Temporomandibular Disorder And Its Influence on Cervical Mobility, Pain Threshold Pressure and Quality of Life in Individual after Stroke
Received: 17-Jun-2020 / Accepted Date: 22-Jul-2020 / Published Date: 29-Jul-2020 DOI: 10.4172/2161-1165.1000385
Abstract
The American Academy of Orofacial Pain defines Temporomandibular Disorder (TMD) as a set of disorders involving the masticatory muscles, the TMJ, and associated structures.In hemiparetic individuals after stroke, due to motor condition, speech, chewing and swallowing functions may be impaired and influence the onset of temporomandibular disorder (TMD). Therefore, the objective of this research was to analyze the relationship of TMD with changes caused by stroke in hemiparetic subjectsand observe pain threshold pressure (PPT), cervical range of motion and the quality of life (QoL). The RDC/TMD was used for TMD diagnosis, PPT Test, fleximeter for cervical range of motion evaluation, to assess QoL, the instrument used was the WHOQOL-bref, with the statistical analysis performed by the SPSS. The sample was divided into groups with TMD (GD) and without TMD (GS). According to the RDC/TMD of the 20 individuals evaluated, 80% (n=16) presented clinical signs and symptoms of TMD, and 43.7% were homolateral to the side affected by stroke. There was a decrease in the range of motion of the cervical spine in GD, a decrease in LDP in sternocleidomastoid (ECOM) and physical domain of QOL only in GD. A high frequency of TMD was observed in the sample, with predominance of diagnosis for the homolateral hemiparesis side.
Keywords: Temporomandibular Joint; Stroke; Temporomandibular Joint Disorders
Introduction
The American Academy of Orofacial Pain defines Temporomandibular Disorder (TMD) as a set of disorders involving masticatory muscles, TMJ and associated structures. This dysfunction is identified as the main cause of pain of non-dental origin in the orofacial region [1,2]. Some clinical signs and symptoms of TMD are: fatigue, tenderness in the muscles of mastication, noise and movement limitation. It is proposed that most of these symptoms appear in an insidious way, evolving over time [3]. When the duration of the symptoms increases, highlighting the painful symptoms, psychosocial factors become more evident, since, generally, limitations and/or chronic pain, negatively affect the quality of life [4]. TMD-related pain is not restricted to the face alone. As the stomatognathic system includes several body segments, the cervical can also be affected, causing pain and limitation in this region, which can negatively interfere in the individual’s social activities. Cranio-cervical changes are believed to interfere with the position of the mandible and vice versa [5]. The functions of speech, chewing and swallowing depend on the synergism of movements of the cervical spine, the TMJ and the skeletal muscle system involved. In hemiparetic individuals, due to the motor condition, these functions may be compromised, influencing the appearance of TMD [6].
Stroke is a common disease with a major impact on public health worldwide, being one of the main causes of chronic disability due to neurological disease. There are several post-stroke sequelae, including motor disorders, speech or language disorders and swallowing disorders, resulting in altered eating patterns. Motor deficits from stroke are characterized by complete paralysis (hemiplegia) or partial/ incomplete (hemiparesis) in the hemibody contralateral to the injury site that occurred in the brain, generating sensory changes and changes in muscle tone [7,8]. Approximately 50% of patients with post-hemiparesis Stroke has deficiency in facial control, weakening of the muscles and orofacial and mandibular functions. Presenting less muscle activity on the affected side and consequently decreased bite strength, being able to cause a reduction in TMJ function. Even the general symptoms of muscle weakness in these patients can influence the alignment and postural balance of the head and neck leading to compensations. Sub-sequently contributing to an impaired physical condition. Thus, the aim of the present study was to investigate the presence of TMD in post-stroke hemiparetic individuals and its possible influence on the range of motion (ROM) of the cervical spine, painpressure threshold (LDP) and quality of life (QOL) of this population.
Methodology
This is a cross-sectional analytical and comparative study, carried out at the State University of Northern Paraná (UENP), located in the city of Jacarezinho-PR. The project was approved by the Ethics and Research Committee with Human Beings of UENP, under opinion No. 3,083,389. 28 volunteers were selected, who were classified and observed on the conditions of participating or not in the research. The inclusion criteria were: To obtain a minimum score of 20 points on the Mini-Mental State Examination (MMSE) and to have the central and lateral incisors of the upper and lower arch. After respecting the inclusion criteria, the sample consisted of 20 volunteers with hemiparesis due to stroke, of both genders and with a mean age of 69.18 ± 6.18. Subsequently, the sample was divided into groups of individuals with TMD (DG) and without TMD (GS).
In the first stage of the research, the volunteers signed the ICF and performed the MMSE, a questionnaire used to assess different cognitive patterns [9]. In a second stage, the volunteers were submitted to the ResearchDiagnosticCriteria for Temporomandibular Disorders questionnaire, or Diagnostic Criteria for Research on Temporomandibular Disorder (RDC / TMD), cervical ROM assessment, LDP assessment, and finally, to the WHOQOL-BREF questionnaire. All assessments were made by a single, trained appraiser. To analyze the presence of TMD, the volunteers were submitted to the RDC/TMD questionnaire. This instrument is considered a reference standard in research on TMD assessment and diagnosis, being characterized as biaxial, composed of two axes. The first axis corresponds to the physical evaluation of the clinical conditions of the TMJ and of the masticatory muscles, including inspection of TMJ noises, altered movements, mouth opening pattern and joint and muscle palpation. The second axis consists of behavioral, psychological and social issues of the individual [10]. The RDC/TMD allowed to classify TMD as particular, muscular and mixed.
The LDP assessment was carried out bilaterally, with the aid of the Wagner PAIN TEST™ algometer device. The algometer was positioned perpendicular to the examined point and an increasing and constant pressure of approximately 0.5 kg/cm2/second was applied, until the moment the patient informed that the pressure exerted became uncomfortable. At that moment, the researcher interrupted the application of the pressure and the algometer recorded the value equivalent to the LDP [11]. The following points were examined: lateral pole of the TMJ, anterior temporal muscle, masseter body, sternocleidomastoid (ECOM) (fibers immediately below the mastoid process) and upper trapezius. The Sanny® Fleximeter was used to assess cervical mobility. From a gravitational pendulum system. The indication of the corresponding value is produced by the effect of gravity, minimizing interpretation errors [12,13]. Active cervical spine movements were measured: flexion, extension, right and left lateral flexion starting from the neutral position, and lateral rotation from the neutral position to the right and left. The movements of flexion, extension and lateral flexion were measured with the volunteers sitting in a chair with support for the trunk and to check the cervical rotation, the volunteers remained in supine position, with hip and knee in flexion and feet supported on the examination Table 1 [14]. The passive movements of the upper cervical spine (segments of movement C1 and C2) were evaluated by Flexion-RotationTest (FRT), in this case, the individual was in supine position, with the lower limbs in flexion, the evaluator passively performed a flexion with rotation of the upper cervical spine [15]. All movements were performed up to the maximum amplitude of the patient. Three measures of each movement were performed, with an interval of 30 seconds between them, recording the mean value at the end of the evaluation. Quality of life was verified through the WHOQOL-BREF questionnaire, consisting of 26 questions, two of which are general quality of life, with four types of response scales: intensity, capacity, frequency and assessment, all graded at five levels. The questions, in turn, compose QOL in four domains: physical (domain 1), psychological (domain 2), social relations (domain 3) and environment (domain 4). The domain scores were converted to a scale of 0 to 100%. The higher the percentage, the better the quality of life [16]. Statistical analysis was performed using Microsoft Excel® software (Version 16.17, Microsoft S.A) and SPSS 25.0, considering their means, medians, standard deviation and percentages. Initially, the Shapiro-Wilk test was applied to verify the normality of the data. To analyze the frequency of TMD and association with the side of hemiparesis, the Chi-square test of independence and Fisher’s exact test were performed. When the data were considered normal, the t-Student Test of independent samples was used to compare the groups GD and GS, described in the form of mean and standard deviation, being applied to verify the cervical ROM and the QOL domains. of the LDP values between the homolateral and contralateral side, hemiparesis in the GD and GS group happened through the Test for related samples considering the Wilcoxon analysis, being described in median and interquartile range (25-75 percentile). The adopted significance was p ≤ 0.05.
| COM DTM GROUP (n=16) | SEM DTM GROUP (n=04) | |
|---|---|---|
| Gender | % n | % n |
| Female | 43.77 | 75.03 |
| Male | 56.2 9 | 25.01 |
| AVE | ||
| Right | 56.2 9 | 50.02 |
| Left | 43.77 | 50.02 |
| DTM classification | ||
| DTM articular | 68.711 | - - |
| DTM muscular | 6.251 | - - |
| DTM mixed | 25.04 | - - |
| DTM side | ||
| Right | 37.5 6 | - - |
| Left | 25.04 | - - |
| Bilateral | 37.56 | - - |
AVE=Accident Vascular Brain; DTM=Dysfunction Temporomandibular.
Table 1: Attribute of the host in relation to the species.
Results
According to the RDC/TMD of the 20 individuals evaluated, 80% (n=16) presented clinical signs and symptoms of TMD, and only 20% (n=4) did not qualify for the diagnosis. In the present sample, the most frequent classification of TMD was articular. Of the individuals with stroke, 55% had hemiparesis in the right hemibody as shown in Table 1.
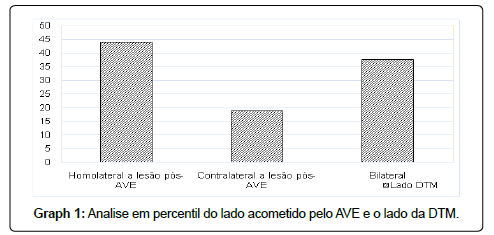
Regarding the side affected by TMD and the side of hemiparesis after stroke, there were no significant associations (p=0.096), however, when analyzing the sample’s percentile, it was possible to observe a higher frequency of TMD homolateral to the hemibody affected by the stroke as shown in Graph 1.
Table 2 shows the values found in the evaluation of low and high cervical ROM. When comparing the GD and GS groups, we noticed a decrease in cervical mobility in the GD, pointing to a statistically significant difference between the groups for the latero-flexion (p=0.002) and FRT (p=0.003) movements homolateral to hemiparesis. When checking the LDP between the homolateral and contralateral sides, hemiparesis in the GD and GS groups, there was a statistical difference in the ECOM (p=0.003) and masseter (p=0.004) points only in the GD, showing lower values of LDP for the homolateral side hemiparesis at these points.
| COM DTM GROUP (n=16) | SEM DTM GROUP (n=04) | P* | |
|---|---|---|---|
| Cervical Movements | |||
| Flexion | 46.2 ± 11.3 | 60.0 ± 10.8 | 0.79 |
| Extension | 43.4 ± 13.9 | 47.5 ± 6.45 | 0.68 |
| Lateral-Flexion homolateral hemishere | 25.3 ± 9.21 | 37.5 ± 6.45 | 0.02* |
| Lateral-Flexion contralateral a hemisphere | 30.9 ± 6.63 | 36.2 ± 2.50 | 0.24 |
| Rotação direita homolateral a hemiparesia | 40.5 ± 15.2 | 52.5 ± 14.4 | 0.14 |
| Rotação esquerda contralateral a hemiparesia | 50.6 ± 12.9 | 66.2 ± 19.3 | 0.17 |
| FRT right homolateral hemisphere | 53.8 ± 14.5 | 71.2 ± 14.9 | 0.03* |
| FRT left contralateral hemisphere | 61.5 ± 10.8 | 66.2 ± 10.3 | 0.43 |
FRT= Passive rotation flexion test of the cervical; p ≤ 0.05*Student t-test
Table 2: Variance in mean and standard deviation of cervical ROM (degrees) between groups with and without TMD.
With respect to QV results obtained by WHOQOL-bref, the physical domain (p=0.001) provides QV short scores for GD with significant differences between the GD and GS groups. These domains do not display differences as they are displayed at Tables 3 & 4.
| COM DTM GROUP (n=16) | SEM DTM GROUP (n=04) | |||||
|---|---|---|---|---|---|---|
| Homolateral a hemiparesia | Contralateral a hemiparesia | P* | Homolateral a hemiparesia | Contralateral a hemiparesia | P* | |
| ATM | 1.80(1.30-2.10) | 1.75(1.20-1.97) | 0.93 | 2.15(1.20-2.77) | 2.20(0.95-2.37) | 0.46 |
| MASSETER | 1.50(1.00-1.85) | 1.70(1.40-1.90) | 0.04* | 1.80(1.32-2.35) | 1.60(1.12-1.85) | 0.1 |
| TEMPORAL | 1.95(1.62-2.40) | 1.85(1.55-2.27) | 0.6 | 2.00(1.60-2.32) | 1.85(1.40-2.37) | 0.7 |
| ECOM | 1.30(0.92-1.72) | 1.65(1.12-2.00) | 0.03* | 1.55(1.30-1.95) | 1.50(1.27-1.57) | 0.35 |
| TRAPÉZIO | 1.95(1.57-2.27) | 2.05(1.57-2.47) | 0.06 | 2.35(2.30-2.47) | 2.20(1.87-2.45) | 0.19 |
AVE=Accident Vascular Brain; ATM=Articulate Temporomandibular; ECOM=Esternocleidomastóideo. p ≤ 0.05* Wilcoxon test.
Table 3: Values exposed in median and interquartile range of the LDP (kg/cm2) of the TMJ. masticatory and cervical muscles for comparison between the homolateral and contralateral sides to hemiparesis in the groups with and without TMD of the post-stroke individuals.
| DOMAINS Q | COM DTM GROUP (n=16) | SEM DTM GROUP (n=04) | P* |
|---|---|---|---|
| Physical | 52.3 ± 11.9 | 70.0 ± 13.5 | 0.01* |
| Psychological | 59.7 ± 13.8 | 56.2 ± 13.8 | 0.82 |
| Social relationship | 50.8 ± 17.1 | 59.2 ± 10.8 | 0.17 |
| Environment | 61.4 ± 13.7 | 67.2 ± 12.8 | 0.49 |
AVE=Accident Vascular Brain; Q=Quality of life; p≤0.05*Student test
Table 4: Comparative analysis of mean and standard deviation of the domains of the WHOQOL-bref quality of life questionnaire in both groups diagnosed with TMD.
Discussion
The TMD study has been carried out in several populations, among them, the most studied are children and young adults [17]. When it comes to TMD in the elderly or in populations with some neurological impairment, the literature is scarce, since the individuals who composed the sample were aged between 60 and 80 years, we observed a predominance of joint TMD, approaching the results found in the literature for that same age group [18,19], also, conditions called osteoarthrosis and arthralgia of the TMJ were identified as the most frequent, and may be related to the physiological process of aging, since, with advancing age, the intra-articular disc of the TMJ loses its viscoelastic property, and its vascularization decreases, making the fibrocartilaginous bundles more dense and increasing the signs of degenerative changes of the ATM [20]. It is known that hemiparesis and spasticity are the main changes observed in the individual who suffered a stroke and that imply impairment of musculoskeletal functions and disabilities to perform activities of daily living [21]. In TMJ when there is hypertonia, the intra-articular disc is pulled in an antero-medial direction, causing imbalances in the functional relationship of the TMJ. Furthermore, failures in motor control can lead to compensations in the TMJ, due to the close anatomical and functional relationship between cerebellum, pons and TMJ. Knowing that the bridge innervates the TMJ through the trigeminal nerve and that the cerebellum is the center where we have a greater flow of proprioceptive information, it is assumed that when we have any change in motor control at the level of the cerebellum, it will be transmitted directly to the bridge and consequently for ATM [22]. These changes, in tone and motor control, may be associated with the result of the present study, where it was found that most hemiparetic individuals after stroke had a diagnosis of TMD and that they were homolateral to the hemibody affected by the stroke. The significant non-association between the TMD side and the CVA side can probably be explained by the small sample size. Changes in mobility and muscle strength are also attributed to deficits in postural motor control in individuals affected by stroke. These changes cause a decrease in body awareness, with less movement to the affected side [23]. Some cervical muscles influence the stomatognathic system by balancing the cranio-cervical complex and the mandible. During cervical movements, changes occur in the TMJ intra-articular space, which justifies the importance of studying cervical ROM in TMD in this population.
The weakness of the flexor muscles of the neck and the shortening of the ECOM muscle can influence an anterior head posture, this posture is frequently observed in hemiparetics, which can interfere with cervical mobility and TMJ functioning, causing hyperactivity in the suboccipital muscles and ECOM, and changes in the position of the mandibular condyle [24,25]. When there is hyperactivity in the suboccipitals, the intravertebral spaces of C1 and C2 are reduced, resulting in hypomobility of the upper cervical spine, this relationship may contribute to explain the decrease in FRT in the DG found in the study in question.
The ECOM muscle plays a fundamental role in the mobility of the cervical spine and in the balance between the chest and head, especially in individuals with TMD, in addition to being an important lateral flexor and neck rotator. In the present study, there were decreases in cervical ROM for latero-flexion and FRT movements homolateral to hemiparesis in the DG. Lower LDP values were also observed for the ECOM muscle and masseter homolateral to hemiparesis only in the GD, such findings can be related to the diagnosis of TMD, which most of them were found on the side affected by the stroke. For Amaral, TMD influences the functionality of the cervical, being a factor that restricts its mobility mainly of the segments of C1 and C2, due to the proximity between the TMJ, occipital bone and first cervical vertebrae [26-28].
Changes in mobility and muscle strength are also attributed to deficits in postural motor control in individuals affected by stroke. These changes cause a decrease in body awareness, with less movement to the affected side. Some cervical muscles influence the stomatognathic system by balancing the cranio-cervical complex and the mandible. During cervical movements, changes occur in the TMJ intra-articular space, which justifies the importance of studying cervical ROM in TMD in this population.
The weakness of the flexor muscles of the neck and the shortening of the ECOM muscle can influence an anterior head posture, this posture is frequently observed in hemiparetics, which can interfere with cervical mobility and TMJ functioning, causing hyperactivity in the suboccipital muscles and ECOM, and changes in the position of the mandibular condyle. When there is hyperactivity in the suboccipitals, the intravertebral spaces of C1 and C2 are reduced, resulting in hypomobility of the upper cervical spine, this relationship may contribute to explain the decrease in FRT in the DG found in the study in question.
The ECOM muscle plays a fundamental role in the mobility of the cervical spine and in the balance between the chest and head, especially in individuals with TMD, in addition to being an important lateral flexor and neck rotator. In the present study, there were decreases in cervical ROM for latero-flexion and FRT movements homolateral to hemiparesis in the DG. Lower LDP values were also observed for the ECOM muscle and masseter homolateral to hemiparesis only in the GD, such findings can be related to the diagnosis of TMD, which most of them were found on the side affected by the stroke. For Amaral, TMD influences the functionality of the cervical, being a factor that restricts its mobility mainly of the segments of C1 and C2, due to the proximity between the TMJ, occipital bone and first cervical vertebrae.
Conclusion
A high frequency of TMD was observed in the sample, with a predominance of diagnosis for the side homolateral to hemiparesis. TMD had negative influences on cervical mobility, with decreased movements of latero-flexion and FRT homolateral to hemiparesis, in LDP with increased pain sensitivity in masseter and ECOM and in the impairment of the physical domain of QOL in the population in question. Due to the scarcity in the literature on the influence of TMD on post-stroke hemiparetic patients, further studies are needed, with a larger sample size, which analyze TMJ and its disorders, as well as LDP, cervical mobility and QOL in a given population for better statements.
References
- Â Biasotto-Gonzalez DA, Mendes PCC, Jesus LA de, Martins MD (2009) Quality of life in patients with temporomandibular disorders - a cross-sectional study. Journal of the Institute of Health Sciences 27:128-132.
- Leeuw R, Klasser GD (2013) Orofacial pain: guidelines for assessment, diagnosis, and management. (5th ed), Hanover Park (IL): Quintessence Publishing.
- Bonjardim LR, Lopes RJF, Amado G, Albuquerque RLJ, Gonçalves SR (2009) Association between symptoms of temporomandibular disorders and gender, morphological occlusion, and psychological factors in a group of university students. Indian J Dent Res 20: 190-194.
- Cavalcanti MOA, Gomes I, Goldim JR (2015) Evaluation of the perception of coercion in non-institutionalized elderly people submitted to research for the diagnosis of temporomandibular disorder. Rev Gaúcha Enferm 36: 28-34.
- Viana MO, Ferreira EICBMF, Menezes JNR, Olegario NBC (2015) Assessment of signs and symptoms of temporomandibular disorder and its relationship with cervical posture. Rev odontol UNESP 44: 125-130.
- Silva FC, Palácio PRC, Gomes AO, Politti F, Lima GR, et al (2018) Electromyographic evaluation of masticatory muscles in individuals with hemiparesis and temporomandibular disorder. Cogent Medicine 5: 1.
- Oh DW, Kang TW, Kim SJ (2013) Effect of stomatognathic alignment exercise on temporomandibular joint function and swallowing function of stroke patients with limited mouth opening. J. Phys. Ther. Sci. 25: 1325-1329.
- Macena RH, Maia M (2016) Cognitive function of elderly women living in long-term care facilities: effects of a physiotherapy program. Rev Bras Geriatr Gerontol 19: 57-70.
- Schiffman E, Ohrbach R, Truelove E, Look J, Anderson G, et al. (2014) Diagnostic Criteria for Temporomandibular Disorders (DC/TMD) for clinical and research applications: recommendations of the International RDC/TMD Consortium Network and Orofacial Pain Special Interest Group. J Oral Facial PainHeadache 28: 6-27.
- Sydney PBH, Conti PCR (2011) Guidelines for somatosensory assessment in patients with temporomandibular disorders and orofacial pain. Rev Dor 12: 349-353.
- Siqueira GR, Alencar GG, Oliveira ECM, Teixeira VQM (2015) Pilates effect on trunk flexibility and ultrasound measurements of abdominal muscles. Brazilian Journal of Sports Medicine 21: 139-143.
- Junior AA, Franco R, Silva VP, Martins FV, Guarigla DA (2013) Comparison and agreement of instruments to assess the range of motion of the cervical spine of university students. Magazine of Physical Education / UEM 24: 609-616.
- Florencio LL, Pereira PA, Silva ERT, Pegoretti KS, Goncalves MC, et al. (2010) Agreement and reliability of two non-invasive methods for assessing cervical range of motion in young adults. Brazilian Journal of Physical Therapy 14: 175-181.
- Grondin F, Municipal T, Laurentjoye H, Ella B (2015) Upper cervical range of motion is impaired in patients with temporomandibular disorders. Cranio 33: 91-99.
- Chachamovich E, Fleck MP, Trentini C, Power M (2008) Brazilian WHOQOL-OLD Module version: a Rasch analysis of a new instrument. Rev. de Saúde Pública 42: 308-316.
- Sasa S and Ljiljana K (2012) Prevalence of temporomandibular dysfunctions symptoms in children and in adults. Healthmed 31: 1779-1785.
- Almeida LHM, Farias ABL, Soares MSM, Cruz JSA, Cruz RES, et al. (2008) Disfunçãotemporomandibularemidosos 1:35-38.
- Segù M and Manfredini D (2019) Temporomandibular Joint Disorders in the Elderly. Oral Rehabilitation for compromised and Elderly Patients 63-79.
- Zilli F, Lima ECBA and Kohler MC (2014) Neuroplasticity in the rehabilitation of patients affected by spastic stroke. Rev. Ter. Ocup. 63: 317-322.
- Schuster RC (2011) Correlation between motor and respiratory disorders in stroke. Rev Neurocienc 19: 587-588.
- Farias NC, Rech I, Ribeiro BG, Oliveira CS, Menna W, et al. (2009) Postural assessment in hemiparetics across the application of software SAP: case report.ConScientiae Saúde 8: 649-54.26.
- Von PH, Pudelko A, Danzeisen M, Hall T, Ballenberger N (2016) Do subjectswith acute/subacute temporomandibular disorder have associated cervical impairments: a cross-sectional study. Man Ther 26: 208-215.
- Ferrão MIB and Traebert J (2008) Prevalence of temporomandibular disfunction in patients with cervical pain under physiotherapy treatment. Fisioter Mov 21: 63-70.
- Smith K, Hall T, Robison K (2008) The influence of age, gender, life style factors and sub-clinical neck pain on the cervical flexion-rotation test and cervical range of motion. Man Ther 13: 552-559.
- Milanesi JM, Corrêa ECR, Borin GS, Souza AJ, Pasinato F (2011 Electrical activity of the cervical muscles and range of motion of the cervical spine in individuals with and without TMD. Physiotherapy and Research 18: 317-322.
- Daher CRM, Cunha LF da, Ferreira APL de, Souza AISO de, Rêgo TAM, et al. (2018) Pain threshold, sleep quality and anxiety levels in individuals with temporomandibular disorders. Rev CEFAC 20: 450-458.
- Pinto RGS, Leite WMA, Sampaio LS, Sanchez MO(2017)Association between temporomandibular signs and symptoms and depression in undergraduate students: descriptive study. Rev Dor 8: 217-224.
- Resende CMBM, Alves ACM, Coelho LT, Alchieri JC, Roncalli AG, Barbosa GAS (2013) Quality of life and general health in patients with temporomandibular disorders. Braz Oral Res 27: 116- 121.
Citation: Camila CA, Natali MF, Tiago TDA, Mariana AR, Joyce K MS (2020) Temporomandibular Disorder And Its Influence on Cervical Mobility, Pain Threshold Pressure and Quality of Life in Individual after Stroke. Epidemiol Sci 10: 385 DOI: 10.4172/2161-1165.1000385
Copyright: © 2020 Camila CA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 3030
- [From(publication date): 0-2020 - Dec 07, 2025]
- Breakdown by view type
- HTML page views: 2149
- PDF downloads: 881