The Cholesterol 24-Hydroxylase Enzyme, CYP46A1, Reduces Overexpressed Alpha-Synuclein Proteins in Human Cellular Models of Parkinsonâs Disease
Received: 11-Oct-2024 / Manuscript No. DPO-24-150181 / Editor assigned: 14-Oct-2024 / PreQC No. DPO-24-150181 (PQ) / Reviewed: 28-Oct-2024 / QC No. DPO-24-150181 / Revised: 04-Nov-2024 / Manuscript No. DPO-24-150181 (R) / Published Date: 11-Nov-2024 DOI: 10.4172/2476-2024.1000240
Abstract
Background: A growing body of evidence suggests a correlation between cholesterol metabolism and the pathogenesis of Parkinson's Disease (PD). We and others have demonstrated that the activation of the cholesterol 24-hydroxylase enzyme, CYP46A1, responsible for converting cholesterol to 24S-Hydroxycholesterol (24-OHC) in the brain, is an effective therapeutic strategy for several neurodegenerative diseases as Alzheimer's disease, huntington’s disease and spinocerebellar ataxia type 3. Nevertheless, there is still much to be elucidated regarding the role of CYP46A1 in PD. Alpha-Synuclein, the symbol of pathological protein of PD, exhibits a pronounced affinity for binding to lipid membranes, especially in cholesterol-rich regions and contains a high-affinity cholesterol-binding motif in the 67-78 a region. To further our understanding of CYP46A1’s actions, we assessed not only the expression of wild-type alpha-syn but also that of the A53T alpha-syn mutant associated with the familial form of the disease as well as mutants lacking presumed cholesterol-binding sites in conjunction with CYP46A1.
Methods: We established several models of PD in human neuroblastoma SH-SY5Y cells through transient transfection with either wild-type or mutant alpha-syn or by inhibiting key genes involved in the disease using Small Interfering RNA (siRNA). The effects of CYP46A1 co-transfection on the alpha-syn expression were then analyzed by Western blot.
Results: Overexpression of human CYP46A1 leads to a significant reduction in wild-type alpha-syn protein levels in SH-SY5Y cells. Our investigations suggest that this decrease is mediated through the autophagy-lysosomal pathway. Additionally, our findings indicate that CYP46A1 may also reduce the levels of alpha-syn proteins carrying mutations in the cholesterol-binding domain or at the residue A53T, which is associated with familial pathology. Moreover, CYP46A1 retains its functionality in a cellular model of PD associated with GBA1. The gene GBA1 is involved in lipid metabolism and its deficiency represents the most prevalent genetic factor associated with an elevated risk of PD.
Conclusion: These findings offer valuable insights into the pathogenesis of PD and reveal potential therapeutic avenues involving CYP46A1, highlighting its ability to reduce alpha-syn expression, which could benefit patients with PD.
Keywords: Parkinson’s disease; CYP46A1; Alpha-synuclein; Autophagy; Human neuroblastoma cells (SH-SY5Y)
Introduction
Alpha-Synuclein (aSyn) plays a central role in the development of Parkinson's Disease (PD) [1]. A key characteristic of PD is the degeneration of dopaminergic neurons in the substantia nigra pars compacta. The remaining neurons display an accumulation of alpha- syn proteins, forming aggregates in Lewy bodies. The SNCA gene encodes a 14.5-kDa protein, alpha-syn, which has been implicated as an important factor in synaptic vesicle trafficking and dopamine release.
However, its physiological function remains incompletely understood. Overexpression of the alpha-syn protein, resulting from duplications or triplications of the SNCA locus or point mutations in the SNCA gene, is associated with familial forms of PD [2-4]. While many of these genetic alterations are either rare or confer a variable risk for developing PD, they highlight that the exclusive overexpression of alpha-syn can contribute to the onset of the disease. The spread of aggregated alpha-syn from cell to cell is currently considered as a mechanism explaining the pathological progression of the disease. Due to the aberrant assembly of alpha-syn being a common feature in the development of PD, significant efforts have been dedicated to understanding and inhibiting this phenomenon.
Recent findings indicate that Lewy bodies in addition of the high level of alpha-syn proteins are also characterized by a high lipid content and degenerated organelles suggesting that lipids, which are the main constituents of cell membranes, may contribute to the development of the disease [5,6]. Over the last two decades, accumulating evidence suggests a transient binding of alpha-syn to lipid membranes particularly in cholesterol-rich regions [7-10]. Cholesterol may affect the binding of alpha-syn to the membrane and its abnormal aggregation [10-12]. Studies have also demonstrated that alpha-syn significantly stimulates cholesterol efflux [13].
The brain, being the highest cholesterol-rich organ in mammals, maintains a major balance of cholesterols levels through a precisely regulated process called brain cholesterol homeostasis. Cholesterol, is an integral component of membranes, contributing to their structure and function and acts as a precursor of metabolites involved in various metabolic pathways. The cholesterol is mainly found in the myelin sheaths of the oligodendrocytes, as well as in the membranes of glial cells and neurons. Astrocytes synthesize cholesterol and export it through Adenosine Triphosphate (ATP)-binding cassette transporters, primarily via ATP Binding Cassette Subfamily a Member 1 (ABCA1) transporter. The transportation of cholesterol to neurons is facilitated by APOE, which is also synthesized by astrocytes [14].
Studies have shown disruptions in this balance during neurodegenerative diseases such as Alzheimer's Disease (AD) [15-17], Huntington’s Disease (HD) [18,19] and Amyo-trophic Lateral Sclerosis (ALS) as well as in brain disorders such as epilepsy [20,21]. Previous research has demonstrated that regulating cholesterol homeostasis through the over expression of cytochrome P450 family 46 subfamily A member 1 (CYP46A1), a brain-specific enzyme that converts cholesterol into 24-Hydroxycholesterol (24-OHC), has protective effects against AD, HD, Spino-Cerebellar Ataxia Type 3 (SCA3) [22-26]. The role of CYP46A1, primarily located in neurons, is major because cholesterol itself cannot cross the Blood-Brain Barrier (BBB), whereas 24-OHC can pass through BBB into the systemic circulation. Acting as a ligand for Liver X Receptors (LXR), 24-OHC subsequently regulates the transcription of cholesterol transport proteins such as ABCA1, which are located on the plasma membrane. Consequently, this process effectively regulates cholesterol efflux. However, these studies have revealed that the effects of CYP46A1 activity extend beyond cholesterol maintenance, leading to a reduction in aggregated proteins implicated in these diseases [27]. These findings underscore the pivotal role of cholesterol turnover and 24-OHC in neurological disorders.
Despite this, only a few studies investigated the role of CYP46A1 in PD. Recent research has revealed reduced levels of CYP46A1 proteins in induced Pluripotent Stem Cell (iPSC)-derived dopaminergic neurons from patients carrying the SNCA triplication or the A53T, SNCA mutation, both associated with familial PD onset [28]. Notably, CYP46A1 overexpression has been observed to elevate levels of 24- OHC and 24 (S), 25-epoxycholesterol (24, 25-EC) in the developing midbrain. This process has been proposed as a potential mechanism for preventing dopaminergic loss in PD [29]. These data suggested that CYP46A1 could be an important factor in PD.
The aim of our study was to investigate whether the overexpression of CYP46A1 could also be beneficial for PD and lead to a decrease in overexpressed alpha-syn. In the present study, we aimed to investigate whether the expression of CYP46A1 could reduce the levels of overexpressed alpha-syn proteins in neuroblastoma SH-SY5Y cells. To deepen our comprehension of CYP46A1’s actions, we evaluated not only the expression of wild-type alpha-syn but also the expression of A53T alpha-syn mutant associated with the familial form of the disease [2], as well as mutants lacking presumed cholesterol-binding sites, all in the presence of CYP46A1. Additionally, we investigated the impact of CYP46A1 on alpha-syn overexpression by inhibiting autophagiclysosome functions using autophagic inhibitors and by reducing the expression of the lysosomal enzyme encoded by the GBA1 gene, which are among the most common genetic factors associated with an increased risk of PD [30,31].
Materials and Methods
Generation of untagged alpha-synuclein constructs
Human alpha-synuclein (WT or A53T) cDNA was amplified by PCR from previously described vectors kindly provided by Add gene (plasmids #102361 and 105727). The PCR products were cloned in the HindIII/XbaI sites of the pcDNA3.1 vector using primers described.
Cell culture and co-transfections
Human SH-SY5Y neuroblastoma cells (ATCC: CRL-266), were cultured in Dulbecco's Modified Eagle Medium (DMEM) with Glutamax (Gibco), supplemented with 4.5 g/L D-glucose and sodium pyruvate, 10% heat-inactivated fetal bovine serum (Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco), in a 5% CO2 atmosphere at 37°C. Cells were transiently transfected with plasmids using Lipofectamine 2000 (Invitrogen), following to the manufacturer’s instructions. The pcDNA3-EGFP plasmid was obtained from Add gene (plasmid #13031) while pAAV-HA-CYP46A1 or pCMV-CYP46A1-Flag plasmids were provided by Askbio France. For autophagy inhibition, treatments were initiated either 6 hours or 24 hours post-transfection with 3-MA or BafA1, respectively. Cells were then incubated with 3-MA for 42 hours or with BafA1 for 24 hours. 3-MA (189-490, Millipore) was prepared at a concentration of 50 mM in Opti-MEM (Gibco), sterilized by filtration through a syringe filter with a 0.2 μM pore size and added to a final concentration of 5 mM in the cell medium. BafA1 (B1793, Sigma) was dissolved as a 10 uM solution in DMSO and added to the cell medium to achieve a final concentration of 50 nM. For proteasome inhibition, MG132 (474790, Sigma) was prepared as a 20 mM solution in DMSO. Cells were treated with 20 μM MG132 42 hours post-transfection for 6 hours. The concentrations of these inhibitors were selected based on data from other studies and preliminary experiments.
Protein extraction and western-blot analysis
Cell pellets were homogenized in RIPA buffer (150 mM NaCl, 50 mM Tris pH 8, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with protease inhibitors for 30 min at 4°C. Whole lysates were clarified by centrifugation at 10,000 rpm for 15 min. Protein concentrations were quantified using Pierce BCA Protein Assay Kit (Thermo Fisher Scientific) with Bovine Serum Albumin (BSA) standards for comparison. Equal volumes of each sample were separated by SDS-PAGE. Protein samples were denatured for 3 min at 95°C and separated into 4%-20% SDS-polyacrylamide gels, then transferred to nitrocellulose membranes (Bio-Rad). Membranes were blocked with 5% BSA in Tris-Buffered Saline (TBS) pH 8 for 1 h, followed by overnight incubation with primary antibodies (dilution 1:1000) at 4°C. After washing three times for 10 min each in TBS with 0.1% Tween-20, membranes were incubated with Li-COR fluorescent secondary antibodies (dilution 1:6000) for 1 hour at room temperature and washed three more times with TBS with 0.1% Tween-20. Finally, images of the membranes were captured using a Li-COR Odyssey fluorescence scanner. The protein ladder used was precision plus protein standards Kaleidoscope ladder, 10 to 250 kD (Bio-Rad).
Immunofluorescence microscopy
For immunostaining, SH-SY5Y cells were cultured on glass coverslips. Twenty-four hours post-transfection, cells were washed twice with Phosphate-Buffered Saline (PBS), fixed with 4% paraformaldehyde in PBS, quenched for 10 min with 0.1 M glycine in PBS and permeabilized for 30 min at room temperature with 0.05% saponin, PBS, 0.5% bovine serum albumin. The coverslips were incubated for 2 h at room temperature with the primary antibodies (see below) at dilution 1:250 in PBS containing 0.5% BSA, 0.05% saponin. After washing, cells were subsequently incubated for 2 hours at room temperature with the secondary antibodies (see below) at dilution 1:500, covered from light. Cells were washed three times with PBS and mounted using Vectashield with DAPI or without (Vector Laboratories). Fluorescent images of cells were captured with a 60X objective using a Zeiss Apotome 2 microscope with the structured illumination system equipped of a Zeiss Axiocam camera. Image acquisition was performed with the ZEN 2 (Zeiss) software. The images were merged and analyzed using Image J.
Antibodies
The following primary antibodies were used: Rabbit anti-actin (Sigma, A2066), rabbit anti-GFP (Sigma, SAB301138), rabbit anti- HA (Cell signaling, 3724), mouse anti-alpha-synuclein antibody (Invitrogen, AHB0261), mouse anti-Flag (Sigma, F3165), mouse antiubiquitin (Invitrogen, 14-6078-82), mouse anti-Lamp2A (Abcam, Ab25631), rabbit anti-LC3B (Abcam, 51520), rabbit anti-GBA1 (Sigma, G4171). Secondary antibodies for immunoblotting were appropriate goat anti-mouse or anti-rabbit IRDye 680RD or 800CW IgG (Odyssey LI-COR). For immunofluorescence labelling Alexa-488 or Alexa-555, conjugated donkey, anti-mouse or anti-rabbit, (life technologies) were used.
RNA isolation and quantitative PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen), treated with DNase I and reverse-transcribed using the one script qPCR RT Kit (Ozyme). cDNA was amplified using SYBR Green on the Roche Light Cycler 480 for quantification. The relative expression levels of mRNA were normalized to S18 ribosomal RNA levels (RibS18).
The following primers were used: Human CYP46A1 forward (5’CGAGTCCTGAGTCGGTTAAGAAGTT3’) and re-verse (5‘AGTCTGGAGCGCACGGTACAT3’), human alpha-syn forward (5’ACCAAACAGGGTGTGGCAGAAG3’) and reverse (5’CTTGCTCTTTGGTCTTCTCAGCC3’) and human Ribs18 forward (5’GAGGATGAGGTGGAACGTGT3’) and reverse (5’GGACCTGGCTGTATTTTCCA3’). The program consisted of an initial hot start cycle at 95°C for 2 min, followed by 45 cycles at 95°C for 10 s, 65°C for 20 s and 72°C for 20 s with a final extension at 72°C for 10 min. Each sample was assayed in triplicate.
Site-direct mutagenesis
The human alpha-syn-pcDNA3.1 plasmid was subjected to sitedirected mutagenesis to introduce specific amino acid changes within the domain spanning residues 67 to 78, as listed in Supplementary Table 1. Various plasmids were generated including: Plasmid 38 with mutations A69G, V70L, T72S, G73R; plasmid 39 with mutations A69T, V70R, G73Y, V74W, A76S; plasmid 40 with mutations V71S, T72A, T75A, A76S and plasmid 42 with mutation A69K. Forward and reverse primers, each of 25-40 nucleotides long, were designed to introduce the desired mutations (see Supplementary Table 2). Additional primers were necessary for cloning into the HindIII/XbaI sites of the pcDNA3.1 vector (see Supplementary Table 2). PCR was performed using the mutation primers (forward or reverse), cloning primers (HindIII or XbaI primers) and the alpha-syn-pcDNA3.1 plasmid as a template, along with a high-fidelity thermostable Taq DNA polymerase (Thermo Fisher, Master Mix Dream Taq Green PCR). The PCR conditions involved an initial denaturation at 94°C for 3 min, followed by 30 cycles of denaturation at 94°C for 30 s, annealing at 66°C for 30 s and extension at 72°C for 30 s, with a final extension step at 72°C for 7 min. For each construct, a final PCR was conducted using the two PCR fragments isolated on agarose gels with a gel extraction kit (Qiagen), along with the two HindIII and XbaI primers. The resulting PCR product was isolated using the Qiaquick PCR purification kit (Qiagen), digested with HindIII and XbaI and then ligated into the pcDNA3.1 plasmid, which had been previously digested with HindIII and XbaI. The plasmids were transformed into competent bacteria via heat shock. Single colonies were selected, grown overnight and plasmid DNA was isolated using a miniprep kit (Qiagen). Sequencing of the plasmids was conducted to confirm the introduced base changes.
Reduction of Lamp2A or GBA1 expression by RNA silencing
To downregulate the levels of Glucocerebrosidase (GCase) encoded by the GBA1 gene or Lamp2A proteins, siRNAs were purchased from thermo fisher scientific (Ambion by life technologies). Three siRNAS targeting human GBA1 (GBA1a (GBA-s501316), GBA1b (GBA-s534767) and GBA1c (GBA-s534768)) were tested. The siRNA targeting human Lamp2A is Lamp2-s8086. As a negative control, siRNA s18483 obtained from Thermofisher was used. The specific siRNAs, at a concentration of 50 nM, were co-transfected with Lipofectamine 2000 (Invitrogen) along with alpha-syn and GFP or CYP46A1 plasmids for 48 hours into SH-SY5Y cells.
Statistical analysis
Statistical analysis were conducted using GraphPad Prism. The specific statistical tests used are indicated in the figure legends. Values are presented as means ± SEM. A p-value of less than 0.05 was considered statistically significant.
Results
Decrease in alpha-syn expression following co-transfection with CYP46A1 in neuroblastoma SH-SY5Y cells
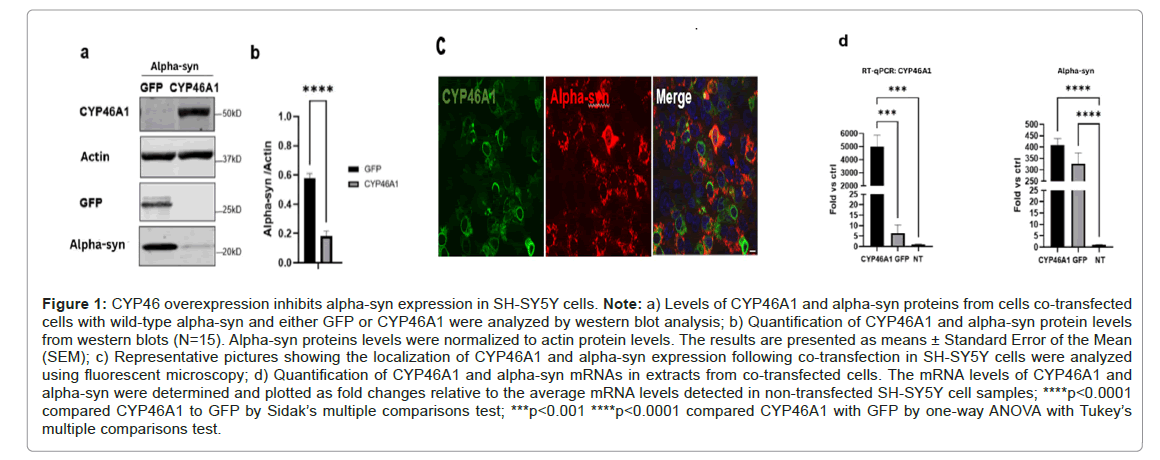
To explore the impact of CYP46A1 on alpha-syn in PD, we generated a plasmid harboring human Wild-Type (WT) alpha-syn cDNA. This construct was then co-transfected with a plasmid expressing either CYP46A1 or GFP into human neuroblastoma SH-SY5Y cells. Fortyeight hours post-transfection, the presence of CYP46A1 led to a significant threefold reduction in the levels of alpha-syn proteins. Immunofluorescence analysis of CYP46A1 and alpha-syn conducted 48 hours post-transfection revealed cytoplasm localization for alpha-syn, characterized by a vesicular pattern. CYP46A1 predominantly exhibited localization around the nuclear membrane and within the cytoplasm, likely associated with the membrane of the endoplasmic reticulum, as previously demonstrated [32]. Next, we investigated whether the reduction in alpha-syn expression occurred at the transcriptional level by performing real-time RT-QPCR analysis. Analysis of alphasyn mRNA levels from cells co-transfected with alpha-syn and either CYP46A1 or GFP showed no significant difference. These findings suggest that the difference in alpha-syn levels is not attributable to transcriptional regulation by CYP46A1. In conclusion, we have demonstrated that while CYP46A1 does not reduce the level of alphasyn transcripts, it does affect the level of alpha-syn proteins (Figures 1A-1D).
Figure 1: CYP46 overexpression inhibits alpha-syn expression in SH-SY5Y cells. Note: a) Levels of CYP46A1 and alpha-syn proteins from cells co-transfected cells with wild-type alpha-syn and either GFP or CYP46A1 were analyzed by western blot analysis; b) Quantification of CYP46A1 and alpha-syn protein levels from western blots (N=15). Alpha-syn proteins levels were normalized to actin protein levels. The results are presented as means ± Standard Error of the Mean (SEM); c) Representative pictures showing the localization of CYP46A1 and alpha-syn expression following co-transfection in SH-SY5Y cells were analyzed using fluorescent microscopy; d) Quantification of CYP46A1 and alpha-syn mRNAs in extracts from co-transfected cells. The mRNA levels of CYP46A1 and alpha-syn were determined and plotted as fold changes relative to the average mRNA levels detected in non-transfected SH-SY5Y cell samples; ****p<0.0001 compared CYP46A1 to GFP by Sidak’s multiple comparisons test; ***p<0.001 ****p<0.0001 compared CYP46A1 with GFP by one-way ANOVA with Tukey’s multiple comparisons test.
Impact of CYP46A1 overexpression on A53T alpha-syn mutant and mutants devoid of presumed putative cholesterolbinding sites
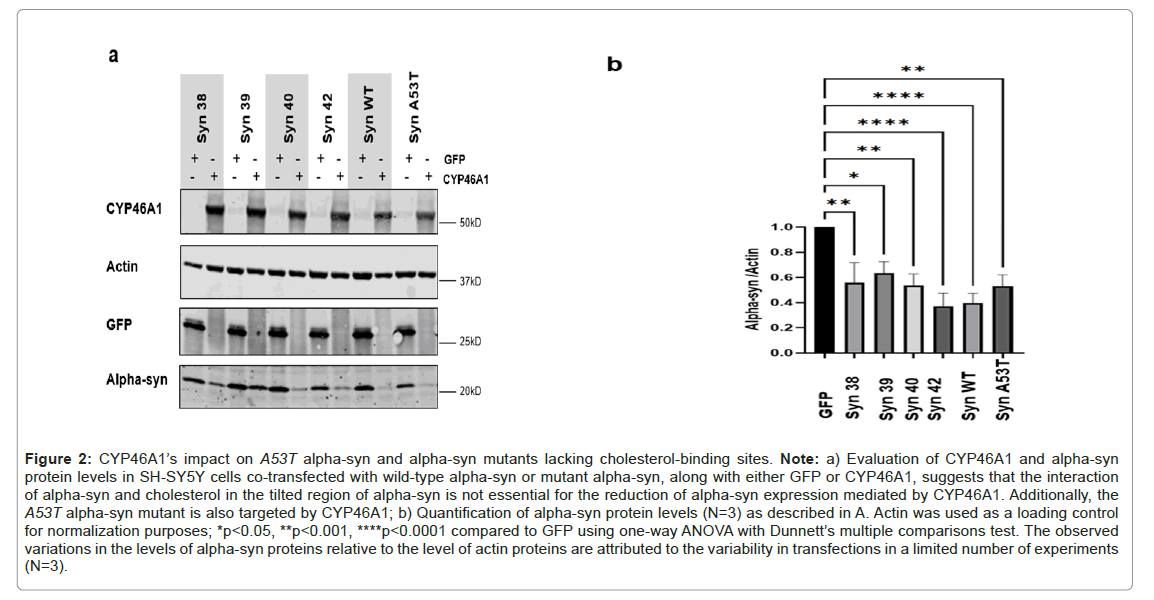
Some findings underscore the essential role of cholesterol in mediating interactions between physiologically relevant membranes and alpha-syn in neurological diseases. Disruption of this association redistributes alpha-syn away from the synapse and impairs its cellular function [7]. Furthermore, alpha-syn extracellular has been discovered to significantly enhance cholesterol efflux from neuronal cells [13]. We investigated whether interaction of alpha-syn and cholesterol is a prerequisite for the reduction of alpha-syn expression mediated by CYP46A1. Through the utilization of a panel of recombinant fragments and synthetic peptides, it has been discovered that alpha-syn interacts with cholesterol with high affinity through a binding domain called the titled domain [33,34]. To disrupt the interaction between cholesterol and alpha-syn, we engineered mutants within the titled domain (residues 67-78) and assessed the resulting reduction in alpha-syn expression. The amino acid residues of wild-type human alpha-syn involved in cholesterol binding are indicated in bold in the sequence GGAVVTGVTAVAQ (Supplementary Table 1) [34,35]. Four mutants (Syn38, Syn39, Syn40 and Syn42) were designed by site-directed mutagenesis. Among these mutants, Syn40, was specifically engineered based on the peptide mutant SynuM53 of alpha-syn, as devised by Crowet and colleagues using molecular modeling methodologies [33]. This mutant is suggested to adopt a parallel orientation to the lipid layer rather than a tilted orientation. Its sequence is GGAVSAGVASVTQ. The other three mutants were designed to alter the essential amino acids identified in these interactions by Fantini et al., (Supplementary Table 1). We also included the A53T mutation linked to familial parkinsonism, which has been shown in in vitro studies to have no effect on lipid binding [7,36,37]. SH-SY5Y cells were co-transfected with each alpha-syn mutant along with either GFP or CYP46A1, or with wildtype alpha-syn or the A53T alpha-syn mutant along with either GFP or CYP46A1. Forty-eight hours post-transfection, we compared the level of alpha-syn protein levels by western blot analysis between the cells cotransfected GFP or CYP46A1. We observed a significant reduction in alpha-syn levels for wild-type or each mutant alpha-syn in the presence of CYP46A1 compared to the presence of GFP. In our study revealed that the decrease of alpha-syn protein levels in cells overexpressing CYP46A1 is not dependent on the interaction between cholesterol and the region 67-78 of alpha-syn. Indeed, CYP46A1 significantly reduced the expression level of alpha-syn mutants at potential cholesterolbinding sites compared to cells co-transfected with GFP. Furthermore, we observed that CYP46A1 can decrease the expression not only of wild-type alpha-syn but also the A53T alpha-syn mutant associated with the familial form of the disease (Figures 2A and 2B).
Figure 2: CYP46A1’s impact on A53T alpha-syn and alpha-syn mutants lacking cholesterol-binding sites. Note: a) Evaluation of CYP46A1 and alpha-syn protein levels in SH-SY5Y cells co-transfected with wild-type alpha-syn or mutant alpha-syn, along with either GFP or CYP46A1, suggests that the interaction of alpha-syn and cholesterol in the tilted region of alpha-syn is not essential for the reduction of alpha-syn expression mediated by CYP46A1. Additionally, the A53T alpha-syn mutant is also targeted by CYP46A1; b) Quantification of alpha-syn protein levels (N=3) as described in A. Actin was used as a loading control for normalization purposes; *p<0.05, **p<0.001, ****p<0.0001 compared to GFP using one-way ANOVA with Dunnett’s multiple comparisons test. The observed variations in the levels of alpha-syn proteins relative to the level of actin proteins are attributed to the variability in transfections in a limited number of experiments (N=3).
CYP46A1 facilitates the reduction of Wild-Type alpha-syn (WT) protein levels via the macroautophagy pathway
Evidence suggests that dysfunction in the autophagy pathway is prevalent in numerous neurodegenerative diseases [38-41]. In PD, alpha-syn has been demonstrated to undergo degradation through macroautophagy, chaperone-mediated autophagy or the proteasome [42-44]. We investigated whether CYP46A1 could enhance macroautophagy, chaperone-mediated autophagy or the proteasome to reduce the expression of alpha-syn proteins in SH-SY5Y cells.
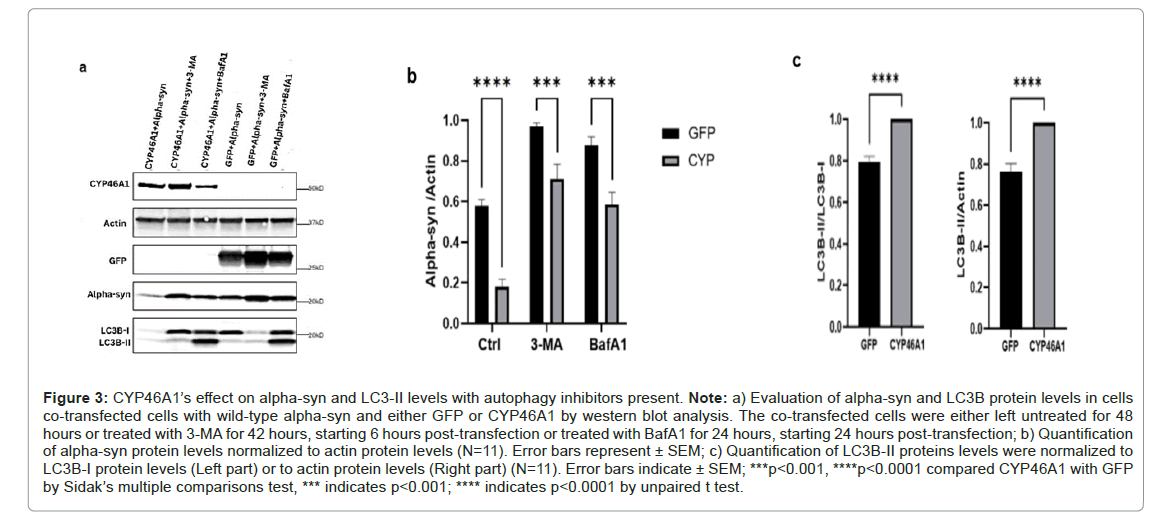
Macroautophagy is a common form of autophagy characterized by the formation of auto phagosome and is effective in removing abnormal proteins and protein aggregates [45]. To investigate the impact of macroautophagy on alpha-syn clearance mediated by CYP46A1, we treated the co-transfected cells with autophagy inhibitors, 3-Methyladenine (3-MA) and Bafilomycin A1 (BafA1). As expected, when cells are co-transfected with alpha-syn and GFP, the presence of these autophagy inhibitors resulted in increased alpha-syn levels. This finding is consistent with the established knowledge that alpha-syn is regulated by macroautophagy. Furthermore, when alpha-syn and CYP46A1 were co-expressed, an increase of alpha-syn proteins was observed in the presence of 3-MA or BafA1. However, this increase in alpha-syn levels was notably lower compared to when alpha-syn and GFP were co-expressed in the presence of the inhibitors. This suggests that CYP46A1 retains its ability to reduce alpha-syn levels even in the presence of autophagy inhibitors. These results were further supported by the quantitative analysis of western blot data from 11 independent experiments.
By inhibiting the fusion of the auto phagosome and the lysosome, BafA1 affects LC3 formation. Through western blot analysis, we observed a significant increase in the LC3B-II/LC3B-I ratio in cells coexpressing alpha-syn and CYP46A1 compared to those co-expressing alpha-syn and GFP, as illustrated in the representative western blot and the data obtained from 11 western blots. These findings strongly suggest that CYP46A1 enhances the induction of macroautophagy of alpha-syn (Figures 3A-3C).
Figure 3: CYP46A1’s effect on alpha-syn and LC3-II levels with autophagy inhibitors present. Note: a) Evaluation of alpha-syn and LC3B protein levels in cells co-transfected cells with wild-type alpha-syn and either GFP or CYP46A1 by western blot analysis. The co-transfected cells were either left untreated for 48 hours or treated with 3-MA for 42 hours, starting 6 hours post-transfection or treated with BafA1 for 24 hours, starting 24 hours post-transfection; b) Quantification of alpha-syn protein levels normalized to actin protein levels (N=11). Error bars represent ± SEM; c) Quantification of LC3B-II proteins levels were normalized to LC3B-I protein levels (Left part) or to actin protein levels (Right part) (N=11). Error bars indicate ± SEM; ***p<0.001, ****p<0.0001 compared CYP46A1 with GFP by Sidak’s multiple comparisons test, *** indicates p<0.001; **** indicates p<0.0001 by unpaired t test.
We also examined whether CYP46A1 could influence alphasyn expression through the Chaperone Mediated Autophagy (CMA) pathway. CMA is a distinctive pathway by which cytosolic protein aggregates are selectively directed to lysosomes for degradation. Wildtype alpha-syn, containing a CMA-targeting motif within its sequence, can undergo degradation in lysosomes via the CMA pathway [43]. The substrate-chaperone complex is then transported to lysosomal membranes, where interactions with Lamp2A receptors facilitate CMA activities [46]. To examine whether CYP46A1 can induce the CMA pathway to degrade alpha-syn, SH-SY5Y cells were co-transfected with Lamp2A siRNA or scrambled control siRNA along with alpha-syn accompanied by either GFP or CYP46A1. The reduction of Lamp2A by siRNA is directly related to CMA activity. Lamp2A protein levels were determined by western blotting (Supplementary Figure 1). The protein levels of alpha-syn detected with GFP or with CYP46A1 did not change in cells where Lamp2A protein levels were reduced by 2.5-fold with siRNA compared to cells co-transfected with siRNA control. These results suggest that CYP46A1 enhances alpha-syn degradation through the macroautophagy pathway independently of CMA autophagy (Supplementary Figure 1).
To explore the impact of CYP46A1 on proteasomal activity in the alpha-syn clearance, SH-SY5Y cells were co-transfected with alphasyn and either GFP or CYP46A1 and subsequently, treated with the proteasome inhibitor MG132 at 20 μM for 6 hours. Our observations revealed an increase in alpha-syn protein levels following MG132 treatment, regardless of the presence of GFP or CYP46A1. This finding is consistent with established knowledge that alpha-syn is degraded not only by autophagy but also by the proteasome [42]. However, the ratio of alpha-syn protein levels in the presence of CYP46A1 compared to those detected with GFP remains unchanged with or without MG132, indicating that CYP46A1 reduces the levels of alpha-syn protein independently of proteasome inhibition (Supplementary Figure 2).
Impact of CYP46A1 on alpha-syn expression in glucocerebrosidase-deficient cells
Mutations in the GBA1 gene constitute the most significant genetic risk factor numerically for the development of PD, increasing the risk by approximately fourfold [30,47]. GBA1 gene encodes the lysosomal enzyme Glucocerebrosidase (GCase), which plays an important role in converting glucosylceramide into glucose and ceramide within the lysosome. GCase-deficient cells display disrupted lysosomal recycling and the accumulation of dysfunctional lysosomes. Given that lysosomes serve as the principal degradative compartment within the cell, their biogenesis and recycling are essential for cellular function and the autophagy-lysosomal pathway. The loss of GCase activity exacerbates autophagy impairment and facilitates the accumulation of alpha-syn [48-50].
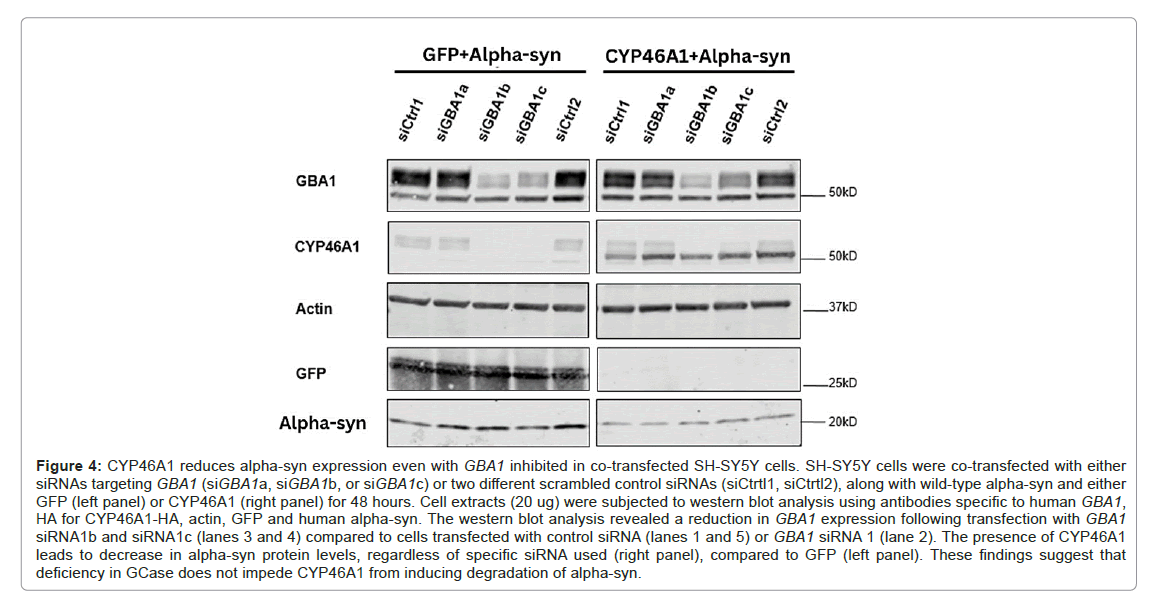
As our study suggests that the overexpression of CYP46A1 decreases alpha-syn protein levels through the macroautophagy pathway, we aimed to investigate whether CYP46A1 could still reduce alpha-syn expression in PD pathological conditions where the expression of the lysosomal enzyme GCase, which regulates autophagy, is diminished. In this study, SH-SY5Y cells were co-transfected with GBA1 siRNAs or scrambled control siRNAs along with alpha-syn and either GFP or CYP46A1 for 48 hours. Two out of three GBA1 siRNAs (siGBA1b and 1c) inhibited the GBA1 gene by factors of 4 and 3, respectively, compared to both scrambled control siRNAs (siCrtl1 and siCrtl2), or siGBA1a, as indicated by western blot analysis. The expression levels of alpha-syn detected using CYP46A1 were observed to decrease compared to those detected using GFP. However, no change in alpha-syn expression was noted in cells with GBA1 deficiency using siRNA siGBA1b or siGBA1c compared to cells with control siRNA. Our experiments demonstrate that CYP46A1 can still effectively reduce alpha-syn expression, even when the expression of the lysosomal enzyme GCase is reduced (Figure 4).
Figure 4: CYP46A1 reduces alpha-syn expression even with GBA1 inhibited in co-transfected SH-SY5Y cells. SH-SY5Y cells were co-transfected with either siRNAs targeting GBA1 (siGBA1a, siGBA1b, or siGBA1c) or two different scrambled control siRNAs (siCtrtl1, siCtrtl2), along with wild-type alpha-syn and either GFP (left panel) or CYP46A1 (right panel) for 48 hours. Cell extracts (20 ug) were subjected to western blot analysis using antibodies specific to human GBA1, HA for CYP46A1-HA, actin, GFP and human alpha-syn. The western blot analysis revealed a reduction in GBA1 expression following transfection with GBA1 siRNA1b and siRNA1c (lanes 3 and 4) compared to cells transfected with control siRNA (lanes 1 and 5) or GBA1 siRNA 1 (lane 2). The presence of CYP46A1 leads to decrease in alpha-syn protein levels, regardless of specific siRNA used (right panel), compared to GFP (left panel). These findings suggest that deficiency in GCase does not impede CYP46A1 from inducing degrad ation of alpha-syn.
Discussion
Considerable evidence suggests that increased expression of alphasyn is involved in the pathogenesis of both sporadic and familial Parkinson's Disease (PD), indicating that the observed changes likely represent some of the earliest events in the progression of PD. Defects in cholesterol metabolism have been associated with neurodegenerative diseases [51,52]. Cholesterol's impact on the membrane binding of alpha-syn may facilitate the formation of beta-sheet structures in alpha-syn, thus promoting the generation of abnormal alpha-syn fibrils [10-12]. Several studies have emphasized a close association between alpha-synuclein (alpha-syn) and cholesterol, both intracellularly and extracellularly. Notably, research has identified a high-affinity cholesterol binding domain in the 67-78 amino acid region of alphasyn [33,34]. While the majority of alpha-syn remains intracellular, extracellular forms of this protein also exist, as it can propagate from cell to cell in a "prion-like" manner [53]. Extracellular alpha-syn has been demonstrated to decrease membrane cholesterol levels and stimulate cholesterol efflux from cells [13,54].
We and others have shown that the activation of CYP46A1 in various brain disease models leads to a decrease in aggregated proteins and improved performance in motor, memory and/or cognitive tests [21-26,55]. However, much less is known about the role of CYP46A1 in PD [27,56]. In our study, we demonstrate that heightened expression of the enzyme CYP46A1, responsible for converting cholesterol into 24S-OHC, results in decreased levels of both wild-type and mutant A53T human alpha-syn proteins in neuroblastoma SH-SY5Y cells (Figures 1 and 4). Additionally, we explored various mutations within the amino acids 67-78, which were hypothesized to influence interactions between cholesterol and alpha-syn based on the findings of Crowet et al., and Fantini et al., [33,34]. However, our results indicate that these mutations in the alpha-syn sequence had no discernible impact. CYP46A1 continued to induce a significant reduction in alphasyn levels, regardless of whether the alpha-syn variant was wild-type or mutant (Figure 2).
These findings suggest that the interplay between alpha-syn and cholesterol, particularly within the 67-78 region, is not essential for CYP46A1's ability to diminish alpha-syn protein levels.
The degradation of alpha-syn is believed to occur through multiple pathways, including macroautophagy, chaperone-mediated autophagy and the proteasome-ubiquitin system [42-44]. Our study suggests that CYP46A1 enhances the macroautophagy pathway, thereby reducing the levels of alpha-syn proteins. Specifically, we observed a significant increase in LC3B-II levels in SH-SY5Y cells overexpressing both CYP46A1 and alpha-syn proteins compared to cells overexpressing GFP and alpha-syn proteins when treated with the autophagy inhibitor BafA1 (Figure 3). These findings are consistent with prior research demonstrating that CYP46A1 promotes autophagy of mutant ataxin-3 or the huntingtin proteins in SCA3 disease and HD, respectively [25,26,57]. Interestingly, even in the presence of autophagy inhibitors such as 3-MA or BafA1, CYP46A1 retains its ability to decrease the expression of alpha-syn. Hence, overexpression of CYP46A1 appears to be a promising therapeutic strategy for the treating accumulated alpha-syn (Figure 3). Our study did not find any effect of CYP46A1 on CMA pathway or on the proteasome-ubiquitin system for the alpha-syn degradation (Supplementary Figures 1 and 2). Overall, our data suggest that CYP46A1 degrades alpha-syn via macroautophagy. However, additional studies focusing on autophagy using molecular or pharmacological approaches in this cellular model could further strengthen the observed results.
Strikingly, one of the most significant genes in PD is GBA1, which is involved in lipid metabolism. Variants in this gene are present in 5%- 20% of PD patients across different populations. It is well-documented that mutations in the GBA1 gene can lead to decreased activity of the lysosomal enzyme Glucocerebrosidase (GCase) [30,47,58]. This phenomenon is believed to result from impaired lysosomal function, leading to the accumulation of alpha-syn [48-50]. We conducted co-transfections of SH-SY5Y cells with GBA1 siRNA, resulting in a fourfold reduction in GBA1 expression, in combination with human alpha-synuclein and either CYP46A1 or GFP. Our observations revealed that CYP46A1 could reduce the level of alpha-synuclein by the same efficacy, regardless of the expression of GBA1. These findings demonstrate that CYP46A1 maintains its ability to suppress alpha-syn expression even in the presence of impaired lysosomal function, as illustrated in Figure 4.
Conclusion
This study shows that the role of CYP46A1 extends beyond cholesterol clearance, promoting the reduction of overexpressed alpha-syn. Our in vitro results demonstrate its effectiveness across various cellular models of Parkinson's disease, including those with overexpression of alpha-syn with or without GBA1 deficiency, or overexpression of the A53T mutated alpha-syn. This suggests that CYP46A1 holds promise as a therapeutic strategy for PD by mitigating the pathological effects of alpha-syn accumulation. Additional data from the author suggest that overexpression of CYP46A1 may prevent dopaminergic loss in PD. Moving forward, it will be important to ascertain in in vivo PD models whether CYP46A1 facilitates neuroprotection and symptom reduction in PD.
Acknowledgements
The authors would like to express their thanks for laboratory support to Dr. Nathalie Cartier. The CYP46A1 vectors were a generous gift from Askbio France. Cell work was performed at the CELIS platform and we thank Laetitia Strehl involved in this platform.
Authors’ Contributions
CBG: Conceptualization methodology, validation, formal analysis, investigation, writing-original draft, visualization.
LR and EA: Methodology, validation, formal analysis, investigation, writing, visualization.
FC: Conceptualization, resources, writing, review.
FP: Resources, writing, review and editing, supervision, funding acquisition. All authors have read and approved the final manuscript.
Funding
This work was supported by the Paris Brain Institute (ICM) and by the French National Institute of Health and Medical Research (INSERM). CBG was supported by INSERM, LR, EA and FC by grants from ICM.
References
- Devine MJ, Gwinn K, Singleton A, Hardy J (2011) Parkinson’s disease and α‐synuclein expression. Mov Disord 26:2160‑2168.
[Crossref] [Google Scholar] [PubMed]
- Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, et al. (1997) Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science 276:2045‑2047.
[Crossref] [Google Scholor] [PubMed]
- Krüger R, Kuhn W, Müller T, Woitalla D, Graeber M, et al. (1998) Ala30Pro mutation in the gene encoding a-synuclein in Parkinson’s disease. Nat Genet 18:106‑108.
[Crossref] [Google Scholar] [PubMed]
- Singleton A, Farrer M, Johnson J, Singleton A, Hague S, et al. (2003) Alpha-Synuclein locus triplication causes Parkinson’s disease. Science 302:841.
[Crossref] [Google Scholar] [PubMed]
- Shahmoradian SH, Lewis AJ, Genoud C, Hench J, Moors TE, et al. (2019) Lewy pathology in Parkinson’s disease consists of crowded organelles and lipid membranes. Nat Neurosci 22:1099‑1109.
[Crossref] [Google Scholar] [PubMed]
- Mahul-Mellier AL, Burtscher J, Maharjan N, Weerens L, Croisier M, et al. (2020) The process of Lewy body formation, rather than simply α-synuclein fibrillization, is one of the major drivers of neurodegeneration. Proc Natl Acad Sci USA 117:4971‑4982.
[Crossref] [Google Scholar] [PubMed]
- Fortin DL, Troyer MD, Nakamura K, Anthony MD, Edwards RH, et al. (2004) Lipid rafts mediate the synaptic localization of α-Synuclein. J Neurosci 24:6715‑6723.
[Crossref] [Google Scholar] [PubMed]
- van Maarschalkerweerd A, Vetri V, Vestergaard B (2015) Cholesterol facilitates interactions between a-synuclein oligomers and charge-neutral membranes. FEBS Lett. 589;2661‑2667.
[Crossref] [Google Scholar] [PubMed]
- Kachappilly N, Srivastava J, Swain BP, Thakur P (2022) Interaction of alpha-synuclein with lipids. Methods Cell Biol 169:43‑66.
[Crossref] [Google Scholar] [PubMed]
- Qi Z, Wan M, Zhang J, Li Z (2023) Influence of cholesterol on the membrane binding and conformation of α-synuclein. J Phys Chem B 127:1956‑1964.
[Crossref] [Google Scholar] [PubMed]
- Man WK, de Simone A, Barritt JD, Vendruscolo M, Dobson CM, et al. (2020) A role of cholesterol in modulating the binding of α-synuclein to synaptic-like vesicles. Front Neurosci 2020;14:18.
[Crossref] [Google Scholar] [PubMed]
- Jakubec M, Bariås E, Furse S, Govasli ML, George V, et al. (2021) Cholesterol‐containing lipid nanodiscs promote an α‐synuclein binding mode that accelerates oligomerization. FEBS J 288:1887‑1905.
[Crossref] [Google Scholar] [PubMed]
- Hsiao JH, Halliday G, Kim W (2017) α-synuclein regulates neuronal cholesterol efflux. Molecules 22:1769.
[Crossref] [Google Scholar] [PubMed]
- Mahley RW (2016) Central nervous system lipoproteins: ApoE and regulation of cholesterol metabolism. ATVB 36:1305‑1315.
- Bogdanovic N, Bretillon L, Lund EG, Diczfalusy U, Lannfelt L, et al. (2001) On the turnover of brain cholesterol in patients with Alzheimer’s disease. Abnormal induction of the cholesterol-catabolic enzyme CYP46 in glial cells. Neurosci Lett 314:45‑48.
- Desai P, DeKosky ST, Kamboh MI (2002) Genetic variation in the cholesterol 24-hydroxylase (CYP46) gene and the risk of Alzheimer’s disease. Neurosci Lett 328:9‑12.
[Crossref] [Google Scholar] [PubMed]
- Brown J, Theisler C, Silberman S, Magnuson D, Gottardi-Littell N, et al. (2004) Differential expression of cholesterol hydroxylases in Alzheimer’s disease. J Biol Chem 279:34674‑34681.
[Crossref] [Google Scholar] [PubMed]
- del Toro D, Xifró X, Pol A, Humbert S, Saudou F, et al. (2010) Altered cholesterol homeostasis contributes to enhanced excitotoxicity in Huntington’s disease 115:153‑167.
[Crossref] [Google Scholar] [PubMed]
- Kreilaus F, Spiro AS, McLean CA, Garner B, Jenner AM, et al. (2016) Evidence for altered cholesterol metabolism in Huntington’s disease post mortem brain tissue. Neuropathol Appl Neurobiol 42:535‑546.
[Crossref] [Google Scholar] [PubMed]
- Abdel-Khalik J, Yutuc E, Crick PJ, Gustafsson JA, Warner M, et al. (2017) Defective cholesterol metabolism in amyotrophic lateral sclerosis. J Lipid Res 58:267‑278.
[Crossref] [Google Scholar] [PubMed]
- Chali F, Djelti F, Eugene E, Valderrama M, Marquer C, et al. (2015) Inhibiting cholesterol degradation induces neuronal sclerosis and epileptic activity in mouse hippocampus. Eur J of Neuroscience 41:1345‑1355.
[Crossref] [Google Scholar] [PubMed]
- Hudry E, van Dam D, Kulik W, de Deyn PP, Stet FS, et al. (2010) Adeno-associated virus gene therapy with cholesterol 24-hydroxylase reduces the amyloid pathology before or after the onset of amyloid plaques in mouse models of Alzheimer’s disease. Mol Ther 18:44‑53.
[Crossref] [Google Scholar] [PubMed]
- Burlot MA, Braudeau J, Michaelsen-Preusse K, Potier B, Ayciriex S, et al. (2015) Cholesterol 24-hydroxylase defect is implicated in memory impairments associated with Alzheimer-like Tau pathology. Hum Mol Genet 24:5965‑5976.
[Crossref] [Google Scholar] [PubMed]
- Boussicault L, Alves S, Lamazière A, Planques A, Heck N, et al. (2016) CYP46A1, the rate-limiting enzyme for cholesterol degradation, is neuroprotective in Huntington’s disease. Brain 139:953‑970.
[Crossref] [Google Scholar] [PubMed]
- Kacher R, Lamazière A, Heck N, Kappes V, Mounier C, et al. (2019) CYP46A1 gene therapy deciphers the role of brain cholesterol metabolism in Huntington’s disease. Brain 142:2432‑2450.
[Crossref] [Google Scholar] [PubMed]
- Nóbrega C, Mendonça L, Marcelo A, Lamazière A, Tomé S, et al. (2019) Restoring brain cholesterol turnover improves autophagy and has therapeutic potential in mouse models of spinocerebellar ataxia. Acta Neuropathol 138:837‑858.
[Crossref] [Google Scholar] [PubMed]
- Moutinho M, Nunes MJ, Rodrigues E (2016) Cholesterol 24-hydroxylase: Brain cholesterol metabolism and beyond. Biochimica et Biophysica Acta (BBA)-Molecular and Cell Biology of Lipids 1861:1911‑1920.
- Zambon F, Cherubini M, Fernandes HJR, Lang C, Ryan BJ, et al. (2019) Cellular α-synuclein pathology is associated with bioenergetic dysfunction in Parkinson’s iPSC-derived dopamine neurons. Hum Mol Genet 28:2001‑2013.
[Crossref] [Google Scholar] [PubMed]
- Theofilopoulos S, Abreu de Oliveira WA, Yang S, Yutuc E, Saeed A, et al. (2019) 24(S), 25-Epoxycholesterol and cholesterol 24S-hydroxylase (CYP46A1) overexpression promote midbrain dopaminergic neurogenesis in vivo. J Biol Chem 294:4169‑4176.
[Crossref] [Google Scholar] [PubMed]
- Neumann J, Bras J, deas E, O’Sullivan SS, Parkkinen L, et al. (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132:1783‑1794.
[Crossref] [Google Scholar] [PubMed]
- Brockmann K, Srulijes K, Pflederer S, Hauser A, and Schulte C, et al. GBA ‐associated Parkinson’s disease: Reduced survival and more rapid progression in a prospective longitudinal study. Mov Disord 2015;30:407‑411.
- Russell DW, Halford RW, Ramirez DMO, Shah R, Kotti T, et al. (2009) Cholesterol 24-Hydroxylase: An enzyme of cholesterol turnover in the brain. Annu Rev Biochem 78:1017‑1040.
[Crossref] [Google Scholar] [PubMed]
- Crowet JW, Lins L, Dupiereux I, Elmoualija B, Lorin A, et al. (2007) Tilted properties of the 67-78 fragment of α‐synuclein are responsible for membrane destabilization and neurotoxicity. Proteins 68:936-947.
- Fantini J, Carlus D, Yahi N (2011) The fusogenic tilted peptide (67-78) of α-synuclein is a cholesterol binding domain. Biochim Biophys Acta 1808:2343‑2351.
[Crossref] [Google Scholar] [PubMed]
- Fantini J, di Scala C, Baier CJ, Barrantes FJ (2016) Molecular mechanisms of protein-cholesterol interactions in plasma membranes: Functional distinction between topological (tilted) and consensus (CARC/CRAC) domains. Chem Phys Lipids 199:52‑60.
[Crossref] [Google Scholar] [PubMed]
- Perrin RJ, Woods WS, Clayton DF, George JM (2000) Interaction of human α-Synuclein and Parkinson’s disease variants with phospholipids. J Biol Chem 275:34393‑34398.
[Crossref] [Google Scholar] [PubMed]
- Jo E, Fuller N, Rand RP, St George-Hyslop P, Fraser PE, et al. (2002) Defective membrane interactions of familial Parkinson’s disease mutant A30P α-synuclein. J Mol Biol 315:799‑807.
[Crossref] [Google Scholar] [PubMed]
- Ravikumar B, Vacher C, Berger Z, Davies JE, Luo S, et al. (2004) Inhibition of mTOR induces autophagy and reduces toxicity of polyglutamine expansions in fly and mouse models of Huntington disease. Nat Genet 36:585‑595.
[Crossref] [Google Scholar] [PubMed]
- Nixon RA, Wegiel J, Kumar A, Yu WH, Peterhoff C, et al. (2005) Extensive involvement of autophagy in Alzheimer disease: An immuno-electron microscopy study. J Neuropathol Exp Neurol 64:113-122.
[Crossref] [Google Scholar] [PubMed]
- Sarkar S, Perlstein EO, Imarisio S, Pineau S, Cordenier A, et al. (2007) Small molecules enhance autophagy and reduce toxicity in Huntington’s disease models. Nat Chem Biol 3:331‑338.
[Crossref] [Google Scholar] [PubMed]
- Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131:1969‑1978.
[Crossref] [Google Scholar] [PubMed]
- Webb JL, Ravikumar B, Atkins J, Skepper JN, Rubinsztein DC, et al. (2003) α-synuclein is degraded by both autophagy and the proteasome. J Bio Chem 278:25009‑25013.
- Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D, et al. (2004) Impaired degradation of mutant α-synuclein by chaperone-mediated autophagy. Science 305:1292‑1295.
[Crossref] [Google Scholar] [PubMed]
- Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type α-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Bio Chem 283:23542‑23556.
[Crossref] [Google Scholar] [PubMed]
- Glick D, Barth S, Macleod KF (2010) Autophagy: Cellular and molecular mechanisms. J Pathol 22:3‑12.
[Crossref] [Google Scholar] [PubMed]
- Cuervo AM, Dice JF (2000) Unique properties of lamp2a compared to other lamp2 isoforms. J Cell Sci 113:4441‑4450.
[Crossref] [Google Scholar] [PubMed]
- Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, et al. (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361:1651‑1661.
[Crossref] [Google Scholar] [PubMed]
- Murphy KE, Gysbers AM, Abbott SK, Tayebi N, Kim WS, et al. (2014) Reduced glucocerebrosidase is associated with increased α-synuclein in sporadic Parkinson’s disease. Brain 137:834‑848.
[Crossref] [Google Scholar] [PubMed]
- Magalhaes J, Gegg ME, Migdalska-Richards A, Doherty MK, Whitfield PD, et al. (2016) Autophagic lysosome reformation dysfunction in glucocerebrosidase deficient cells: Relevance to Parkinson disease. Hum Mol Genet 25:3432‑3445.
[Crossref] [Google Scholar] [PubMed]
- Gündner AL, Duran-Pacheco G, Zimmermann S, Ruf I, Moors T, et al. (2019) Path mediation analysis reveals GBA impacts Lewy body disease status by increasing α-synuclein levels. Neurobiol Dis 121:205‑213.
[Crossref] [Google Scholar] [PubMed]
- Liu JP, Tang Y, Zhou S, Toh BH, McLean C, et al. (2010) Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol Cell Neurosci 43:33‑42.
[Crossref] [Google Scholar] [PubMed]
- Dai L, Zou L, Meng L, Qiang G, Yan M, et al. (2021) Cholesterol metabolism in neurodegenerative diseases: Molecular mechanisms and therapeutic targets. Mol Neurobiol 58:2183‑2201.
[Crossref] [Google Scholar] [PubMed]
- Vargas JY, Grudina C, Zurzolo C (2019) The prion-like spreading of α-synuclein: From in vitro to invivo models of Parkinson’s disease. Ageing Res Rev 50:89‑101.
[Crossref] [Google Scholar] [PubMed]
- Ronzitti G, Bucci G, Emanuele M, Leo D, Sotnikova TD, et al. (2014) Exogenous-synuclein decreases raft partitioning of Cav2.2 channels inducing dopamine release. J Neurosci 34:10603‑10615.
[Crossref] [Google Scholar] [PubMed]
- Djelti F, Braudeau J, Hudry E, Dhenain M, Varin J, et al. (2015) CYP46A1 inhibition, brain cholesterol accumulation and neurodegeneration pave the way for Alzheimer’s disease. Brain 138:2383‑2398.
[Crossref] [Google Scholar] [PubMed]
- Alavi MS, Karimi G, Ghanimi HA, Roohbakhsh A (2023) The potential of CYP46A1 as a novel therapeutic target for neurological disorders: An updated review of mechanisms. Europ J Pharmacol 949:175726.
[Crossref] [Google Scholar] [PubMed]
- Nóbrega C, Conceição A, Costa RG, Koppenol R, Sequeira RL, et al. (2020) The cholesterol 24-hydroxylase activates autophagy and decreases mutant huntingtin build-up in a neuroblastoma culture model of Huntington’s disease. BMC Res Notes 13:210.
[Crossref] [Google Scholar] [PubMed]
- Gan-Or Z, Amshalom I, Kilarski LL, Bar-Shira A, Gana-Weisz M, et al. (2015) Differential effects of severe vs. mild GBA mutations on Parkinson disease. Neurol 84:880‑887.
[Crossref] [Google Scholar] [PubMed]
Citation: Besnard-Guérin C, Rousselot L, Audouard E, Chali F, Piguet F, et al. (2024) The Cholesterol 24-Hydroxylase Enzyme, CYP46A1, Reduces Overexpressed Alpha-Synuclein Proteins in Human Cellular Models of Parkinson’s Disease. Diagnos Pathol Open 9:240 DOI: 10.4172/2476-2024.1000240
Copyright: © 2024 Besnard-Guérin C, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 891
- [From(publication date): 0-0 - Nov 30, 2025]
- Breakdown by view type
- HTML page views: 620
- PDF downloads: 271