The Antioxidative and Antiapoptotic Effects of Chlorophyllin on a Female Rat Spleen Exposed to Localized Gastric Radiotherapy
Received: 16-Jan-2018 / Accepted Date: 02-Feb-2018 / Published Date: 09-Feb-2018
Abstract
This study evaluated the antioxidative and antiapoptotic effects of chlorophyllin (CHL) on a rat spleen exposed to localized gastric radiotherapy. The stomach region of female rats was exposed to 300 kV X-rays at a dose rate of 2.8 Gy/min either alone or co-treated with oral intake of CHL (15.0 mg kg-1BW) for 4 days. The Catalase (CAT) and Superoxide Dismutase (SOD) activities and Malondialdehyde (MDA) concentrations in the spleen homogenates were conducted. The splenic tissue was examined histologically while localization of CD3, B220, Iba1, PCNA, ss- DNA, IL-1β and TNF-α was determined immunohistochemically. Morphometrical measurements of the indices of proliferative cells and apoptotic cells were done with image J software. For data analyses, ANOVA's method followed by Tukey’s test was applied for multiple comparisons. In radiated group, a significant decrease in CAT and SOD and a significant increase in MDA were recorded. Histopathologically, cellular depletion of the germinal center and appearance of the apoptotic bodies and tingible body macrophages were noted. Statistically, a significant decrease (0.32 ± 0.020 P˂0.05) in the index of proliferative cells and a significant increase in the index of apoptotic cells (0.62 ± 0.034 P˂0.05) were detected. Administration of CHL reversed the oxidative stress levels as evidenced by significant increases of the antioxidant biomarkers and a significant decrease of the oxidant biomarker. In addition, structural and immunohistochemical ameliorations were proved via increased cellular proliferation (0.56 ± 0.032 P˂0.05) and decreased apoptosis (0.13 ± 0.010 P˂0.05). In conclusion, localized radiotherapy is not safe on neighboring organs. Adding of CHL modulated the radiotherapy damage. Maintenance of regular dietary consumption of CHL is recommended during localized radiotherapy to provide a constant supply of potent antioxidants that might prevent any alterations.
Keywords: Antioxidants; Cytokines; T cells; X-rays
Abbreviations
ROS: Reactive Oxygen Species; CHL: Chlorophyllin; H&E: Hematoxylin and Eosin; SOD: Superoxide Dismutase; CAT: Catalase; MDA: Malondialdehyde; IL1β: Interleukin 1 β; TNF- α: Tumor Necrotic Factor α; PCNA: Proliferating Cell Nuclear Antigen; ssDNA: Single Strand DNA
Introduction
In most instances, total body exposure to ionizing irradiation often shows cardinal signs of inflammation. These inflammatory responses are commonly mediated through generation of reactive oxygen species (ROS), chemokines cytokines and growth factors [1]. In medicine, the superiority of localized radiotherapy over whole body irradiation is documented. As it decreases normal tissue injuries and allows a higher dose of radiation to the tumor cells. Furthermore, the curative rate is higher. Unfortunately, many cases of immunosuppression were detected during therapeutic localized irradiation, even in low doses or low rate. The spleen is deemed as the "meter of the immunity" owing to the presence of B and T lymphocytes. Its T-cells is one of the highly radiosensitive cells due to the presence of polyunsaturated fatty acid in their cell membranes. To date, the spleen is still considered a neglected organ and most concerns are confined to hepatic and renal damage [2-8].
In fact, the endogenous antioxidant defense mechanisms might be insufficient to fully scavenge post-irradiated free radicals from the body. Therefore, preventive measures during radiotherapy are preferred. Many radioprotector strategies were tried to avoid or overcome unwanted tissue injuries including; herbal extracts like Vernonia cinerea and Nasturtium officinale . Owing to their side effects, their uses are limited [3,5,9].
Considerable evidence have been suggested that dietary antioxidants could inactivate ROS and keep them only at the lower level necessary to maintain normal cell function including apoptosis cell proliferation and differentiation and regulation of redox-sensitive signal transduction pathways. In addition, continuous consumption of vegetables and fruits especially, the green colored containing antioxidants could prevent certain diseases and even fight cancer cells [10-13].
Chlorophyllin (CHL) is the water-soluble derivative of chlorophyll (the green photosynthetic pigment found in fruits vegetables and greens algae and cyanobacteria). Chemically, the CHL is different from chlorophyll. Whereas in CHL the magnesium ion of chlorophyll is replaced by copper. While methyl and phytyl ester groups are replaced by sodium and potassium. The phytol tail of chlorophyll is absent in CHL. Functionally, CHL is found to have same properties of chlorophyll. However, CHL is more stable than chlorophyll [14]. CHL cleans the circulatory digestive immune and detoxification systems. The antigenotoxic and immunomodulatory properties of CHL have been demonstrated in many studies. The mechanism of action is believed to be mediated via its porphyrin ring [15-17]. The superiority of CHL in conferring a higher degree of protection against oxidative stress pushed us to test the chlorophyllin as a radioprotective on the spleen of rats exposed to localized gastric radiotherapy.
Materials and Methods
This study was performed in the Radiology center and Histology & Cell Biology department, Faculty of Medicine, Zagazig University, El- Zagazig, Egypt under the guidelines of the Institutional Animal Care and the Research Ethics Committee of Zagazig University.
Animals
Healthy 21 adult female Wistar albino rats (160-195 g aged 2-4 months) were purchased from the animal farm, Faculty of Veterinary Medicine, Zagazig University, Egypt. To ensure adequate adaptation, the rats were left for one week under the optimal environment (22-25ᵒC temperature 12 hour light/12 hour dark allowed liquid food and water ad-libitum). Then, they were divided into three groups (n=7 each): group I (control) received 0.9% saline orally, group II (radiated) exposed to gastric X-rays while group III received the same dose of Xrays concomitant with oral intake of 15.0 mg kg-1 of body weight CHL 1 hour before radiation. All rats were killed after two days of the last dose.
Treatment with CHL
The green CHL powder (Sigma-Aldrich Chemical Co USA) was obtained from Algomhuria Company, El Mohafza St, Zagazig, Al Sharquia, Egypt. CHL was dissolved in 0.9% saline. The dosage is adjusted as 15.0 mg kg-1 of body weight and was given via oral route to rats of group III 1 hour before radiation [18].
Irradiation protocol
The irradiation protocol was performed in the Radiology center Zagazig Egypt. In brief, after fasting for 12 hour of food only, the animals of group II and III were anesthetized via intravenous injection of pentobarbital (6 mg/kg) prior 10 minutes of exposure. The rats' bodies were fixed in a vertical position and slightly rotated to the left side for protection of their spinal cord. Their abdomens were covered with a lead sheet (8 × 5 cm) having a window (1.5 × 3 cm). At 30 cm distance, the rats were exposed to 300 kV X-rays (filtered with 0.6 mm Cu and 1 mm Al) at a dose rate of 2.8 Gy/min once a day for 4 days.
Tissue preparation for histological analysis and homogenate
The rats were euthanized by decapitation. Their spleens were dissected and cut into three pieces. Some specimens were fixed in 10% neutral buffered formalin processed using an automatic tissue processor. About 3-5 μm thick sections were cut stained with Hematoxylin and Eosin (H&E) examined and photographed. The other splenic specimens were fixed in 4% paraformaldehyde at 4ᵒC for immunohistochemical staining [19]. For homogenates preparation, small pieces of the spleen of each rat (about 0.5 g) were added to 5 ml phosphate buffer solution at 4ᵒC and centrifuged at 3000 rpm for 15 min. Then, the cell debris was discarded and collected supernatant was preserved (at −80ᵒC) until further use for anti-oxidants and oxidative stress biomarkers assay.
Anti-oxidants and oxidative stress biomarkers assay in the spleen homogenates
The Catalase (CAT) and Superoxide Dismutase (SOD) activities and Malondialdehyde (MDA) concentrations were determined in the spleen homogenates.
The CAT was measured by use of a commercially available kit (Biodiagnostic, Cairo, Egypt) [20]. This method depends on the reaction between catalase and a known quantity of hydrogen peroxide (H2O2). Then, after 1 min the reaction was stopped by adding CAT inhibitor. If peroxidase is present, a chromophore with color intensity inversely proportional to the amount of CAT in the sample will be formed due to the reaction of the remaining H2O2 with 35-dichloro-2- hydroxybenzene sulfonic acid and 4-aminophenazone. The absorbance was measured at 510 nm.
The SOD was measured by a commercially available kit (Biodiagnostic, Cairo, Egypt) [21]. The principle depends on the ability of SOD to inhibit the phenazine methosulphate-mediated reduction of nitroblue tetrazolium dye.
The MDA (the main end product of lipid peroxidation) was measured by a commercially available kit (Biodiagnostic, Cairo, Egypt) [22]. This method depends on the reaction between thiobarbituric acid with MDA in an acidic medium at 95ᵒC for 30 minutes to form a thiobarbituric acid-reactive product. The absorbance was measured at 534 nm.
Immunohistochemistry
The immunohistochemical staining was performed by a streptavidin-biotin-peroxidase method [23]. We stained the sections of all studied groups with anti-CD3 (1:200 ab828 Abcam Tokyo Japan), B220 (1:600 NBP1-43408 APC Novus Biologicals Canada), anti-Iba1 (ab107159 Abcam Tokyo Japan), anti-proliferating cell nuclear antigen (PCNA) (1:500 PC10Thermo Fisher Scientific Tudor Road Manor Park UK), anti-single strand DNA (ssDNA) ( 1:200F7-26 Enzo Life Sciences New York USA), anti-interleukin 1 β (IL-1β) (1:200 Ab82558 Abcam Tokyo Japan) and anti-tumor necrotic factor α (TNF-α ) (1:500 ab6671 Abcam Tokyo Japan) to detect pan T-lymphocytes, B-lymphocytes, macrophages, proliferative cells, apoptotic cells and proinflammatory cytokines respectively. All chemicals were purchased from Al Gomhuria Company, El Mohafza St, Zagazig, Egypt.
In brief, the paraffin sections with 3-5 μm thickness were immersed with xylol to remove paraffin. Then, rehydrated in a descending graded alcohol series up to 50% and washed twice with distilled water for 5 minutes. Antigen retrieval, endogenous peroxidase block, antibodies dilutions and different heat conditions were performed according to manufacturers' instructions. The 3,3′-diaminobenzidine (Dakopatts, Glostrup, Denmark) at pH 7.0 for 3 minutes was used as a chromogen. The Sections were washed in distilled water and counterstained with Harris hematoxylin and mounted with DPX. One negative control section from each animal was incubated in 0.01 M PBS without addition of the primary antibody examined and not photographed.
Histoplanimetry
Light digital micrographs (400X magnification) of four paraffin sections per rat (n=6 rats/group) stained with anti-PCNA, anti-ssDNA and anti-TNFα were prepared for histoplanimetry. The positive cell index was calculated using manual counting in Digimizer 4.3.2. image analysis software (MedCalc software, bvba, Belgium). From selected three well oriented white pulp areas/a section in a frame area=[64126.84 μm2], we counted number of immunopositive cells. The index of +ve cells were calculated according to following equation: number of immunopositive cells/total cells' number. The average of the indices for each immunostained marker was presented.
Statistical analysis
The statistical analysis of all studied parameters between the control, radiated without CHL and radiated with CHL groups was expressed as means ± standard error (SE). The data were analyzed using SPSS 16.0 computer program (one way ANOVA). Post hoc comparison of means was performed by Tukey's test. Significant differences were considered when P ≤ 0.05, n=5 rats/ group.
Results
The activity of antioxidant biomarkers and concentrations of oxidative stress among studied groups
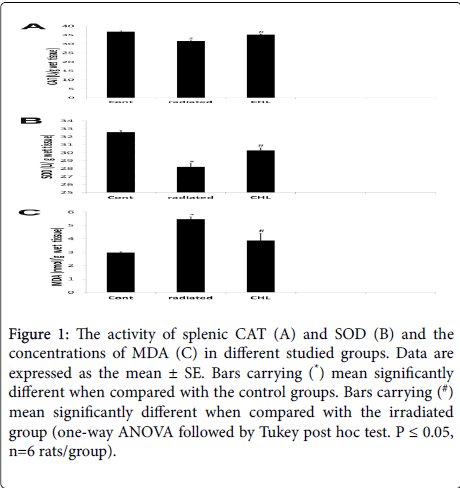
As shown in Figure 1, in the radiated group, the activities of CAT and SOD was significantly decreased (31.57 ± 0.746; 28.23 ± 0.44) while the concentration of MDA was significantly increased (5.46 ± 0.23) when compared with the control group (36.91 ± 0.60; 32.59 ± 0.20; 2.97 ± 0.09 respectively). In the CHL treated group, these data was reversed where a significant increase in CAT and SOD activities (35.32 ± 0.4; 30.26 ± 0.34 respectively) and a significant decrease in MDA concentration (3.88 ± 0.05) was demonstrated in comparison with the irradiated group.
Figure 1: The activity of splenic CAT (A) and SOD (B) and the concentrations of MDA (C) in different studied groups. Data are expressed as the mean ± SE. Bars carrying (*) mean significantly different when compared with the control groups. Bars carrying (#) mean significantly different when compared with the irradiated group (one-way ANOVA followed by Tukey post hoc test. P ≤ 0.05, n=6 rats/group).
Histological features of the spleen among the studied groups
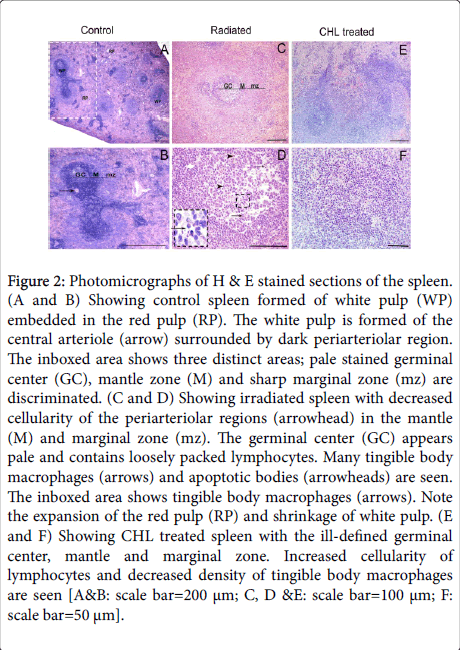
Light microscopic examination of the H&E stained control spleen sections showed two distinctive areas: white pulp and red pulp (Figure 2A). In the white pulp, the cells were heavily condensed around the central arteriole and in the mantle area. The density of cells was less in the germinal center and marginal zone (Figure 2B). In rats receiving radiation, expansion of the red pulp (RP) and shrinkage of white pulp areas was obviously seen. Varied degrees of decreased cellularity especially in the germinal center of the majority of follicles was demonstrated (Figure 2C). An apparent number of tingible body macrophages and apoptotic bodies were seen in the majority of germinal centers (Figure 2D). In CHL treated group, expansion of the white pulp and an increased cellular density was noted. In some white pulps, the three zones were ill-defined (Figure 2E). A decrease density of tingible body macrophages was demonstrated (Figure 2F).
Figure 2: Photomicrographs of H & E stained sections of the spleen. (A and B) Showing control spleen formed of white pulp (WP) embedded in the red pulp (RP). The white pulp is formed of the central arteriole (arrow) surrounded by dark periarteriolar region. The inboxed area shows three distinct areas; pale stained germinal center (GC), mantle zone (M) and sharp marginal zone (mz) are discriminated. (C and D) Showing irradiated spleen with decreased cellularity of the periarteriolar regions (arrowhead) in the mantle (M) and marginal zone (mz). The germinal center (GC) appears pale and contains loosely packed lymphocytes. Many tingible body macrophages (arrows) and apoptotic bodies (arrowheads) are seen. The inboxed area shows tingible body macrophages (arrows). Note the expansion of the red pulp (RP) and shrinkage of white pulp. (E and F) Showing CHL treated spleen with the ill-defined germinal center, mantle and marginal zone. Increased cellularity of lymphocytes and decreased density of tingible body macrophages are seen [A&B: scale bar=200 μm; C, D &E: scale bar=100 μm; F: scale bar=50 μm].
Immune cell populations among studied groups
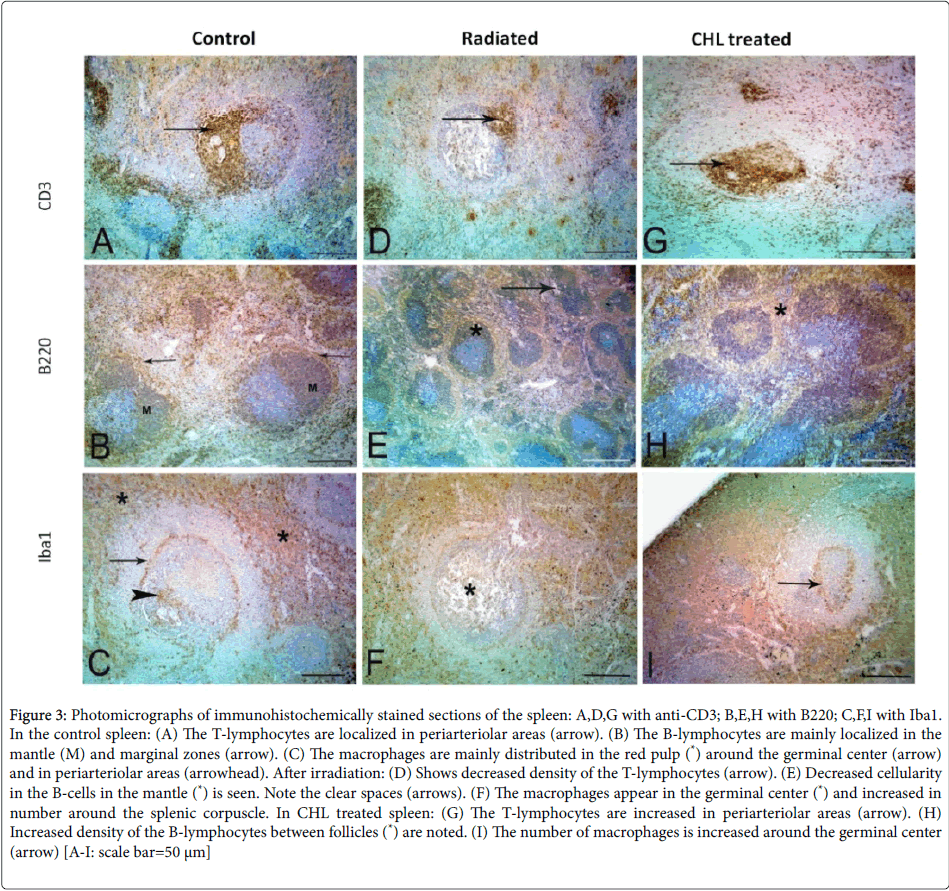
The immunohistochemical staining of normal spleen showed that the CD3+ve T-cells were condensed around the central arterioles. While the B-cells were localized in the mantle and marginal zone. The macrophages were distributed in the red pulp, marginal zone and around the central arteriole (Figures 3A-3C respectively). After receiving radiation, the spleen revealed variable decreases in cellularity and distribution of both CD3+ve T and B-cells. While the macrophages appeared to be more in the germinal center (Figures 3D-3F respectively). In CHL treated rats, there was an increase in CD3+ve Tcells, B- cells and macrophages but no changes in the distribution of the cells could be noted (Figures 3G-3I).
Figure 3:Photomicrographs of immunohistochemically stained sections of the spleen: A,D,G with anti-CD3; B,E,H with B220; C,F,I with Iba1. In the control spleen: (A) The T-lymphocytes are localized in periarteriolar areas (arrow). (B) The B-lymphocytes are mainly localized in the mantle (M) and marginal zones (arrow). (C) The macrophages are mainly distributed in the red pulp (*) around the germinal center (arrow) and in periarteriolar areas (arrowhead). After irradiation: (D) Shows decreased density of the T-lymphocytes (arrow). (E) Decreased cellularity in the B-cells in the mantle (*) is seen. Note the clear spaces (arrows). (F) The macrophages appear in the germinal center (*) and increased in number around the splenic corpuscle. In CHL treated spleen: (G) The T-lymphocytes are increased in periarteriolar areas (arrow). (H) Increased density of the B-lymphocytes between follicles (*) are noted. (I) The number of macrophages is increased around the germinal center (arrow) [A-I: scale bar=50 μm]
Proliferating cells among studied groups
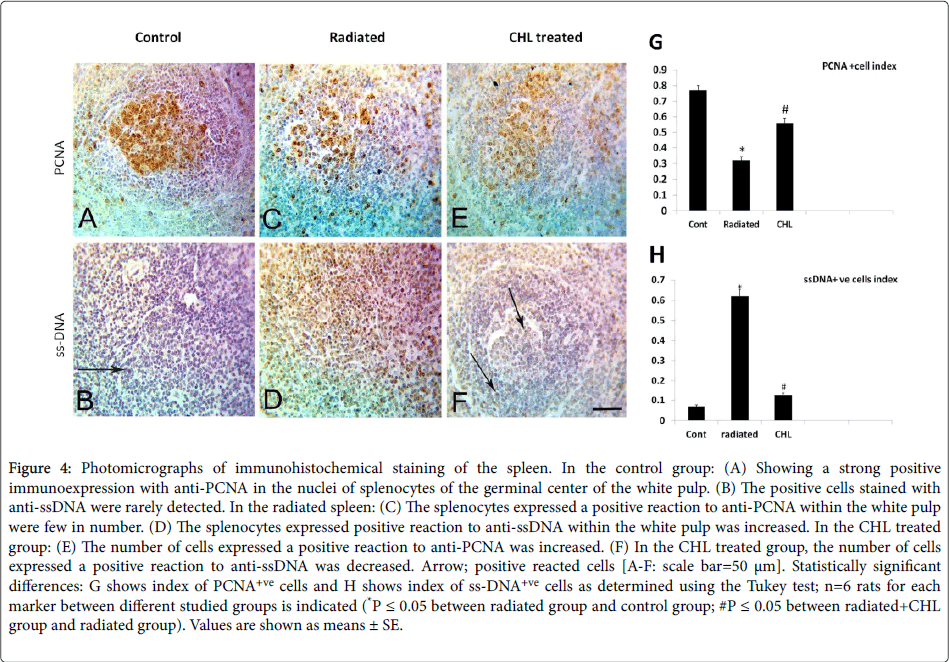
In the control group, nuclear immunostaining with anti-PCNA revealed that the positively stained cells were localized mainly in the germinal center (Figure 4A, 4C and 4E). In comparison with the control group (0.77 ± 0.031), a significant decrease in the index of positively reacted cells (0.32 ± 0.020 P˂0.05) was detected (Figure 4G). After CHL administration, the index of positively reacted cells with anti-PCNA showed a significant increase (0.56 ± 0.032; P˂0.05) (Figures 4E,4G).
Figure 4: Photomicrographs of immunohistochemical staining of the spleen. In the control group: (A) Showing a strong positive immunoexpression with anti-PCNA in the nuclei of splenocytes of the germinal center of the white pulp. (B) The positive cells stained with anti-ssDNA were rarely detected. In the radiated spleen: (C) The splenocytes expressed a positive reaction to anti-PCNA within the white pulp were few in number. (D) The splenocytes expressed positive reaction to anti-ssDNA within the white pulp was increased. In the CHL treated group: (E) The number of cells expressed a positive reaction to anti-PCNA was increased. (F) In the CHL treated group, the number of cells expressed a positive reaction to anti-ssDNA was decreased. Arrow; positive reacted cells [A-F: scale bar=50 μm]. Statistically significant differences: G shows index of PCNA+ve cells and H shows index of ss-DNA+ve cells as determined using the Tukey test; n=6 rats for each marker between different studied groups is indicated (*P ≤ 0.05 between radiated group and control group; #P ≤ 0.05 between radiated+CHL group and radiated group). Values are shown as means ± SE.
Apoptotic cells among studied groups
The immunostained cells with nuclear anti-ssDNA in the control group were rarely seen (Figure 4B). A significant increase in the index of the positively reacted cells number with anti-ssDNA (00.62 ± 0.034 P˂0.05) was noted (Figures 4D,4H) in irradiated group. After CHL administration, the index of apoptotic cells number expressed ss-DNA was significantly decreased (0.13 ± 0.010; P˂0.05) (Figures 4F,4H).
Expression of pro-inflammatory cytokines among studied groups
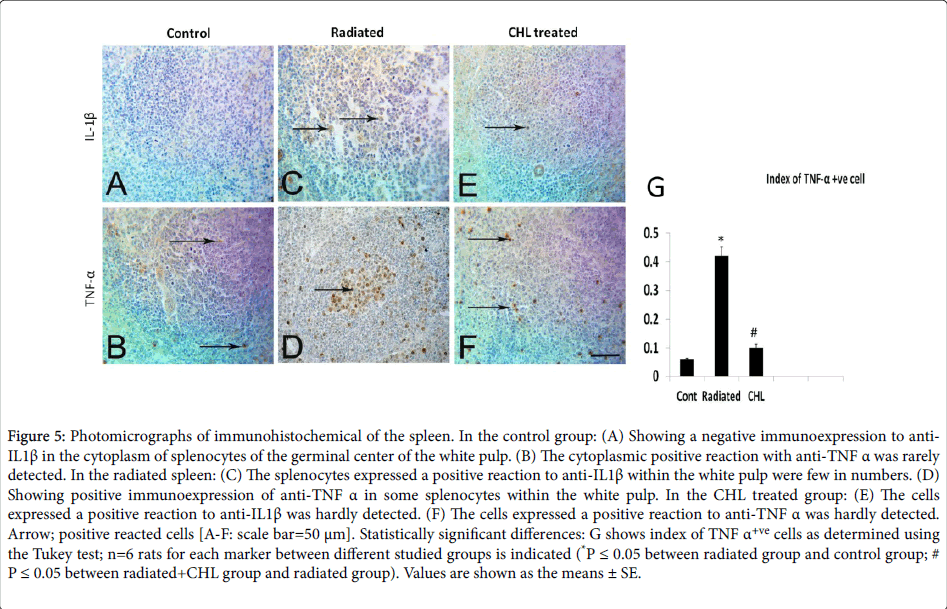
In the control group, the cells expressed cytoplasmic IL-1β and TNF-α were rarely detected (Figures 5A and 5B respectively). In the radiated group, the number of positively stained cells with anti-IL1β was trivial (Figure 5C and 5D). While the cell index of positively reacted cells to anti-TNFα (Figure 5G) was significantly increased (0.42 ± 0.031; P˂0.05) in comparison with the control group (0.06 ± 0.003). In CHL treated group, the positively stained cells with anti- IL1β were rarely seen (Figure 5E and 5F) while the cell index of anti- TNFα positive population was significantly decreased (0.10 ± 0.012; P˂0.05) as shown in Figure 5G.
Figure 5: Photomicrographs of immunohistochemical of the spleen. In the control group: (A) Showing a negative immunoexpression to anti- IL1β in the cytoplasm of splenocytes of the germinal center of the white pulp. (B) The cytoplasmic positive reaction with anti-TNF α was rarely detected. In the radiated spleen: (C) The splenocytes expressed a positive reaction to anti-IL1β within the white pulp were few in numbers. (D) Showing positive immunoexpression of anti-TNF α in some splenocytes within the white pulp. In the CHL treated group: (E) The cells expressed a positive reaction to anti-IL1β was hardly detected. (F) The cells expressed a positive reaction to anti-TNF α was hardly detected. Arrow; positive reacted cells [A-F: scale bar=50 μm]. Statistically significant differences: G shows index of TNF α+ve cells as determined using the Tukey test; n=6 rats for each marker between different studied groups is indicated (*P ≤ 0.05 between radiated group and control group; #P ≤ 0.05 between radiated+CHL group and radiated group). Values are shown as the means ± SE.
Discussion
The dietary antioxidants play a very important role in alleviating the injurious effects of oxidative stress on cells. Many researchers considered CHL as a superior component to drug derifil in the treatment of many disorders related to oxidant-antioxidant imbalance [16]. Our aim in this study was to determine the possible structural alterations of spleen after localized gastric irradiation and radioprotective effects of Chlorophyllin administration.
According to the presented data, the safety of localized irradiation is doubtful. This is fueled in part by a significant decrease of antioxidants and a significant increase of oxidant biomarkers. Excess oxidants (ROS) production could occur via activated macrophages and neutrophils. These ROS might be deleterious to immune cells via increased sensitivity of T-cells to apoptosis by decreasing expression of anti-apoptotic gene bcl-2 [24]. The endogenous defense mechanisms via antioxidants are not always sufficient to fully scavenge oxidative stress. So, dietary components with antioxidant properties are necessary to be fortify the activity of endogenous antioxidants.
Administration of CHL significantly reversed the oxidative stress levels in this study. Similar data were reported after adding of water extract chlorophyll compounds on human lymphocytes culture exposed to H2O2 [25]. They assumed that chlorophyll compounds enhance the ability of lymphocytes to resist H2O2 induced oxidative damage. Moreover, reducing ROS production after CHL intake in irradiated mice prevented splenic lymphocytes apoptosis [17]. Zhang et al. believe that CHL confers protection against oxidative stress by activating nuclear factor (erythroid-derived 2) signaling pathway [26]. A recent study showed that chlorophyll compounds act both as free radical scavengers and as Fe (II) chelators. Furthermore, the authors confirmed the ability of chlorophyll compounds to prevent the catalysis of lipid peroxidization [27].
The presence of varying degrees of low cellularity of germinal centers (depressed white pulp) in this work supports the possibility of decreased lymphoid activity that has resulted from strong exhaustive immune activity in this region [28]. ROS is destructive to DNA lipids and proteins. Consequently, alterations in signal transduction and gene expression to mitogenesis, transformation mutagenesis and finally apoptosis of cells [29]. It was reported that radiation-induced apoptotic bodies in mice spleen was via generation of excess ROS intracellular [30].
After localized radiation, the alterations of germinal centers in this study were similar to the data recorded after whole body radiation [16]. The increase of the tingible body macrophages, condensation of macrophages and hemosiderin pigments in the germinal centers and marginal zone of irradiated group denoting radio-resistance of macrophages and increased phagocytosis of apoptotic bodies [31].
The marginal zone is responsible for screening pathogens from systemic circulation. Its cells are involved in both innate and adaptive immunity to these antigens [32]. In rats exposed to radiation in this study, the cellular density of the marginal zone was lower compared to the control group. These data might indicate disturbances in interactions between migratory and resident cells of splenic regions [33].
Immunohistochemically, the present study revealed that the most affected cells after irradiation were T-lymphocytes. Such data may be suggestive to higher radiosensitivity of T-cells [8]. Increased cellularity in the B-cell areas might be due to the migration of proliferated immature B cells or immunoblasts (in response to radiation stimuli) into the red pulp after maturation into immunoglobulin-producing plasma cells [33]. Further investigations are needed.
Expression of PCNA is not confined to cellular proliferation, it can get an idea of DNA synthesis and DNA repair especially in injured cells. Also, PCNA is responsible for chromatin remodeling and epigenetics [34,35]. The positively reacted cells with anti-PCNA was darkly stained and decreased in number after exposure to localized irradiation in the present work. After CHL intake the expansion of lymphocytes was observed. In line, increased homing of lymphocytes in the spleen was reported [16]. They referred cellular increment to the enhancement of anti-apoptotic related genes bcl-2 and bcl-xL expression in lymphocytes. So, they supposed that CHL protects against immunosuppression induced by radiation. Increased stem cell activity and/or modulation of hematopoiesis by immune-stimulatory contents of CHL might be another cause of the expansion of lymphocytes in white pulp [36].
The present observations may thus appear contradictory to the published data of Chiu et al. who proved antiproliferative properties of CHL on human breast cancer MCF-7 cells [37]. In addition, Kumar et al. reported retardation of cell proliferation following CHL administration [17]. Moreover, Chiu et al. found that CHL inhibits the proliferation and growth of MCF-7 breast carcinoma cells by inducing apoptosis after deactivating the extracellular signal-regulated kinases [37].
Regarding apoptosis, this study corroborated the radiosensitivity of splenocytes even with the localized radiotherapy. The proposed mechanism of apoptosis is via mainly induction of DNA single-strand breaks and to some extent via activation of TNF-α. Controversy, Takahashi et al. referred apoptosis after irradiation to Bax formation [30]. Activation of tumor suppressor p53 after induction of doublestrand breaks in DNA is another cause of apoptosis after irradiation [38]. These contradictory data might relate to differences in experimental procedures used.
In the current work, CHL inhibited apoptosis via reduction of single-strand breaks in DNA. In the line of our work, adding of chlorophyllin to human lymphocytes culture exposed to H2O2 reduced ss-DNA breaks [25]. Moreover, oral administration of CHL was shown to decrease apoptosis via protection against induction of DNA singlestrand breaks (an apoptotic marker) in C57BL/6J mice treated with benzo[a]pyrene [39]. Thus, CHL seems to have a dual effect on protection of splenocytes, stimulating their proliferation and/or decreasing their apoptosis. Kumar et al. attributed improvement of splenic structure receiving CHL to the formation of complexes between CHL and the mutagens or carcinogens. Also, CHL fasts their excretion and diminishes their bioavailability [17]. Therefore, CHL could reduce cytogenetic damage induced by radiation. In conclusion, localized radiotherapy is not safe on neighboring organs. Adding of CHL modulated the radiotherapy damage. Maintenance of regular dietary consumption of CHL is recommended during localized radiotherapy to provide a constant supply of potent antioxidants that might prevent any alterations.
Acknowledgements
We would like to thank Dr. Amira Elwan, the lecturer of Oncology Department, Faculty of Medicine, Zagazig University for her technical help and Dr. Sami Al Said, Prof. Dr. of English Department, Faculty of Art, Zagazig University for language revision.
Funding
This research is personally funded.
Potential conflict of interest
The authors have no conflicting financial interest.
References
- Schaue D, Micewicz ED, Ratikan JA, Xie MW, Cheng G, et al. (2015) Radiation and inflammation. Semin Radiat Oncol 25: 4-10.
- Lei R, Zhao T, Li Q, Wang X, Ma H, et al. (2015) Carbon ion irradiated neural injury induced the peripheral immune effects in vitro or in vivo. Int J Mol Sci 16: 28334-28346.
- Pratheeshkumar P, Kuttan G (2011) Protective role of Vernonia cinerea L. against gamma radiation-induced immunosupression and oxidative stress in mice. Hum Exp Toxicol 30: 1022-1038.
- Ashry OM, El Shahat A, Abou el Khier I, Abd el Sammad H (2011) Hematopoiesis stimulating role of IL-12 enabling bone marrow transplantation in irradiated rats. Egypt J Rad Sci 24: 234-255.
- Karami M, Nosrati A, Naderi M, Makhloogh M, Shahani S, et al. (2015) Protective effects of Nasturtium officinale against gamma-irradiation-induced hepatotoxicity in C57 mice. Res J Pharmacog 2: 19-25.
- Akleyev AV, Akushevich IV, Dimov GP, Veremeyeva GA, Varfolomeyeva TA, et al. (2010) Early hematopoiesis inhibition under chronic radiation exposure in humans. Radi Envir Biophy 49: 281-291.
- Khozouz RF, Huq SZ, Perry MC (2008) Radiation-induced liver disease. J Clin Oncol 26: 4844-4845.
- An HJ, Seong SJ (2006) Proteomics analysis of apoptosis-regulating proteins in tissues with different radiosensitivity. J Radiat Res 47: 147-155.
- Bjelakovic G, Nikolova D, Gluud LL, Simonetti RG, Gluud C, et al. (2007) Mortality in randomized trials of antioxidant supplements for primary and secondary prevention: systematic review and meta-analysis. Jama 297: 842-857.
- Ammar, Amal AA (2015) A study on the preventive effect of mulberry (Morus alba l.) fruits in rats exposed to gamma radiation. Egyptian J Hospital Med 61: 383-388.
- Wambi C, Sanzari J, Wan XS, Nuth M, Davis J, et al. (2008) Dietary antioxidants protect hematopoietic cells and improve animal survival after total-body irradiation. Radiat Res 169: 384-396.
- Choi S, Lee S, Kim H, Lee I, Kozukue N, et al. (2010) Changes in free amino acid phenolic chlorophyll carotenoid and glycoalkaloid contents in tomatoes during 11 stages of growth and inhibition of cervical and lung human cancer cells by green tomato extracts. J Agric Food Chem 58: 7547-7556.
- Weydert CJ, Cullen JJ (2010) Measurement of superoxide dismutase catalase and glutathione peroxidase in cultured cells and tissue. Nat Protoc 5: 51.
- Boloor K, Kamat J, Devasagayam T (2000) Chlorophyllin as a protector of mitochondrial membranes against γ-radiation and photosensitization. Toxicology 155: 63-71.
- Shepherd M, McLean S, Hunter CN (2005) Kinetic basis for linking the first two enzymes of chlorophyll biosynthesis. FEBS J 272: 4532-4539.
- Sharma D, Kumar SS, Sainis KB (2007) Antiapoptotic and immunomodulatory effects of chlorophyllin. Mol Immunol 44: 347-359.
- Kumar SS, Shankar B, Sainis KB (2004) Effect of chlorophyllin against oxidative stress in splenic lymphocytes in vitro and in vivo. Biochem Biophys Acta 1672: 100-111.
- Leite SV, Oliveira RJ, Kanno T, Mantovani MS, Moreira E, et al. (2013) Chlorophyllin in the intra-uterine development of mice exposed or not to cyclophosphamide. Acta Scientiarum Health Sci 35: 201-210.
- Spencer LT, Bancroft JD, Bancroft J, Gamble M (2013) Tissue processing. Bancroft's Theory and Practice of Histological Techniques Expert Consult. Bancroft's Theory and Practice of Histological Techniques pp: 105.
- Nishikimi M, Appaji N, Yagi K (1972) The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46: 849-854.
- Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95: 351-358.
- Jackson P, Blythe D (2008) Immunohistochemical techniques. Teoksessa Bancroft JD & Gamble M.(toim.) Theory and practice of histological techniques 6: 433-472.
- Hildeman DA, Mitchell T, Aronow B, Wojciechowski S, Kappler J, et al. (2003) Control of Bcl-2 expression by reactive oxygen species. Proc Natl Acad Sci 100: 15035-15040.
- Hsu CY, Yang CM, Chen CM, Chao PY (2005) Effects of chlorophyll-related compounds on hydrogen peroxide induced DNA damage within human lymphocytes. J Agric Food Chem 53: 2746-2750.
- Zhang Y, Guan L, Wang X, Wen T, Xing J, et al. (2008) Protection of chlorophyllin against oxidative damage by inducing HO-1 and NQO1 expression mediated by PI3K/Akt and Nrf2. Free Radic Res 42: 362-371.
- Hsu CY, Chao PY, Hu SP, Yang CM (2013) The antioxidant and free radical scavenging activities of chlorophylls and pheophytins. Food Nutr Sci 4: 1-8.
- Mirkov I, Zolotarevski L, GlamoÄlija J, Kataranovski D, Kataranovski M, et al. (2008) Experimental disseminated aspergillosis in mice: Histopathological study. Mycol Med 18: 75-82.
- Kujoth G, Hiona A, Pugh T, Someya S, Panzer K, et al. (2005) Mitochondrial DNA mutations oxidative stress and apoptosis in mammalian aging. Science 309: 481-484.
- Takahashi A, Kondo N, Inaba H, Uotani K, Kiyohara Y, et al. (2003) Radiation-induced apoptosis in scid mice spleen after low dose irradiation. Adv Space Res 31: 1569-1573.
- Singh L, Tyagi S, Rizvi MA, Goel HC (2007) Effect of Tinospora cordifolia on gamma ray-induced perturbations in macrophages and splenocytes. J Radiat Res 48: 305-315.
- Mebius RE, Kraal G (2005) Structure and function of the spleen. Nature reviews. Immunol 5: 606.
- Cesta MF (2006) Normal structure function and histology of the spleen. Toxicol Pathol 34: 455-465.
- Moldovan GL, Pfander B, Jentsch S (2007) PCNA the maestro of the replication fork. Cell 129: 665-679.
- Shiraishi M, Ishino S, Yoshida K, Yamagami T, Cann I, et al. (2016) PCNA is involved in the EndoQ-mediated DNA repair process in Thermococcales. Sci Rep 6.
- Suryavanshi S, Sharma D, Checker R, Thoh M, Gota V, et al. (2015) Amelioration of radiation-induced hematopoietic syndrome by an antioxidant chlorophyllin through increased stem cell activity and modulation of hematopoiesis. Free Radic Biol Med 85: 56-70.
- Chiu LC, Kong CK, Ooi VE (2003) Antiproliferative effect of chlorophyllin derived from a traditional Chinese medicine Bombyx mori excreta on human breast cancer MCF-7 cells. Int J Oncol 23: 729-735.
- Lee CL, Blum JM, Kirsch DG (2013) Role of p53 in regulating tissue response to radiation by mechanisms independent of apoptosis. Transl Cancer Res 2: 412-421.
- Gradecka-Meesters D, Palus J, Prochazka G, Segerbäck D, Dziubałtowska E, et al. (2011) Assessment of the protective effects of selected dietary anticarcinogens against DNA damage and cytogenetic effects induced by benzo[a]pyrene in C57BL/6J mice. Food Chem Toxicol 49: 1674-1683.
Citation: Ali Khalifa ME, Gouda ZA (2018) The Antioxidative and Antiapoptotic Effects of Chlorophyllin on Female Rat Spleen Exposed to Localized Gastric Radiotherapy. J Biochem Cell Biol 1: 102.
Copyright: ©2018 Ali Khalifa ME, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License which permits unrestricted use distribution and reproduction in any medium provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Usage
- Total views: 4803
- [From(publication date): 0-2018 - Nov 14, 2025]
- Breakdown by view type
- HTML page views: 3797
- PDF downloads: 1006