The Effects of N-Acetyl Cysteine on Nasal Mucociliary Clearance in Healthy Volunteers: A Randomized, Double-Blind and Placebo-Controlled Study
Received: 22-Nov-2014 / Accepted Date: 22-Dec-2014 / Published Date: 02-Jan-2015 DOI: 10.4172/2161-119X.1000182
Abstract
Background: Mucociliary clearance is an important host defense function of the upper respiratory tract that requires the coordinated beating of cilia and results in the transport of mucus to the oropharynx. N-Acetyl Cysteine is a mucolytic drug currently used in pulmonary diseases. In the present study we sought to assess the effects of N-Acetyl Cysteine nasal mucociliary clearance in healthy volunteers.
Methods: A total of 100 healthy individuals (55 male) with the mean age of 34.21 (± 12.63) years were included in the present randomized, double-blind and placebo-controlled study. Participants were assigned into two groups of case and control. The Mucociliary Clearance Time (MCT) was measured by saccharine test; measuring the time in minutes required for the subject to taste a saccharin particle placed on the inferior turbinate of the nasal. The variables studied were nasal MCT before and after taking the placebo or NAC, age, and sex. Data were analyzed using SPSS V.16.
Results: Wilcoxon signed ranks test demonstrated considerable different of Saccharine Test Time (STT) between before and after taking the NAC (p=0.021), and no significant different of STT between before and after taking the placebo (p=0.723).
Conclusion: N-Acetyl Cysteine exerts measurable effect on nasal mucociliary clearance in healthy volunteers; therefore may be beneficial in conditions associated with disruption of mucociliary clearance such as rhinitis and sinusitis. However, further studies are suggested to achieve more conclusive results.
Keywords: Mucociliary clearance; N-Acetyl cysteine; Saccharin test
249094Introduction
The respiratory system is constantly exposed to environmental pathogens and toxins, spread as aerosol in the environment. The mucociliary system is the first defense of airways against harmful particles and disruptive environmental triggers. Ingredients, bacteria and respiratory viruses are trapped by airways’ mucosa and driven to the larynx by respiratory beats, ultimately egress due to swallowing or coughing [1]. Airways’ clearance by the mucocilary system is the main host defense mechanism of upper or lower respiratory tracks. Defects in this process, either genetically or acquired, make an individual susceptible to contracting chronicnasal, paranasal sinuses’ and airways’ tracks chronic infections [2]. Nose is the major part of the respiratory system that transfers air into and out of the system, playing an important role in providing necessary humidity (100%) and temperature (37 degrees), local defense and filtration of ingredients and gases [3]. A variety of factors affect mucociliary clearance such as increasing age, smoking, chronic nasal and pulmonary diseases (rhinitis, asthma, bronchiectasis, and chronic bstructive pulmonary disease), and anatomical defects of the upper respiratory track (septal deviation, hypertrophy of the concha, etc.) and a history of nasal surgery or trauma. The role of defects in mucociliary function due to either genetic diseases (cystic fibrosis) or acquired (secondary to infection of erosive mucociliary system diseases) has been recognized in many chronic airways tracks’ diseases [4]. For example, defects in one or more parts of mucociliary clearance (epithelium, mucosa and cilia) cause chronic rhinosinusitis and stasis rinosinusial discharges lead to chronic inflammations [5]. Today, respiratory inflammation diseases are at the top of primary referral reasons to clinics. Airways’ discharges are treated using mucoactive drugs which accommodate airways’ clearance. Mucoactive drugs are classified based on their mechanisms; one group directly affects the production and chemical composition of airways’ discharges, resulting in high efficacy on mucociliary clearance. Another group, having no certain impact on mucosa, helps in treatment of unusual discharges by affecting airways’ structures and functions and modifying pathopysiologic mechanisms [6]. The saccharin test is a valid and reliable technique to evaluate the amount of mucociliary clearance times [7]. The saccharin test and similar tests, such as Aspartame are very useful to evaluate the mucociliary system’s function. These tests are simple without the need for complex equipments and do not cause any discomfort for the patient. Results of these tests are dependent on individual factors which are directly related to mucociliary clearance. Verifying the consistency between nasal mucociliary clearance and tracheobronchial tracks suggests qualification of mucociliary clearance by less invasive methods like the saccharin test.
Methods
This study was reviewed and approved by the Institutional Review Board at the Iran University of Medical Sciences, and written informed consent was obtained from all participants. Volunteers enrolled in this clinical trial were scheduled to receive the NAC or the placebo and do STT before and after it in Firoozrar general hospital, Tehran, Iran, from January 2012 to March 2012. All individuals were divided into case and control groups by double-blind method.
A total of 100 healthy volunteers (55 males and 45 females) with a mean age of 34.21 ± 12.63 y/o (ranging from 19 to 86) were included. Range of age for 71% of volunteers was 21-40 y/o, along with 3% under 20 and 3% above 60. The control group consisted of 24 men (48%) and 26 women (52%) with a mean age of 33.72 ± 14.40 y/o (ranging from 18 to 86), resulting in a normal distribution. The case group consisted of 31 men (62%) and 19 women (38%) with a mean age of 34.58 ± 10.75 y/o (ranging from 27 to 58). Although the median was 28.5 and had long distance to end of range (86 y/o), distribution of age data was not normal. Despite the mean age, the mean gender of the control group has a normal distribution, but the case group does not (Figure 1).
136 volunteers (double the required individuals) were selected to participate in the study, where 36 volunteers were excluded. The exclusion criteria consisted of any anatomical defects of the upper respiratory system (septal deviation, hypertrophy concha, etc), history of nasal surgery or trauma, history of chronic nasal and respiratory diseases (asthma, rhinitis, chronic obstructive pulmonary disease, nasal polyps), acute infection of respiratory diseases during the recent six weeks, gustatory defects, history of smoking or use of drug (‘’nonsmoker’’ was defined as a person who has never smoked or has given up smoking for five years), pregnancy and history of taking drugs that influence mucociliary clearance, such as antihistamines, adrenergic drugs, anticholinergic drugs, local and systemic anticongestants, mucolytics, corticosteroids and theophyllin.
The saccharin test was based on Anderson et al. method in 1974 [8]. Saccharin test was done for every individual before taking medication of the NAC or the placebo. Individuals did not need any preparation for STT. For each test, 50 mg of saccharin powder, made by Merk Company, was placed on inferior turbinate, 1 cm away from the tip. Since maximum dose of saccharin is 2.5 mg per kg per day, the amount used in this study was safe for the subjects. The researcher made sure saccharin was precisely located on mucosa, avoiding squamous cell epithelium. All the individuals were in sitting position, bending their heads slightly backwards and breathed normally. All individuals were asked to inform the researcher of sensing any new taste in their pharynx, where they were not aware of the sweet taste of saccharin to prevent false positives. Time measurements were done before and after taking NAC or the placebo. The highest NAC concentration in the mucosa is detected within 2-4 hours. The taste of saccharin powder requires 12-15 hours to be eliminated from the nasopharynx cavity, and doing the second saccharin test sooner than 15 hours could have interfered with the first one. As a result, the test was repeated after 18 hours to avoid overlaps and time measurements were done before and after taking NAC or the placebo.
Data analysis was completed using Statistical Package for Social Sciences version 16 software. For each measured variable, descriptive values are expressed as the mean-standard deviation. Analysis of quantitative variables was done using Wilcoxon signed ranked test. Categorical variables were compared using the Chi square test and relationships were assessed by Pearson. Reported p values are 2-tailed and p<0.05 is considered statistically significant.
Results
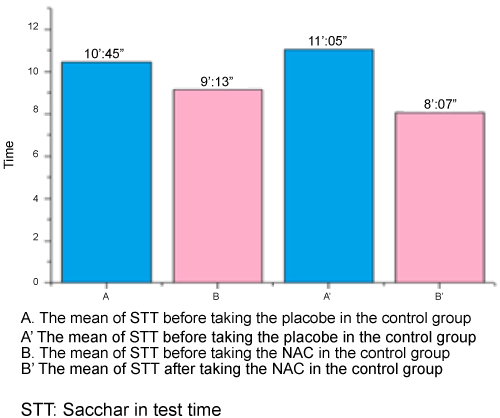
The mean Saccharin Test Time (STT) before taking the placebo or NAC in all 100 volunteers was 9’:59’’ ± 6’:06’’ (ranging from 2’:00” to 14’:00”). The mean time of sensing sweetness of saccharin in the control group before taking the placebo was 10’:45’’ ± 7’:13” (ranging from 2’:00” to 14’:00”), where the mean time of distinguishing saccharin taste in the control group after taking the placebo was 11’:05’’ ± 7’:46’’ (ranging from 1’:30’’ to 17’:00”). So, the mean time of the control group before and after showed only a 00:20’’ difference. In Wilcoxon Signed Ranks test of the control group, there were 3 individuals (6%) among 50 with no fluxion, 23 (46%) with negative fluxion and 24 (48%) with positive fluxion.
The mean time of sensing sweetness of saccharin in the case group before taking the NAC was 9’:13’’ (rangeing from 3’:00 “to 14’:00”), where the mean time of distinguishing saccharin taste in the case group after taking the NAC was 8’:07’’ (ranging from 1’:00” to 15’:00”). Therefore, the mean time of case group before and after taking NAC demonstrated a 00:54’’ difference. Wilcoxon Signed Ranks in case group showed 1 individual (2%) among 50 with no fluxion, 14 (28%) with negative fluxion and 35 (70%) with positive fluxion. There was no relationship between the age and the difference STT before and after taking the placebo and NAC in all 100 volunteers, where related analysis indicated 0.95% assurance (p=0.503) (Figure 2). There was no relationship between the age and the difference of STT before and after taking the placebo in control group (0.95% assurance (p=0.463)), as well as NAC in the case group (0.95% assurance (p=0.309)). There was no relationship between the genders and the difference of STT before and after taking the placebo or NAC in the 100 volunteers, where related analysis showed 0.95% assurance (p=0.153). There was no relationship between the genders and the difference of STT before and after taking the placebo in the control group, as well as NAC in case group with 0.95% assurance ( p=0.271 and p=0.672, respectively).
Discussion
Respiratory system is tremendously resistant to environmental triggers in spite of being exposed to many types of pathogens and toxic chemical substances. Respiratory mucosa provides this highly effective defense by traping inspiratory and removing toxic ingredients from lungs using cilia movements, cilia beats and coughing [9]. Mucous is produced and excreted constantly where mucosa cilia frequency of 12- 15 beats per minute rate is ensures clearance of mucosa layers at rate of one millimeter per minute [10]. The rate of clearance is increased by hydration, as well as higher rate of cilia beats due to adrenergic, cholinergic and adenosine agonist drugs [10,11]. Examination of mucociliary clearance can aid in screening respiratory diseases, where early diagnosis of low mucociliary function can result in faster and more effective treatments [12]. Studies have shown that using substances such as saccharin and aspartame for testing is easy to implement and analyze, without requiring complex equipments or causing discomfort for patients [13-15]. In current study saccharine test was used to evaluate the effect of NAC on mucociliary system. Maximum time of sensing sweet taste (14’:00”) was shorter than that reported by Rev med study (36’:00”) [16] which is explained by race and genetic differences as well as volunteers’ younger age of the current study. Previous studies reported a meaningful relationship between demographic variants (age and gender) and saccharin test time [15,16]. In the current study, there was no relationship between age and the mean STT because there was no normal age distribution by chance. We can improve this error by bigger sample size (Figure 3).
Difference of 00:20’’ between the mean times of the control group before and after application of placebo indicates lack of distinction between them which was confirmed by Wilcoxon Signed Ranks. 48% of individuals who took the placebo sensed the saccharin taste sooner than the first time due to individuals’ autosuggestion. There is no statistical relationship between these variants with 95% assurance (p=0.723). The mean times of case group before and after taking NAC showed a 00:54’’ difference, with a positive fluxion nearly three-fold compared to the control group. There was also a significant difference between STT before and after taking the NAC with 95% assurance (p=0.021), confirming the positive impact of NAC on nasal mucociliary clearance.
Conclusion
We conclude that N-acetyl Cysteine exerts measurable effect on nasal mucociliary clearance in healthy volunteers and therefore is beneficial in conditions associated with disruption of mucociliary clearance such as rhinitis and sinusitis diseases as much as pulmonary diseases as an adjuvant.
References
- Reynolds HY (1994) Respiratory Host Defenses - Surface Immunity Original Research Article. Immunobiology 191: 402-412.
- Sato S, Kiyono H (2012) The mucosal immune system of the respiratory tract. Current Opinion in Virology 2: 225-232.
- Suarez CS, Dintzis SM, FrevertCW (2012) Respiratory Comparative Anatomy and Histology 54:121-134.
- Johnson-Delaney CA, Orosz SE (2011) Ferret Respiratory System: Clinical Anatomy, Physiology, and Disease. Veterinary Clinics of North America: Exotic Animal Practice 14: 357-367.
- Kyd JM, RuthFoxwell A, Cripps AW (2001) Mucosal immunity in the lung and upper airway.Vaccine 19:2527-2533.
- Miyata T, Kai H, Isohama Y,Takahama K (1998) Current opinion of muco-active drug research: strategies and problems. EurRespir J 11: 480-491
- Marttin E, Schipper NGM, Verhoef JC(1998) Nasal mucociliaryclearanceas a factor in nasal drug delivery. Adv Drug Deliv Rev 29: 13-38.
- Andersen IB, Camner P, Jensen Pl (1974) Acomparison of nasal and tracheobronchial clearance. ArchEnvironHealth 19:290-293.
- JanHolmgren (1991) Mucosal immunity and vaccination.FEMS Microbiology Letters 89: 1-9.
- Salathe M (2007) Regulation of mammalianciliary beating. Annu Rev Physiol 69:401-422
- Thornton DJ, Sheehan JK (2004) Frommucins to mucous: toward a more coherent understanding of this essential barrier.Proc Am ThoracSoc 32: 54-61.
- Ellerman A, Bisgaard H (1997) Longitudinal study of lung function in acohort of primary ciliary dyskinesia. EurRespir J 10: 2376-2379.
- Canciani M, Barlocco EG, Mastella G (2005) The saccharin method for testing mucociliary function in patients suspected of having primary ciliarydyskinesia.Pediatric Pulmonology 5: 210-214.
- Plaza Valia P, Carrion Valero F, MarinPardo J (2013) Saccharin test for the study of mucociliary clearance: reference values for a Spanish population. US National Library of Medicine.Archivos de Bronconeumologia 49: 131-175.
- Rev med Brux (2011) Airway clearance techniques in chronic obstructive pulmonary syndrome 32: 381-387
- Kenneth Yu, John R. Stram (1995) Nasal mucociliary clearance and the saccharin tablet test: What does it measure?Otolaryn gology - Head and Neck Surgery 113: 170-171.
Citation: Elahi M, Elahi H (2015) The Effects of N-Acetyl Cysteine on Nasal Mucociliary Clearance in Healthy Volunteers: A Randomized, Double-Blind and Placebo-Controlled Study. Otolaryngology 5:182. DOI: 10.4172/2161-119X.1000182
Copyright: © 2015 Elahi M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 24245
- [From(publication date): 1-2015 - Aug 30, 2025]
- Breakdown by view type
- HTML page views: 19348
- PDF downloads: 4897