admitted to the Emergency Unit with acute chest pain and cough. His medical history included hypertension, hyperlipidemia, and a childhood history of pulmonary TB. | |
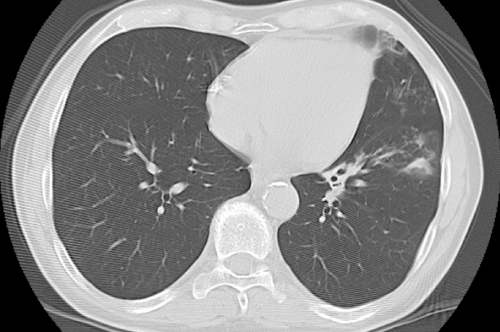
| On admission, the patient was apyretic, with no evidence of abnormal physical findings. Chest radiograph was negative, whereas a chest CT revealed two nodules located in the lingular segment, and one nodule in the left lower lobe (Figure 1A). Because there were no signs of active infection, a strategy of close clinical and radiological monitoring was adopted. Two weeks later, the patient developed a productive cough without fever, and empirical levofloxacin was initiated, followed by rapid resolution of symptoms. A second chest CT performed two months after the first revealed a progression of the radiological findings, with extension of the three nodular densities and appearance of bronchiectasis (Figure 1B). Because the patient was asymptomatic, these findings were interpreted as a radiological scar of the recent infectious pulmonary event. However, considering the history of TB, three consecutive sputum samples were collected in March 2008, which were negative on direct smear microscopy for acid-fast bacilli (AFB), and nucleic acid amplification test (NAAT) for M. tuberculosis. | |
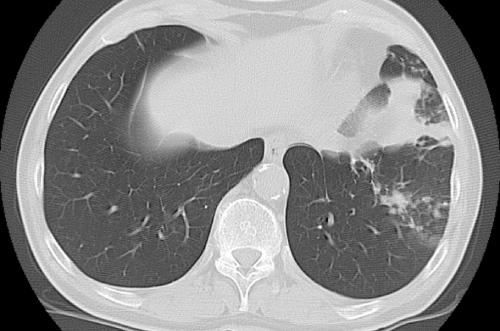
| At the end of March 2008, the patient presented severe cough with sticky yellowish sputum. Direct sputum microscopy revealed AFBs, whereas the NAAT for M. tuberculosis was negative. The patient was admitted to the infectious diseases clinic with suspected pulmonary TB. On admission, the chest CT revealed new zones of nodular thickening associated with dense streaks in the left lower lobe, with a gross area of parenchymal consolidation (Figure 1C). | |
| The result of a direct smear for AFB in the bronchoalveolar lavage (BAL) was positive; NAATs for M. tuberculosis and culture for bacteria were negative. At that time, the culture on both solid (Löwenstein– Jensen) and liquid (MGIT™ Becton Dickinson, Sparks, MD USA) media of the first sputum sample collected in March, yielded the presence of acid-fast bacilli result. The culture isolate was identified by analysis of its characteristics on culture (time for growth, colony morphology, pigment production, ability of the isolate to grow at various temperatures on 5% sheep blood agar and Löwenstein–Jensen slants), as M. abscessus [4]. Of the three sputum samples collected in April, two were positive for M. abscessus. Subsequently, BAL specimens further confirmed the diagnosis of NTM lung disease due to M. abscessus. | |
| Empirical treatment was started intravenously with amikacin (700 mg once-daily), cefoxitin (4 g every 8 h), and oral clarithromycin (500 mg twice daily). Sotalol, ramipril, atorvastatin and acetyl salicylic acid were also prescribed to the patient. Antibiotic susceptibility testing of M. abscessus revealed resistance to ciprofloxacin, moxifloxacin, cotrimoxazole, doxycycline, susceptibility to clarithromycin and amikacin, and indeterminate susceptibility to cefoxitin and linezolid. The patient’s clinical symptoms showed marked improvement, following the initiation of therapy. Follow-up investigations after the initiation of treatment included complete blood cell count, serum creatinine, liver function test and therapeutic drug monitoring (TDM) of amikacin twice weekly, electrocardiogram, and an audiometric test at baseline and monthly. | |
| After three weeks of treatment, the patient showed marked increase in liver enzymes (AST 94 UI/L, ALT 245 UI/L, gamma glutamyl transpeptidase 125 UI/L, alkaline phosphatase 231 UI/L), and thereafter, all the anti-mycobacterial drugs and atorvastatin were discontinued. | |
| Two weeks after discontinuation of therapy, the liver enzymes returned to normal levels, and amikacin and clarithromycin were reintroduced one at a time, uneventfully. Cefoxitin and atorvastatin were not reintroduced, being considered as the most likely cause of hepatotoxicity [5,7]. | |
| On May 20, 2008, the patient was discharged, continuing daily administration of antimycobacterial drugs at the outpatient clinic. After 8 months of treatment, the patient experienced hearing loss potentially associated with use of amikacin, which led to termination of antimycobacterial therapy. Two months after the treatment course, a chest CT scan showed complete resolution of the nodules. In addition, a bronchoscopy was performed, with negative result of direct smear for AFB and mycobacterial culture. | |
| During follow-up, the patient began experiencing episodes of fever and productive cough, with yellow-green sputum compatible with acute pneumonia. The chest CT scan performed during each episode revealed the appearance of new and different radiological abnormalities compatible with bacterial pneumonia. The culture of sputum collected during each episode relived the growing, in order of S. aureus, M. catarrhalis and P. fluorescens. Sputum examinations for AFB and mycobacterial culture were always negative, to confirm the cure of M. abscessus pulmonary disease. | |
| Discussion | |
| This case report confirms that M. abscessus lung disease can affect immunocompetent subjects, with a history of previous TB. Lyu et al. [8] showed that 61% of patients with M. abscessus lung disease presented a history of pulmonary TB. | |
| Secondly, the treatment of M. abscessus lung disease is complex. In vitro, M. abscessus is susceptible only to parenteral agents and newer oral macrolides [4-5]. Since monotherapy with oral clarithromycin could result in the acquisition of macrolide resistance, this drug should always be combined with parenteral antibiotics [4,8,9]. Jeon et al. [5] assessed the efficacy of 24 months of clarithromycin, ciprofloxacin and doxycycline, along with an initial 4-week course of amikacin and cefoxitin in 65 patients with M. abscessus lung disease. Interestingly, only 58% of these cases underwent culture conversion, 12 months following the treatment period [5]. | |
| A recent retrospective investigation evaluated the clinical and microbiologic outcome in 107 patients with M. abscessus lung disease, treated with antibiotic combination therapy (4.6 drugs per patient, with a median duration of intravenous administration of six months), and surgical resection, or antibiotic combination therapy alone. Of these, only 38% of the patients were culture negative for at least 1 year, without experiencing relapses. Of note, the outcome was significantly worse in patients who received antibiotic therapy only, without adjunctive surgical treatment [10]. Another retrospective study compared two regimens, including a macrolide, and one parenteral agent (amikacin), or macrolide, and two parenteral agents (amikacin and cefoxitin, or imipenem), for 511 days [8]. Parenteral drugs were used for a minimum of 2-4 months, and a maximum of up to 20 months. The success rate of treatment was 80.5% at a median of 151 days, without significant differences in treatment success, and relapses rates between the groups receiving one and two parenteral agents [8]. | |
| Our treatment regimen involved the administration of amikacin and clarithromycin for 8 months, with the addition of cefoxitin in the first 3 weeks. The symptoms resolved rapidly, and long-term sputum conversion was observed during the subsequent 24 months. Our management suggests that prompt, individualized and protracted treatment can be curative, in cases of mild disease. However, these drugs are reportedly toxic [5,10]. One study showed the onset of adverse reactions in 43.9% of the cases treated with multidrug regimens [8], the most frequent being drug-induced liver injury (17.1%). Others have reported a discontinuation of cefoxitin, owing to development of hepatotoxicity in 60% of the cases after a median of 22 days, requiring substitution with imipenem [5]. | |
| In conclusion, although M. abscessus lung disease was successfully cured, it might still predispose to other secondary infectious complications. The patient presented recurrent episodes of pneumonia, following the mycobacterial lung disease, suggesting that bronchiectasis can be both a risk for, and a consequence of M. abscessus infection [4]. | |
| Acknowledgements | |
| We thank Gabriele Pinsi for technical assistance. | |
| References | |
References
- De Groote MA, Huitt G (2006) Infections due to rapidly growing mycobacteria. Clin Infect Dis 42: 1756-1763.
- Griffith DE (2010) Nontuberculous mycobacterial lung disease. Curr Opin Infect Dis 23: 185-190.
- Daley CL, Griffith DE (2010) Pulmonary non-tuberculous mycobacterial infections. Int J Tuberc Lung Dis 14: 665-671.
- Griffith DE, Aksamit T, Brown-Elliot BA, Catanzaro A, Daley C, et al. (2007) An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175: 367-416.
- Jeon K, Kwon OJ, Lee NY, Kim BJ, Kook YH, et al. (2009) Antibiotic treatment of Mycobacterium abscessus lung disease: a retrospective analysis of 65 patients. Am J Respir Crit Care Med 180: 896-902.
- Koh WJ, Kwon OJ, Lee KS (2002) Nontuberculous mycobacterial pulmonary disease in immunocompetent patients. Korean J Radiol 3: 145-157.
- Russo MW, Scobey M, Bonkovsky HL (2009) Drug-induced liver injury associated with statins. Semin Liver Dis 29: 412-422.
- Lyu J, Jang HJ, Song JW, Choi CM, Oh YM, et al. (2011) Outcome in patients with Mycobacterium abscessus pulmonary disease treated with long-term injectable drugs. Respir Med 105: 781-787.
- Wallace RJ Jr, Meier A, Brown BA, Zhang Y, Sander P, et al. (1996) Genetic basis for clarithromycin resistance among isolates of Mycobacterium chelonae and Mycobacteriumabscessus. Antimicrob Agents Chemother 40: 1676-1681.
- Jarand J, Levin A, Zhang L, Huitt G, Mitchell JD, et al. (2011) Clinical and microbiologic outcomes in patients receiving treatment for Mycobacterium abscessus pulmonary disease. Clin Infect Dis 52: 565-571.
Figures at a glance
 |
 |
 |
||
| Figure 1a | Figure 1b | Figure 1c |
Relevant Topics
- Advanced Therapies
- Chicken Pox
- Ciprofloxacin
- Colon Infection
- Conjunctivitis
- Herpes Virus
- HIV and AIDS Research
- Human Papilloma Virus
- Infection
- Infection in Blood
- Infections Prevention
- Infectious Diseases in Children
- Influenza
- Liver Diseases
- Respiratory Tract Infections
- T Cell Lymphomatic Virus
- Treatment for Infectious Diseases
- Viral Encephalitis
- Yeast Infection
Recommended Journals
Article Tools
Article Usage
- Total views: 24390
- [From(publication date):
June-2013 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 19525
- PDF downloads : 4865
