Research Article Open Access
3-Aminopropyltrimethoxysilane and 3-Glycidoxypropyltrimethoxysilane Mediated Synthesis of Graphene and its Nanocomposite: Potential Bioanalytical Appliactions
Prem C Pandey*, Arvind Prakash, Ashish K Pandey and Digvijay Pandey
Department of Chemistry, Indian Institute of Technology, Banaras Hindu University, Varanasi, India
- *Corresponding Author:
- Prem C Pandey
Department of Chemistry
Indian Institute of Technology
Banaras Hindu University
Varanasi-221005, India
Tel: +91 9415813018
Fax: +91 542 2368428
E-mail: pcpandey.apc@iitbhu.ac.in
Received Date: March 18, 2014; Accepted Date: April 23, 2014; Published Date: April 25, 2014
Citation: Pandey PC, Prakash A, Pandey AK, Pandey D (2014) 3-Aminopropyltrimethoxysilane and 3-Glycidoxypropyltrimethoxysilane Mediated Synthesis of Graphene and its Nanocomposite: Potential Bioanalytical Appliactions. J Anal Bioanal Tech S7:012. doi: 10.4172/2155-9872.S7-012
Copyright: © 2014 Pandey PC. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Analytical & Bioanalytical Techniques
Abstract
We report herein, 3-aminopropyltrimethoxysilane (3-APTMS) and 3-glycidoxypropyltrimethoxysilane (3-GPTMS) mediated reduction of graphene oxide into graphene. The resulting graphene has been characterized by UV-VIS and Raman spectroscopy. The as synthesized graphene is used to make polycrystalline nanocomposite of prussian blue having admirable peroxidase mimetic ability. The apparent Km value of the graphene-PB nanodispersion with hydrogen peroxide (H2O2) as the substrate is found to be 6.8 mM. The as synthesized material not only validates the peroxidase-like activity, but also exhibits excellent electrochemical activity for biomedical applications. Electrocatalytic reduction of H2O2 shows electroanalytical sensitivity to the order of 850 μA mM-1cm-2 with lowest detection limit of 10 nM.
Keywords
Functional graphene; Functional alkoxysilanes; Polycrystalline material; Nanocomposite; Peroxidase mimetic; Electrocatalyst
Introduction
Recently, graphene has emerged as a promising material for new technological evolution because continuous network of hexagonally arranged carbon atoms leads to exceptional electronic, mechanical, and thermal properties [1-3]. That is why graphene has played an important role in electronic components, energy-storage materials such as capacitors and batteries, modified electrodes, and mechanical resonators [1-5]. Therefore, tremendous attention has been devoted to the facile chemical synthesis of the graphene with desired properties like crystallinity, solubility along with compatibility in targeted systems [4,5]. However, the controlled synthesis of functional graphene under ambient conditions justifying improved processibility, biocompatibilty and nanogeometry have been prerequisite issues. The use suitable organically functionalized alkoxysilanes precursors during the synthetic protocol of graphene may impart the properties of prerequisite issues followed by introducing the facile rout of graphene synthesis through sol-gel processing. In addition to that the resulting functional graphene may easily be casted into thin films of organically modified silicate (ORMOSIL) [6-9]. The resulting matrix may impart in realizing the potentiality of graphene within nanostructured domains under ambient conditions which has been attempted in the present investigation.
Ormosil materials made from the combination of hydrophilic and hydrophobic components of functional alkoxysialnes have been widely used as nano-structured matrices and have shown potentiality in the design of electrochemical sensors [6-9]. In addition to that some of such functional alkoxysilanes like 3-aminopropyltrimethoxysilane (3-APTMS) and 3- glycidoxypropyltrimethoxysilane (3-GPTMS)) have recently been utilized as reagents and stabilizer during the synthesis of noble metal nanoparticles under ambient [10-12]. Further the active role of 3-APTMS has also been demonstrated during the synthesis of prussian blue nanoparticles (PBNPs) [13,14]. Such reducing and stabilizing ability of 3-GPTMS and 3-APTMS has directed to use the same during the synthesis of graphene from graphene oxide. Accordingly, the results on 3-APTMS and 3-GPTMS mediated conversion of graphene oxide into graphene sol has been attempted in the present investigation.
The use of such 3-APTMS and 3-GPTMS may allow the formation of functional graphene that may possess activity for making nanocomposite with known catalytic material displaying unusual property combinations and unique design possibilities as reported earlier [13,14]. Usually graphene based nanocomposites have been troubled with small specific surface area and low conductivity as well as poor durability, which are ascribed to the inevitable aggregation between individual graphene sheets driven by strong π–π interaction. The use of functional graphene may enforce the formation of nanocomposite with known catalytic material having functional compatibility with as synthesized graphene. Prussian blue naoparticles (PBNPs) are known catalytic material as artificial peroxidase and the functional compatibility of the same with as prepared graphene may enforce the nanocomposite formation imparting exceptional catalytic properties. We have recently reported the synthesis of such functional PBNPs involving the active role of 3-APTMS [13,14]. Such process also allow the synthesis of mixed metal hexacyanoferrate with nearly all combinations of hetero-transition metals [14,15]. Indeed interesting findings on the interaction of PBNPs and graphene has been observed and discussed vide infra. The potential application of as synthesized graphene and its nanocomposite with PBNPs as peroxidase mimetic has been examined. Since the PBNPs display remarkable electrocatalytic behavior during hydrogen peroxide sensing [13,14], the electrochemistry of as prepared graphene-PBNPs nanocomposite has also been studied. Accordingly, present investigation has been attempted to investigate the following: (1) functionalized alkoxysilane mediated controlled synthesis of graphene and its nanocomposite; (2) peroxidase mimetic activity of graphene and its nanocomposite for H2O2 detection; (3) electrocatalytic activity of same for H2O2 reduction. The electrocatalytic and peroxidase mimetic abilities of these nanomaterials are reported in this communication.
Experimental Section
Materials and Methods
3-Aminopropyltrimethoxysilane (3-APTMS), 3-Glycidoxypropyltrimethoxysilane, o-dianisidine, graphite powder (particle size 1–2 mm), Tetrachloroauric acid, and Nujol oil (density 0.838 g mL-1) were obtained from Aldrich Chemical Co., India. Potassium ferricyanide, ethanol, cyclohexanone, hydrogen peroxide ( H2O2) were purchased from Merck, India. The water used in experiments was double distilleddeionized water
All Absorption spectra, kinetic study and electrochemical experiment were conducted in 0.1 M phosphate buffer solution (pH 7.0) containing 0.5 M KCl. The absorption spectra of samples were recorded in corresponding sols using a Hitachi U-2900 Spectrophotometer. Transmission electron microscopy (TEM) studies were performed using Hitachi 800 and 8100 electron microscopes (Tokyo, Japan) with an acceleration voltage of 200 kV. Electrochemical experiments were performed on an Electrochemical Workstation Model CHI660B, CH Instruments Inc., TX, USA, in a three electrode configuration with a working volume of 3 mL. An Ag/AgCl electrode (Orion, Beverly, MA, USA) and a platinum plate electrode served as reference and counter electrodes, respectively. All potentials given in text are relative to the Ag/AgCl. The working electrode was a modified carbon paste electrode (CPE).
Synthesis of graphene
Graphene oxide (GO) was synthesized from as described earlier [16]. It involves the reaction of graphite with strong oxidizers such as sulfuric acid, nitric acid, potassium chlorate, and potassium permanganate. The typical procedure for chemical conversion of graphene oxide to graphene, involves the mixing of 10 ml of 0.5 mg/ ml GO and 100 ml absolute ethanol followed by ultrasonication for 15 min. The resulting exfoliate suspension of GO was further mixed with 1 ml 3-APTMS (0.5 mM) followed by ultrasonication for 30 minute. The resulting suspension was again mixed with 1 ml 3-GPTMS (4 mM) and allowed for stirring for 2-4 h. The yellow suspension of rephene oxide was converted into a dark blackish brown suspension of reduced graphene oxide (RGO)/graphene within 2 hours.
Synthesis of Prussian blue nanoparticles (PBNPs) sol and their composite with graphene
PBNPs were synthesized as described earlier [13]. In a typical procedure, 10 μL 3-APTMS (0.5 M) was added into 50 μL aqueous solution of potassium ferricyanide (0.05 M) under stirring followed by addition of 2 μL of cyclohexanone (9.62 M). The mixture immediately turns to develops bright green color that after vigorous stirring gets converted to deep blue sol of PBNPs. To above, 5 μL HCl (6.5 M) was added and again stirred for 2 min. The synthesis of graphene-PB nanocomposite involves the mixing of graphene dispersion and PBNPs. As a typical case, the graphene dispersion and PBNPs was mixed in the ratio of 1:3 followed by vigorous stirring.
Preparation of graphene-PB composite modified electrode
Graphene-PB dispersion (100 μl) was allowed to be adsorbed over spectroscopic grade graphite particles (100 mg, particle size 1-2 μ) following ultrasonication for 10 min. The adsorbed composite material was left to dry overnight at 100°C. The dried graphite powder was used to fabricate active carbon paste electrode (CPE). The composition of graphite paste (w/w) was; graphite powder = 68%, graphene-PBNPs adsorbed on graphite powder = 2%, and nujol oil = 30%.
Results and Discussion
Role of 3-APTMS and 3-GPTMS in the synthesis of Graphene and its nanocomposite
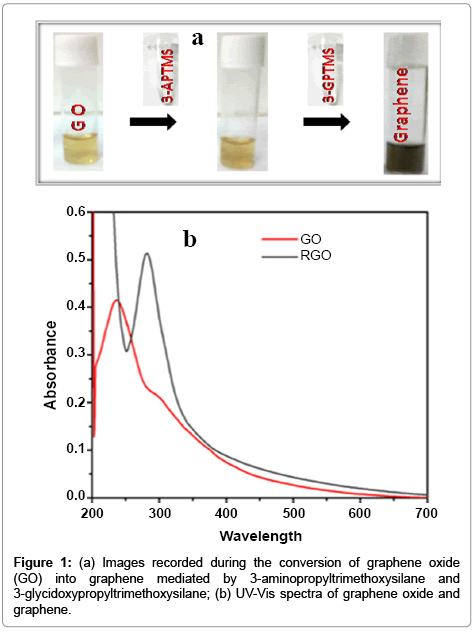
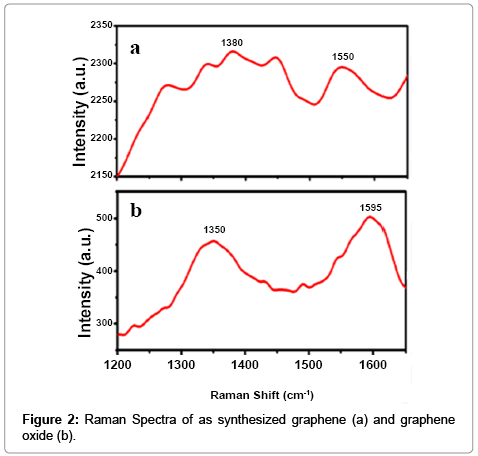
We have studied in details on the role of 3-APTMS and 3-GPTMS during the controlled synthesis of noble metal nanoparticles (AuNPs, AgNPs and PdNPs) [10,11]. It has been found that 3-APTMS capped noble metal salts are converted into their respective nanoparticles in the presence of 3-GPTMS. In addition to that 3-APTMS capped potassium ferricyanide may also be converted into PBNPs of controlled nanogeometry in the presence of cyclohexanone [13]. Such findings directed our attention to investigate 3-APTMS and 3-GPTMS assisted synthesis of graphene dispersion and indeed interesting results on these lines are recorded. The graphene oxide suspension in 3-APTMS undergoes 3-GPTMS mediated conversion at room temperature (25°C) into reduced graphene dispersion. The formation of reduced graphene oxide (RGO) or graphene is confirmed by imaging photography and UV-Vis spectroscopy as shown in Figure 1a and 1b respectively. The images as shown in Figure 1a visually justify the RGO formation and also proved by UV-Vis spectroscopy (Figure 1b). The UV-Vis spectra of graphene represent the characteristic peak at 280 cm-1 that confirm graphene suspension and compared with characteristic peak of GO suspension at 230 cm-1 with a plateau at 304 cm-1 Further, in order to validate the conversion of RGO into graphene, Raman spectroscopy of the same was recorded as shown in Figure 2. The differences of the Raman spectra for the graphene and graphene oxide as shown in Figure 2a and 2b clearly justify the conversion of graphene from graphene oxide. Since the synthesis involves the participation of 3-APTMS, it is important to understand the Raman spectra of 3-Aminopropyltrimethoxysilaneethanol- water system as reported earlier [17]. The D peak and single sharp G peak appear at position of 1380 cm-1 and 1550 cm-1 respectively confirmed the graphene dispersion and neighboring peak at 1280 cm-1 and 1447 cm-1 indicating 3-Aminopropyltrimethoxysilane-ethanolwater system in this dispersion and found consistent with observations reported earlier [17].
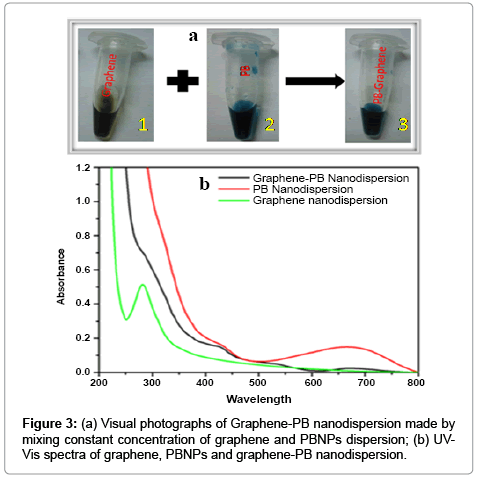
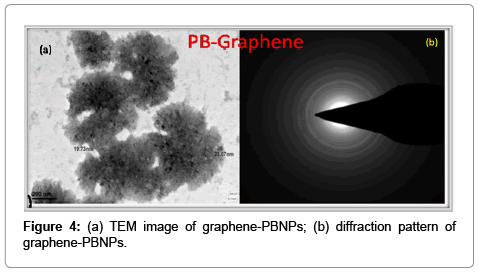
The as prepared graphene or reduced graphene oxide (RGO) is found highly compatible for the synthesis of nanocomposite dispersion with several known nanomaterials. As an example we have been able to make graphene-prussian blue nanoparticles (graphene-PBNPs) nanodispersion having excellent practical utility. We have already studied in details on the synthesis of PBNPs [13]. Under optimum composition the graphene dispersion forms excellent nanocomposite with PBNPs. The images recorded in Figure 3a justify the conversion of dispersion of graphene-PBNPs in an optimal ratio of PBNPs and graphene to the order of 3:1. The UV-Visible spectra of graphene- PBNPs nanocomposite dispersion is shown in Figure 3b that represents the absorption maxima of PBNPs at 666 cm-1 along with a characteristic absorption peak of graphene at 280 cm-1. The resulting graphene- PB nanocomposite justify that PB nanoparticles are embedded in the graphene matrix as shown in TEM (Figure 4a). The diffraction pattern as shown in Figure 4b of the same clearly demonstrates the polycrystalline behavior of graphene-PB nanocomposite. The introduction of polycrystallinity in graphene-PB nanocomposite is basically originated from the use of polycrystalline PB nanoparticles which have greater affinity with as-prepared graphene dispersion. The stability of the material is reasonably good for practical applications with additional option for making the material on requirement within limited time course.
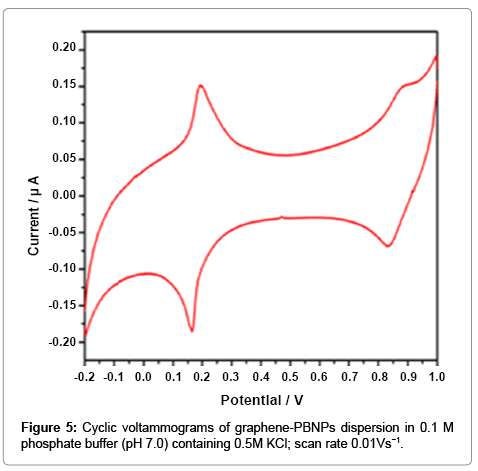
The graphene-PB nanocomposite dispersion show excellent electrochemical behavior for many electroanalytical applications. The cyclic voltammogram of a typical graphene-PB composite modified electrode recorded in phospahate buffer (PBS) (0.1 M, pH 7.0) containing 0.5 M KCl electrolytes at a scan rate of 0.01 V s−1 is shown in Figure 5. Two reversible redox couples (Figure 5) for the graphene- PB are observed between the potential ranges of −0.2 to 1.0 V vs. Ag/ AgCl, showing the oxidation of PB to Berlin green (BG) as well as the reduction of PB to Prussian white (PW), indicating the effective presence of PB on the modified electrode. The formal potentials calculated by the cathodic and anodic peak potential was found to be 0.19V for the redox conversion between PB and PW, and 0.85V for the redox conversion between PB and BG. The peak separation was 0.3 V for first redox couple, justifying the validity of present nanocomposite.
Peroxidase mimetic activity of graphene-PB nanocomposite
Horseradish peroxidase (HRP) has been widely used in the development of ELISA kits and many other bioassays based on the catalytic activity on the reduction of H2O2. However, the biological activity of HRP is susceptible to various experimental parameters and is vulnerable to denaturation. Accordingly, there is a potential need for its replacement using suitable HRP-mimetic material. Many nanomaterials, such as metal oxide, noble metals, PB, graphene and their composites have also been reported to possess intrinsic peroxidaselike activity [18-22]. PB is a well-known metallic complex and has been used as an artificial peroxidase for H2O2 reduction [14,15,23,24]. The large surface area of as synthesized nanocomposite material may facilitate biocatalysis for practical applications. Under this condition a large amount of PBNPs may be well adsorbed on a graphene sheet representing exciting materials for practical applications exhibiting high catalytic activity.
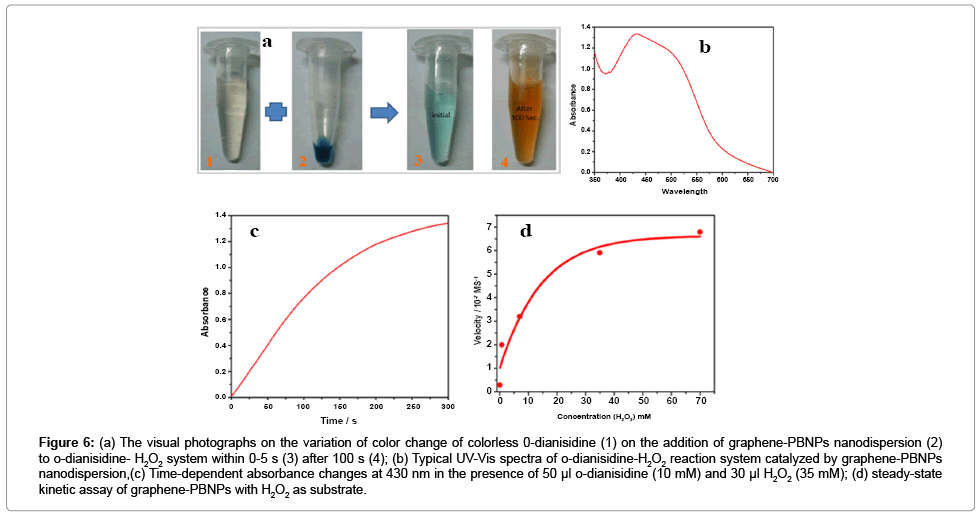
Peroxidase-like activity of the as-synthesized polycrystalline graphene-PB nanodispersion was studied based on probing the oxidation of o-dianisidine by H2O2 in the presence of the as-prepared graphene-PB nanodispersion as catalyst using a Hitachi U-2900 spectrophotometer is reported earlier [14,15]. The results as shown in Figure 6a justify the conversion of colourless o-dianisidine into orange brown solution. The absorbance spectrum of the resulting colored solutions was recorded at 430 nm (ε = 11.3 mM-1cm-1) as shown in Figure 6b. The results indicate that the graphene-PB nanodispersion have peroxidase-like activity towards other typical peroxidase substrates like dopamine and pyrogallol [14,15]. Typical Michaelis– Menten curves can be obtained for the graphene-PB nanodispersion with H2O2 as substrate as shown in Figure 6d. The Michaelis constant (Km) and the maximal reaction velocity (Vmax) are found to be 6.8 mM and 6.27 × 10-7 Ms-1 respectively. The apparent Km value of graphene- PB nano dispersion was compared with HRP recorded under existing experimental conditions, which confirmed that graphene-PB nano dispersion shows significantly enhanced practical usability due to relatively longer stability of the nanocomposite as compared to that of HRP. The apparent Km value of the graphene-PB nano dispersion with H2O2 as the substrate was found to be 6.8 mM whereas it was 3.7 mM for HRP [25]. Our finding further revealed that catalytic efficiency of graphene-PB nano dispersion shows relatively fast catalysis within 100 Sec. The system is found biocompatible and suitable for probing desired biochemical interactions. The selectivity of the nanomaterial as peroxidase mimetic is found to > 95% in the presence of common interfering analytes.
Figure 6: (a) The visual photographs on the variation of color change of colorless 0-dianisidine (1) on the addition of graphene-PBNPs nanodispersion (2) to o-dianisidine- H2O2 system within 0-5 s (3) after 100 s (4); (b) Typical UV-Vis spectra of o-dianisidine-H2O2 reaction system catalyzed by graphene-PBNPs nanodispersion,(c) Time-dependent absorbance changes at 430 nm in the presence of 50 μl o-dianisidine (10 mM) and 30 μl H2O2 (35 mM); (d) steady-state kinetic assay of graphene-PBNPs with H2O2 as substrate.
Electrocatalytic reduction of H2O2
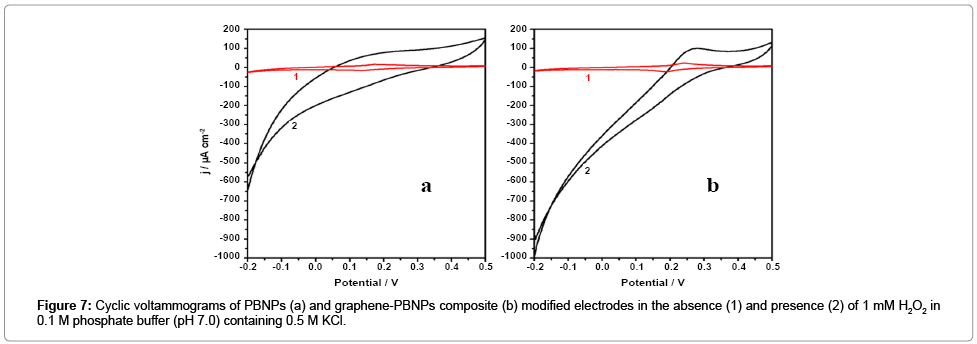
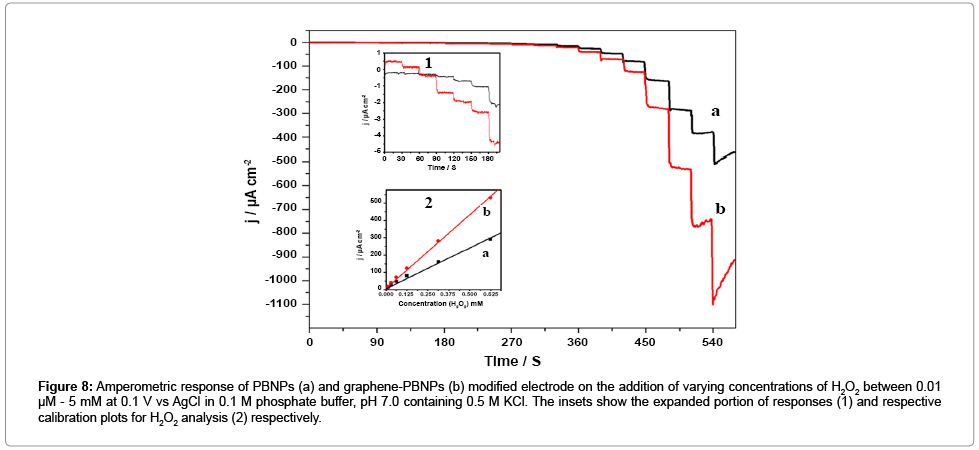
We subsequently examined the electrochemical behavior of Graphene-PB nanocomposite. modified electrode. Figure 7a and 7b shows the cyclic voltammograms of the PBNPs (a) and graphene-PB nanocomposite (b) modified electrode in 0.1 M phosphate buffer (pH 7.0) in absence and the presence of 1 mM H2O2 (Figure 7). There is an increase in cathodic current on the addition of H2O2, the reduction currents for both the electrodes (Figure 7). However, the graphene- PB nanocomposite modified system shows higher reduction current (Figure 7b) as compared to only PB-system (Figure 7a), indicating better electrocatalytic activity of nanocomposite toward H2O2. In order to study the quantitative determination of H2O2, the amperometric responses for these systems were recorded on the addition of varying concentrations of H2O2 (0.01 μM to 5 mM) in 0.1 M phosphate buffer (pH 7.0) at working potential of 0.1 V vs. Ag/AgCl as shown in Figure 8. It is clear from Figure 8 that the response was amplified in case of graphene-PB nanocomposite (Figure 8b) than PBNPs (Figure 8a), again suggesting the good electrocatalytic behaviour of composite material towards the reduction of H2O2. The calibration curves for H2O2 analysis at modified electrodes were constructed using average currents recorded for each concentration point. The inset-2 of Figure 8 depicts the calibration curves for H2O2 at PBNP (curve a), and graphene-PB nanocomposite (curve b) modified electrodes. The sensitivity of PBNPs and graphene-PB nanocomposite modified system towards H2O2 has been calculated from the linear curve with 99.3 % correlation coefficient and found to be 480 ± 10 and 850 ± 17 μAmM−1cm−2 respectively. The inset-1 of Figure 8 also embodies the expanded portion of initial amperometric response. The lowest detection limit for H2O2 analysis was found to be 10 nM for graphene-PB nanocomposite modified system. The present system justify its advantages over earlier reported systems [13-15] in terms of: (1) choice of operating potential, (2) better sensitivity of the systems under present experimental conditions, (3) better limit of detection.
Figure 8: Amperometric response of PBNPs (a) and graphene-PBNPs (b) modified electrode on the addition of varying concentrations of H2O2 between 0.01 μM - 5 mM at 0.1 V vs AgCl in 0.1 M phosphate buffer, pH 7.0 containing 0.5 M KCl. The insets show the expanded portion of responses (1) and respective calibration plots for H2O2 analysis (2) respectively.
Conclusion
In summary, a new method for the synthesis of graphene nanodispersion is reported that involves the participation of 3-APTMS and 3-GPTMS. The as synthesized graphene shows functional activity for making polycrystalline nanocomposite with Prussian blue nanoparticles. The studies on the electrocatalysis of H2O2 and artificial peroxidase like properties of as synthesized nanocomposite with excellent mimetic behaviour show the representative application of the same. In a typical kinetic study, the oxidation of o-dianisidine by H2O2 in the presence of the as-prepared graphene-Prussian blue nanodispersion the Michaelis constant (Km) and the maximal reaction velocity (Vmax) are recorded as 6.8 mM and 6.27 × 10-7 Ms-1. H2O2 sensing at lower operating potential with better lowest detection limit validates the admirable electrocatalytic ability of Graphene-Prussian blue nanocomposite.
References
- Xue M, Chen G, Yang H, Zhu Y, Wang D, et al. (2012) Superconductivity in potassium-doped few-layer graphene. J Am ChemSoc 134: 6536-6539.
- Shao Y, Wang J, Wu H, Liu J, Aksay IA, et al. (2010) Graphene Based Electrochemical Sensors and Biosensors: A Review.Electroanal 22: 1027-1036.
- Wu ZS, Yang S, Sun Y, Parvez K, Feng X, et al. (2012) 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J Am ChemSoc 134: 9082-9085.
- Choi W, Lahiri I, Seelaboyina R, Kang YS (2010) Synthesis of Graphene and Its Applications: A Review. Critical Reviews in Solid State and Materials Sciences 35: 52-71.
- Tsen AW, Brown L, Levendorf MP, Ghahari F, Huang PY, et al. (2012) Tailoring electrical transport across grain boundaries in polycrystalline graphene. Science 336: 1143-1146.
- Pandey PC, Upadhyay S,Pathak HC,Tiwari I, Tripathi VS (1999) Studies on glucose biosensor based on non-mediated and mediated electrochemical oxidation of reduced glucose oxidase.Electroanalysis 11: 1251-1258.
- Pandey PC, Upadhyay S, Tiwari I, Sharma S (2001) A novel Ferrocene-Encapsulated Palladium-Linked Ormosil-based Electrocatalytic Biosensor. The Role of the Reactive Functional Group.Electroanal 14: 1519-1527.
- Pandey PC, Upadhyay S, Sharma S (2003) Functionalized ormosil-based biosensor. Probing a Horseradish peroxidase catalyzed reactions. J ElectrochemSoc 150: H85-H92.
- Pandey PC, Upadhyay S, Shukla NK, Sharma S (2003) Studies on the Electrochemical Performance of Glucose Biosensor based on Ferrocene encapsulated ORMOSIL and Glucose Oxidase Modified Graphite Paste Electrode.BiosensBioelectron18: 1257-1268.
- Pandey PC, Chauhan DS (2012) 3-Glycidoxypropyltrimethoxysilane mediated in situ synthesis of noble metal nanoparticles: application to hydrogen peroxide sensing. Analyst 137: 376-385.
- Pandey PC, Pandey AK, Pandey G (2014) Functionalized Alkoxysilane Mediated Controlled Synthesis of Noble Metal Nanoparticles Dispersible in Aqueous and Non-Aqueous Medium. J NanosciNanotechnol 14: 6606-6613.
- Pandey PC, Pandey G (2014) Tunable functionality and nanogeometry in tetrahydrofuranhydroperoxide and 3-Aminopropyl-trimethoxysilane mediated synthesis of gold nano-particles; Functional application in Glutathione sensing. J Mater Chem B.
- Pandey PC, Pandey AK (2013) Novel Synthesis of Prussian blue Nanoparticles and Nanocomposite sol: Electro-analytical application in Hydrogen Peroxide Sensing.ElectrochimActa 87: 1-8.
- Pandey PC, Pandey AK (2013) Novel synthesis of super peroxidase mimetic polycrystalline mixed metal hexacyanoferrates nanoparticles dispersion. Analyst 138: 2295-2301.
- Pandey PC, Pandey AK (2013) Electrochemical sensing of dopamine and pyrogallol on mixedanalogue of Prussian blue nanoparticles modified electrodes—Role of transition metal on the electrocatalysis and peroxidasemimetic activity, ElectrochimActa 109: 536-545.
- Hummers WSJr, Offeman RE (1958) Preparation of Graphitic Oxide. J Am ChemSoc 80: 1339.
- Ogasawara T, Nara A, Okabayashi H, Nishio E, O’Connor CJ (2000) Time resolved near-infrared and two-dimensional near infrared correlation spectroscopic studies of polymerization process of silane coupling agent. Dyanamicbehaviour of water molecule in the 3-aminopropyltrimethoxysilane-ethanol-water system. Colloid PolymSci 278: 1070-1084.
- Asati A, Santra S, Kaittanis C, Nath S, Perez JM (2009) Oxidase-like activity of polymer-coated cerium oxide nanoparticles. AngewChemInt Ed Engl 48: 2308-2312.
- André R, Natálio F, Humanes M, Leppin J, Heinze K, et al. (2011) V2O5 Nanowires with an Intrinsic Peroxidase-Like Activity.AdvFunct Mater 21: 501-509.
- Jv Y, Li B, Cao R (2010) Positively-charged gold nanoparticles as peroxidase mimic and their application in hydrogen peroxide and glucose detection. ChemCommun (Camb) 46: 8017-8019.
- Zhang X-Q, Gong S-W, Zhang Y, Yang T, Wang C-Y, et al. (2010) Prussian blue modified iron oxide magnetic nanoparticles and their high peroxidase-like activity. J MaterChem 20: 5110-5116.
- Guo Y, Deng L, Li J, Guo S, Wang E, et al. (2011) Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 5: 1282-1290.
- Karyakin AA, Karyakina EE, Gorton L (2000) Amperometric biosensor for glutamate using prussian blue-based "artificial peroxidase" as a transducer for hydrogen peroxide. Anal Chem 72: 1720-1723.
- Moscone D, D'Ottavi D, Compagnone D, Palleschi G, Amine A (2001) Construction and analytical characterization of Prussian-Blue-based carbon paste electrodes and their assembly as oxidase enzyme sensors. Anal Chem 73: 2529-2535.
- Wei H, Wang E (2008) Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem 80: 2250-2254.
Relevant Topics
Recommended Journals
Article Tools
Article Usage
- Total views: 15680
- [From(publication date):
specialissue-2015 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 10965
- PDF downloads : 4715