The Impact of Uterine Neoplastic Tissue in Accurately Examined Fallopian Tubes on Pathological Diagnosis and Clinical Management
Received: 20-Oct-2021 / Accepted Date: 03-Nov-2021 / Published Date: 10-Nov-2021
Abstract
The presence of intraluminal uterine neoplastic tissue or neoplastic cells in accurately examined Fallopian tubes can pose serious diagnostic problems for pathologists.
Indeed, this finding, with the simultaneous presence of neoplastic cells in the intraoperative cytology of peritoneal washing, might suggest the retrograde transtubal spread of a uterine malignancy and, consequently, can be responsible for its upstaging, with an impact on the therapeutic approach.
In a comment on our previously paper, in which we demonstrated this unusual mechanism of metastasis in a case of USC (Uterine Serous Carcinoma), and by a mini-review of the retrograde transtubal spread of uterine carcinoma, we discuss the pathological criteria for this findings and its impact on the management of patients affected by all subtypes of minimally invasive uterine carcinomas.
Keywords: Retrograde transtubal neoplastic spread; Laparoscopic hysterectomy; Hysteroscopy; Technical artifact
Abbreviations
EC: Endometrial Carcinoma; ESGO: European Society of Gynecological Oncology; ESMO: European Society of Medical Oncology; ESTRO: European Society for Radiotherapy and Oncology; LH: Laparoscopic Hysterectomy; USC: Uterine Serous Carcinoma
Description
EC is the sixth most common cancer in women worldwide, with the highest incidence observed between the ages of 55 and 64 years [1,2].
The main risk factors for the development of EC are diabetes, obesity, nulliparity, early-onset menarche, late-onset menopause, and exposure to unopposed oestrogen or tamoxifen therapy [3,4].
Typically, these malignancies are classified based on clinical, morphological, histological and genetic features into two major groups, type I and type II.Type I Ec, referred to as endometrioid tumours, is the more frequent subtype; it is considered a low-grade tumour that often occurs in perimenopausal obese women. Characteristically, this neoplasm is hormone-dependent, and its prognosis is favourable [1,4].
In contrast, type II Ec, which corresponds to a serous histotype, is less common and occurs in elderly women. Type II Ec is hormone- independent and associated with a higher stage of development at
Numerous mechanisms have been proposed to explain why serous carcinoma of the uterus often coexists with intraperitoneal diffusion even in cases with superficial infiltration of the myometrium. These mechanisms include neoplastic embolization of the lymphatic vessels and multifocal neoplastic transformation that can simultaneously involve the endometrial ovarian and peritoneal surface [5-8].
Multifocal papillary serous carcinoma of the peritoneum, in addition to breast and ovarian carcinoma and endometrial serous carcinoma, have usually been suggested in patients with BRCA1 mutations [9].
However, in a recently published paper, we reported an example of a minimally invasive USC in a BRCA1-mutated patient with a history of breast carcinoma and demonstrated that subsequent peritoneal metastases were not related to concomitant ovarian or tubal lesions, but to an unusual mechanism of metastasis that can be considered retrograde transtubal spread [10].
This unusual mechanism of metastasis in serous carcinoma of the endometrium is rare and has an important impact on prognosis but, in our opinion could be underestimated [11-13].
In fact, to identify the presence of uterine carcinoma in the Fallopian tube an accurate histological examination is required with adequate sampling of the adnexa [10,14,15].
Additionally, it is important to perform an immune histochemical analysis with specific markers to demonstrate the serous phenotype and the endometrial origin of neoplastic cells in the tubal lumen [10].
In cases of USC and all high-grade EC (Type II), the presence of neoplastic cells in the Fallopian tubes likely represents a propensity for transtubal dissemination of aggressive subtypes of uterine carcinoma [11-13].
Although transtubal spread is more common among women with the serous subtype and other aggressive EC, data in the literature suggest that this mechanism can also be observed in cases of low-grade EC [16].
In these examples, the spread of endometrial neoplasms to the peritoneal surface, even in the absence of accurate sampling of the Fallopian tubes was demonstrated by the presence of atypical cells in the intraoperative cytology of peritoneal washing and by the presence of peritoneal keratin granulomas in cases of EC with squamous differentiation [16].
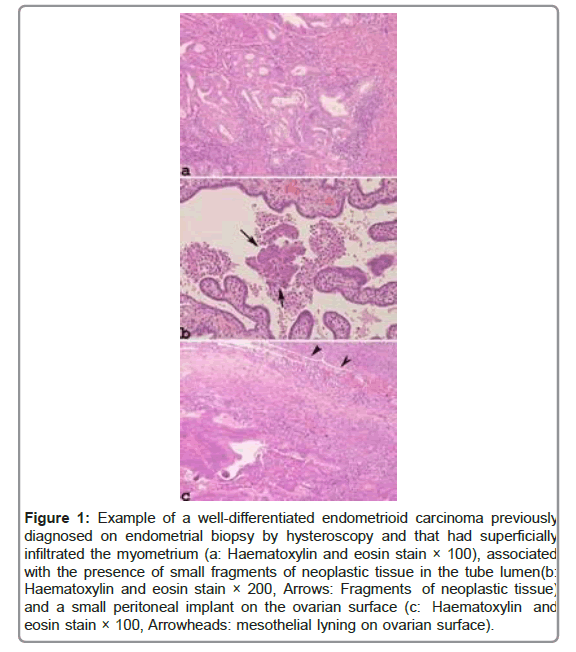
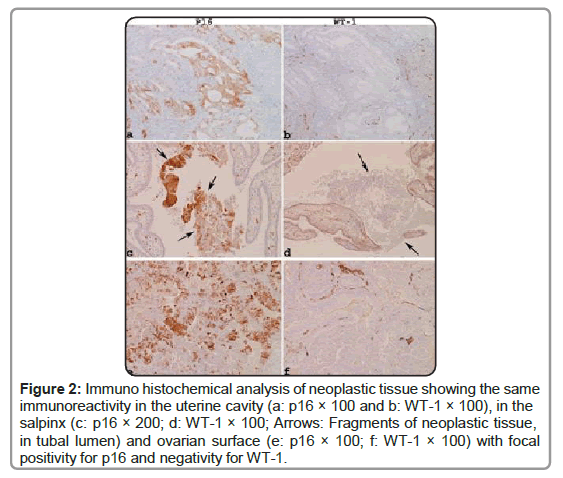
Moreover, the transtubal spread of EC can be observed in cases of minimally invasive low-grade endometrioid carcinoma by accurate sampling of the Fallopian tubes and in the simultaneous presence of neoplastic noninvasive implants on the ovarian surface from a previous hysteroscopy (Figures 1 and 2).
Figure 1: Example of a well-differentiated endometrioid carcinoma previously diagnosed on endometrial biopsy by hysteroscopy and that had superficially infiltrated the myometrium (a: Haematoxylin and eosin stain × 100), associated with the presence of small fragments of neoplastic tissue in the tube lumen(b: Haematoxylin and eosin stain × 200, Arrows: Fragments of neoplastic tissue) and a small peritoneal implant on the ovarian surface (c: Haematoxylin and eosin stain × 100, Arrowheads: mesothelial lyning on ovarian surface).
Figure 2: Immuno histochemical analysis of neoplastic tissue showing the same immunoreactivity in the uterine cavity (a: p16 × 100 and b: WT-1 × 100), in the salpinx (c: p16 × 200; d: WT-1 × 100; Arrows: Fragments of neoplastic tissue, in tubal lumen) and ovarian surface (e: p16 × 100; f: WT-1 × 100) with focal positivity for p16 and negativity for WT-1.
In these cases, in accordance with the recent ESMO-ESGO-ESTRO guidelines, these neoplasms should be placed in the “high” prognostic risk group; therefore, it is necessary to consider performing adjuvant therapy with chemotherapy and radiotherapy [17].
However, it should be noted that in cases of minimally invasive EC, the intraoperative cytology of peritoneal washing might represent a serious diagnostic problem for pathologists and might have important consequences on the patient because of the upstaging of the uterine neoplasm and the treatment approach.
Thus, it is necessary to consider other conditions that might explain this finding, such as uterine manipulation due to total LH, exfoliation of uterine neoplastic tissue produced during hysteroscopy and technical artifact due to the sampling of surgical specimens in the pathology laboratory [18-21].
To avoid the dissemination of neoplastic cells during LH, tubal ligation could be useful [22,23].
Hysteroscopy is a diagnostic method widely used in the diagnosis of endometrial neoplasms and pre neoplastic lesions that cause abnormal genital bleeding. This method, in fact, allows the evaluation of the morphology of the endometrium and the performance of biopsies of suspicious lesions for neoplasms.
The exfoliation of uterine neoplastic tissue and neoplastic diffusion produced during hysteroscopy, as suggested by some authors, could be facilitated by irrigation of the uterus with fluid medium and increased pressure in the uterine cavity during the procedure [19,20].
These authors observed that this mechanism of neoplastic diffusion is more frequent in cases of aggressive subtypes of EC. Moreover, they demonstrated retrograde transtubal spread in the peritoneal cavity and the simultaneous presence of neoplastic cells in the intraoperative cytology of peritoneal washing [19,20].
Regarding histologic artifact, due to the sampling of surgical specfimens in pathology laboratories, which represents a serious potential source for diagnostic error, it is necessary to recognize these artefacts, according to the useful data provided by Layfield et al. [21].
Essentially, these authors state that these artifacts can be recognized, taking into account that the foreign neoplastic tissue present in a histological section is usually observed at the periphery of non-neoplastic tissue or is sometimes located in a different plane; therefore, on microscopic examination, it has a different focus than the surrounding tissue.
Declarations
Acknowledgements
Not applicable.
Authors’ Contributions
The authors declare that they participated in this study as described below and have read and approved the final manuscript. Prof Vito Andrea Capozzi acquired the data pertaining to the cases, reviewed the literature, and drafted the manuscript. Giovanna Giordano was responsible for the concept of the paper, reviewed the available literature, evaluated the pathological data, drafted and edited the manuscript. Dr Roberto Berretta contributed to the data acquisition. All authors agree to be accountable for all aspects of the work.
Funding
Not applicable.
Availability of Data and Materials
All data generated or analysed during this study are included in this published article.
Declarations
Consent for publication the patients agreed to the publication of these cases.
Competing Interests
The authors declare no conflicts of interest.
References
- Siegel RL, Miller KD , Jemal A (2018) Cancer statistics. CA Cancer J Clin 68: 7-30
- Casey MJ (2021) Endometrial Cancer. In Stat Pearls; StatPearls Publishing LLC.: Treasure Island, FL, USA.
- Stuttard PJ, Zhou B, Kontis V, Bentham J, Gunter MJ, et al., (2018) Worldwide burden of cancer attributable to diabetes and high body-mass index: A comparative risk assessment. Lancet Diabetes Endocrinol. 6: e6-e15
- Amant F, Moerman P, Neven P, Timmerman D, Limbergen E et al., (2005) Endometrial cancer. Lancet 366: 491-505.
- Soslow RA, Isacson EPC (2000) Endometrial intraepithelial carcinoma with associated peritoneal carcinomatosisâ€. Am. J. Surg. Pathol. 24:726-732.
- Zheng W, Schwartz PE (2005) Serous EIC as an early form of uterine papillary serous carcinoma: recent progress in understanding its pathogenesis and current opinions regarding pathologic and clinical management. Gynecol Oncol 96: 579-582
- Muto MG, Welch WR, Mok SC, Bandera CA, Fishbaugh PM et al., (1995) Evidence for a multifocal origin of papillary serous carcinoma of the peritoneum. Cancer Res 55: 490-492.
- Kupryjanczyk J, Thor AD, Beauchamp R, Poremba C, Scully RE et al. (1996) Ovarian, peritoneal, and endometrial serous carcinoma: clonal origin of multifocal disease. Mod Pathol 9: 166-173.
- Schorge JO, Muto MG, Welch WR, Bandera CA, Rubin SC, et al., (1998) Molecular evidence for multifocal papillary serous carcinoma of the peritoneum in patients with germline BRCA1 mutations. J Natl Cancer Inst. 90:841-845.
- Giordano G (2020) An unusual mechanism of metastasis in serous carcinoma of the endometrium associated with BRCA1 mutation gene: A case report with clinical and immunohistochemical features. Eur J Gynaecol Oncol 41: 1065-1069
- Snyder MJ, Bentley R, Robboy SJ (2006) Trans-tubal spread of serous adenocarcinoma of the endometrium: an underrecognized mechanism of metastasis. Int J Gynecol Pathol 25:155-160.
- Stewart CS, Doherty DA, Havlat M, M Koay HE, Leung YC et al., (2013) Trans-tubal spread of endometrial carcinoma: correlation of intraluminal cell with grade, peritoneal fluid cytology and extra-uterine metastasis. Pathology 45: 382-387
- Felix AS, Sinnott JA , Vetter MH , Rhoades J , Cohn DE et al., (2018) Detection of endometrial cancer cell in the fallopian tube lumen is associated with adverse prognostic factor and reduce survival. Gynecol Oncol 150:38-43.
- Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ et al., (2006) The tubal fimbria is a preferred site for early adenocarcinoma in women with familial ovarian cancer syndrome Am J Surg Pathol 30:230-236.
- Samimi G, Trabert B, Duggan MA, Robinson JL , Coa KI et al., (2018) Processing of fallopian tube, ovary, and endometrial surgical pathology specimens: A survey of U.S. laboratory practices. Gynecologic Oncology.148: 515-520
- Uehara K, Yasuda M, Ichimura T, Yamaguchi H, Nagata K, et al., (2011) Peritoneal keratin granuloma associated with endometrioid adenocarcinoma of the uterine corpus. Diagn Pathol 6:104.
- Colombo N, Creutzberg C, Amant F, Bosse T, MartÃn AG et al., (2016) “ESMO-ESGO-ESTRO consensus conference on endometrial cancer: Diagnosis, treatment and follow-upâ€. Ann. Oncol 27: 16-41.
- Krizova A, Clarke BA, Bernardini MQ, James S, Kalloger SE et al., (2011) Histologic artifacts in abdominal, vaginal, laparoscopic, and robotic hysterectomy specimens: a blinded, retrospective review. Am J Surg Pathol 35:115–126.
- Rose PG, Mendelsohn G, Kornbluth I (1998) Hysteroscopic dissemination of endometrial cancer. Gynecol Oncol 71: 145-146.
- Obermair A, Geramou M, Gucer F, Denison U, Graf AH et al., (2000) Does hysteroscopy facilitate tumor cell dissemination? Incidence of peritoneal cytology from patients with early stage endometrial carcinoma following dilatation and curettage (D & C) versus hysteroscopy and D & C. Cancer 88:139-143.
- Layfield LJ, Witt BL, Metzger KG (2011)Extraneous tissue: a potential source for diagnostic error in surgical pathology. Am J Clin Pathol136:767-772.
- Li M, Â Zhao L, WangZ, Wang Y Shen D, et al., (2016) Prior tubal ligation might influence metastatic spread of nonendometrioid endometrial carcinoma. Int J Gynecol Cancer 26: 1092-1097.
- Felix AS, Brinton LA, McMeekin DS, Creasman WT, Mutch D et al., (2015) Relationships of tubal ligation to endometrial carcinoma stage and mortality in the NRG Oncology/Gynecologic Oncology Group 210 trial. J Natl Cancer Inst 107:1-9.
Citation: Giordano G, Capozzi VA, Berretta R (2021) The Impact of Uterine Neoplastic Tissue in Accurately Examined Fallopian Tubes on Pathological Diagnosis and Clinical Management. Diagnos Pathol Open S8:032.
Copyright: © 2021 Giordano G, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 2293
- [From(publication date): 0-2021 - Dec 08, 2025]
- Breakdown by view type
- HTML page views: 1606
- PDF downloads: 687