The Role of EPHA3 Mutation in the Prognosis of Non-Small Cell Lung Cancer Patients Receiving Immunotherapy
Received: 10-Dec-2024 / Manuscript No. DPO-24-154670 / Editor assigned: 12-Dec-2024 / PreQC No. DPO-24-154670 (PQ) / Reviewed: 26-Dec-2024 / QC No. DPO-24-154670 / Revised: 02-Jan-2024 / Manuscript No. DPO-24-154670 (R) / Published Date: 09-Jan-2024
Abstract
Immune Checkpoint Inhibitors (ICIs) have changed the treatment mode of Non-Small Cell Lung Cancer (NSCLC) patients, but precise biomarkers are still needed to screen out those could benefit from ICIs. EPHA3 is the gene that codes for the Eph receptor A3 and has been found to be associated with lung cancer, but the relationship between EPHA3 and ICIs still need to be explored. In our study, data of 344 NSCLC patients receiving ICIs and 954 NSCLC patients treated without ICIs were downloaded from the Memorial Sloan Kettering Cancer Center (MSKCC) database and The Cancer Genome Atlas (TCGA) database respectively. Patients were divided into EPHA3-mutant type (EPHA3-Mut) group and EPHA3-wild type (EPHA3-Wt) group by EPHA3 mutation status. Kaplan-Meier survival analysis found that the EPHA3-Mut group (n=36) have got higher Overall Survival (OS) rates than the EPHA3-Wt group (n=308) (median OS: 3 years (95% Confidence Interval (CI)=1 to not reached) vs. 0.917 years (95% CI=0.75 to 1.17, p=0.025) in the MSKCC cohort, while differences of OS (p=0.083) in the TCGA cohort have not been observed. Besides, EPHA3 mutation was related to higher Tumor Mutation Burden (TMB) (p<0.0001), elevated Neoantigen Load (NAL) (p<0.0001) and greater mutation rate in the DNA Damage Response (DDR) pathways. EPHA3-Mugroup showed higher CD8 + T cells (p<0.05) an Natural Killers (NK) cells (p<0.01) infiltration. Gene Set Enrichment Analysis (GSEA) showed that several immune response-related pathways were up-regulated in the EPHA3-Mut group. According to the study, EPHA3 mutation may be related with the effectiveness of ICIs in NSCLC patients.
Keywords: Immunotherapy; Immuno-checkpoint inhibitors; Nonsmall cell lung cancer; EPHA3; Immune
Keywords
Immunotherapy; Immuno-checkpoint inhibitors; Non- small cell lung cancer; EPHA3; Immune
Introduction
ICIs have altered the treatment mode of NSCLC, some patients with NSCLC achieved long-term survival through immunotherapy [1]. However, there are still majority of patients who cannot significantly benefit from immunotherapy [2]. Therefore, we urgently need some biomarkers to screen out patients who could benefit from immunotherapy. Up to now, there are some potential biomarkers such as PD-L1 expression and Tumor Mutation Burden (TMB) already been found out to predict the curative effect of ICIs [3]. However, in some studies, these potential biomarkers cannot accurately predict the curative effect of immunotherapy, for example, some scholars found that NSCLC patients with a PD-L1 expression level of 5% or more did not reach longer Progression-Free Survival (PFS) after treated with Nivolumab [4]. Thus, more precise biomarkers of ICIs remain to be explored. Receptor Tyrosine Kinases (RTKs) is the largest class of enzyme linked receptor proteins, many types of RTKs play a critical part in the development and growth of tumors and are related with curative effect of ICIs. For instance: Epidermal Growth Factor (EGF) Receptor (EGFR) pathway activation could reduce the PD-L1 expression in NSCLC patients and is correlated with immunosuppression, Fibroblast Growth Factor (FGF) Receptor 4 (FGFR4) was one of the main targets for down-regulation of PD-L1 in vitro, while FGFR2 promotes PD-L1 expression in colorectal cancer through JAK/STAT3 signaling pathway, interruption of gastrin at the Cholecystokinin (CCK) receptor may alter tumor immune cells, which may could affects the efficacy of immunotherapy [5-8]. Eph receptor family is the largest known family of RTKs, according to their extracellular domains, Eph receptors are divided into two subgroups, Eph receptor A (EphA) and Eph receptor B (EphB), EphA is consisted of 9 members, while EphB is consisted of 5 members [9]. EPHA3 is the gene that codes for the Eph receptor A3, it has been found to be associated with the development of lung adenocarcinoma and thus, we speculated whether EPHA3 has effect on the curative effect of ICIs.
Therefore, we conducted a comprehensive analysis through an immunotherapy cohort (MSKCC cohort), and TCGA cohort to determine whether EPHA3 is associated with the curative effect of ICIs on NSCLC patients [10]. The result showed that EPHA3 mutation could improve NSCLC patients’ OS treated with ICIs. Besides, EPHA3 mutation is connected with greater immune cell soakage in the Tumor Immune Microenvironment (TIME), higher tumor antigenicity, more gene mutations in the DNA Damage Response (DDR) pathways and more activation of immune-related pathways in NSCLC patients. These results imply that EPHA3 mutation may act as predictive biomarker of immunotherapy efficacy in patients with NSCLC.
Materials And Methods
Clinical cohorts and genome characteristics
To find out the relationship between EPHA3 mutation and ICIs, we downloaded previously published immunotherapy cohort from cBioPortal, as discovery cohort (n=344, EPHA3-Mut group vs. EPHA3-Wt group=36:308), all patients in the cohort were NSCLC patients recruited from MSKCC who were treated with PD-1 or PD- L1 inhibitors [10]. We also downloaded somatic mutation of NSCLC patients who had not treated with ICIs form TCGA database from the Genomic Data Commons (GDC) portal, after excluding the samples with incomplete information, there are 954 (EPHA3-Mut vs. EPHA3- Wt =113:841) samples. Targeted Next-Generation Sequencing (NGS) was used for the analysis of the somatic mutation data from the MSKCC cohort. Maftools package in the R was used to visualize the top 20 most commonly mutated genes and clinical features. Besides, the co- mutation and mutually exclusive mutation of top 25 most commonly mutated genes in MSKCC cohort was explored through Maftools package.
Survival analysis
MSKCC cohort and TCGA cohort are divided to EPHA3-Mut group and EPHA3-Wt group according to EPHA3 mutation status. Kaplan- Meier survival curves analyses were used to compare OS between EPHA3-Mut group and EPHA3-Wt group in both two cohorts. Besides, we performed univariate and multivariate cox regression analysis to explore the relationship between patients’ OS and some frequently mutated genes, including EPHA3, as well as clinical features of NSCLC patients in the MSKCC cohort. In this analysis, TMB was divided into two groups according to dividing line of 10 Mut/Mb [11,12].
Analysis of tumor antigenicity
To investigate whether EPHA3 mutation has an effect on tumor antigenicity, we explored the level of TMB, Neoantigen Load (NAL) as well as mutations in DDR pathways in both EPHA3-Mut group and EPHA3-Wt group. The number of non-synonymous somatic mutations is divided by 38 Mb to obtain the TMB value. NAL data and mutation data in DDR pathways were obtained from researches of Thorsson et al., and Knijnenburg et al., respectively [13,14].
Analysis of immune cell soakage was performed
To explore the connection of EPHA3 mutation and immune soakage, we used the data of 22 kinds of immune cells’ signature matrix obtained from CIBERSORTx, to explore the feature of immune cell soakage in the TIME of patients in the TCGA cohort, we then compared the immune cell soakage status of EPHA3-Mut group and EPHA3-Wt group [15]. Online analytical tool CIBERSORTx was applied to conduct the analysis.
Gene set enrichment analysis
To further study the biological pathways related to EPHA3, we downloaded hallmark gene sets and ontology gene sets from Molecular Signatures Database (MSigDB) of the Broad Institute [16]. Then, clusterProfiler package of R was used for the GSEA. Pathways with p<0.05 were considered significantly different.
Statistical analysis
Fisher’s exact test was used to analyse co-mutation and mutually exclusive mutation of top 25 most commonly mutated genes in MSKCC cohort. Kaplan-Meier method with the log-rank test were applied to graph survival curves. Univariate and multivariate cox regression analysis were conducted to determine the potential of EPHA3 mutation, other common mutated gene mutations and clinical features to predict the efficacy of immunotherapy in the MSKCC cohort. Mann-Whitney U test was used to compare the differences of TMB and NAL between EPHA3-Mut group and EPHA3-Wt group. Gene mutations of DDR pathways in EPHA3-Mut group and EPHA3-Wt group in TCGA cohort were compared using Chi-square test. All statistical tests were two- sided.
Results
Clinical and genetic characteristics of patients
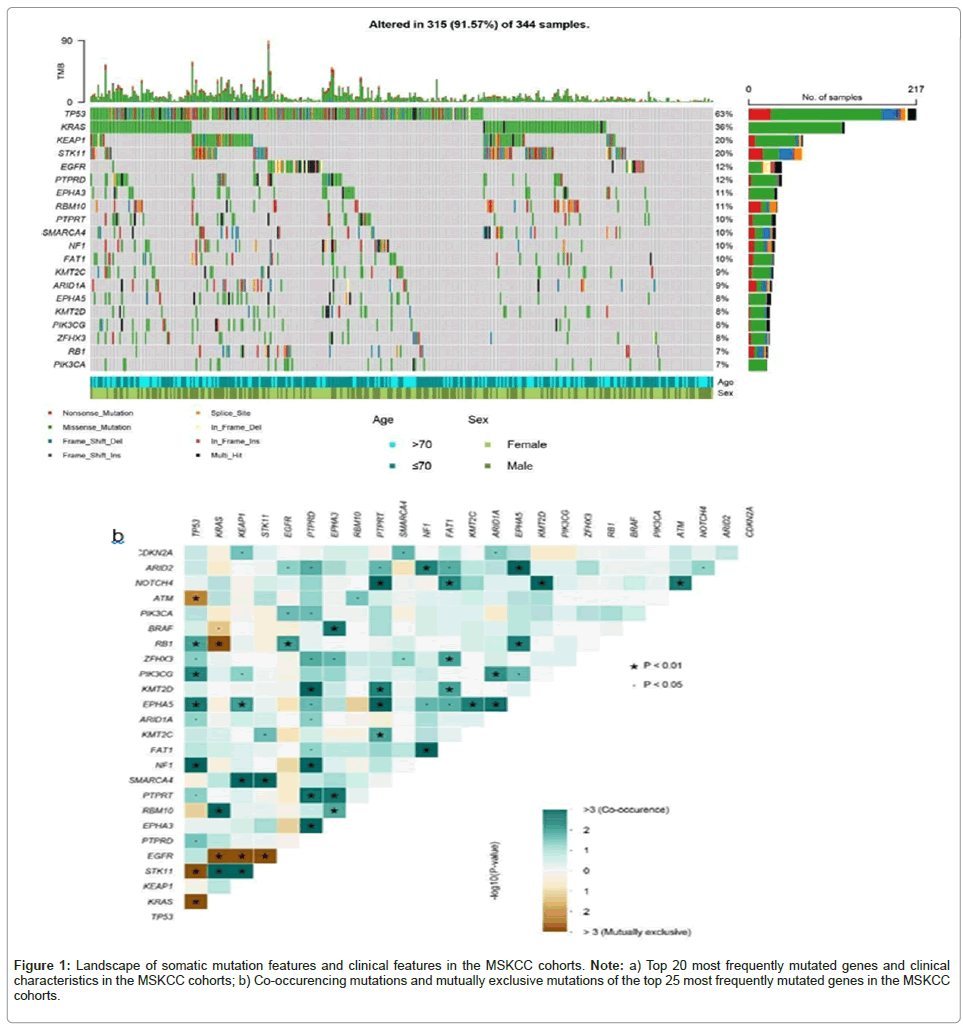
Our discovery cohort is consisted of 344 NSCLC patients receiving ICIs, 119 (35.5%) of them were over 71 years old, 178 (51.7%) of them were men, 166 (48.3%) of them were women, 268 (77.9%) were lung adenocarcinoma, 44 (12.8%) were lung squamous cell carcinoma, other 32(9.3%) of them were other kind of NSCLC. The median TMB was 7.76 mutations/Mb (range, 0-96.5 mutations/Mb). The waterfall plot was used to demonstrate these genes whose mutation rate was in the top 20, as well as clinical features of the NSCLC patients in MSKCC cohort, as shown in the plot, EPHA3 was the seventh most frequently mutated gene. Besides, Gene interaction analysis of MSKCC cohort shows that EPHA3 tended to co-mutate with KRAS, ZFHX3, PTPRT and RBM10 (p<0.05) (Figures 1a and 1b).
Figure 1: Landscape of somatic mutation features and clinical features in the MSKCC cohorts. Note: a) Top 20 most frequently mutated genes and clinical characteristics in the MSKCC cohorts; b) Co-occurencing mutations and mutually exclusive mutations of the top 25 most frequently mutated genes in the MSKCC cohorts.
The relationship of OS and EPHA3 mutation in NSCLC patients receiving ICIs
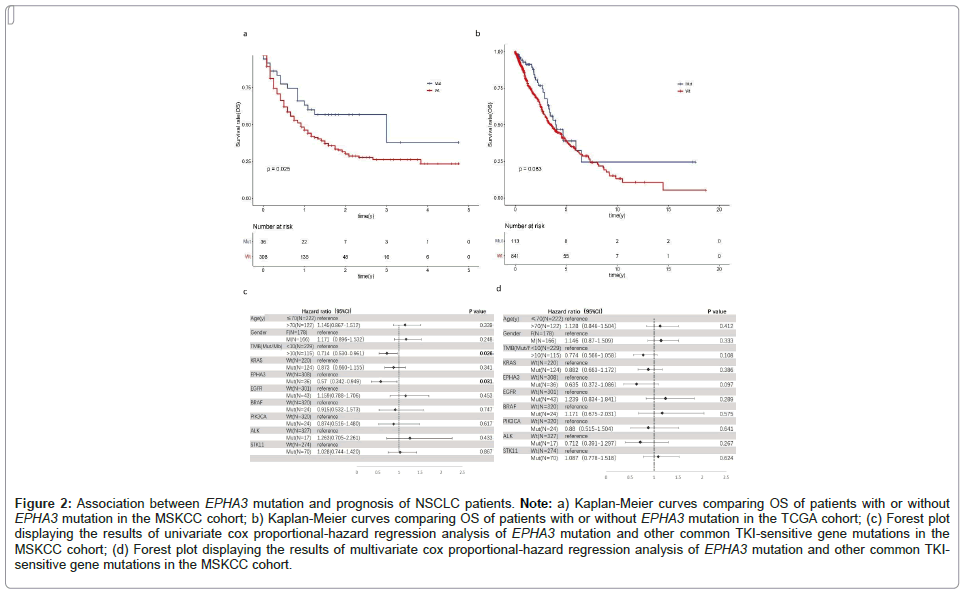
To analyse the contribution of EPHA3 to prognosis of NSCLC patients treated with ICIs, we conducted survival analyses for MSKCC and TCGA cohorts. As showed in the Kaplan-Meier analysis, in the MSKCC cohort, EPHA3-Mut group had observably longer OS (p=0.025). The median OS was 3 years (95% CI=1 to not reached) in EPHA3-Mut group vs. 0.917 years (95% CI=0.75-1.17) in EPHA3-Wt group. Compared with MSKCC cohort, there is no significant differences of OS (p=0.083) between EPHA3-Mut group and EPHA3-Wt group in the TCGA cohort. The median OS was 3.98 years (95% CI=3.3 to not reached) in EPHA3-Mut group vs. 3.53 years (95% CI=3.03-4.5) in the group of EPHA3-Wt group. Univariate cox regression analysis showed that TMB (HR=0.714, 95% CI=0.530-0.961, p=0.026) and EPHA3 (HR=0.57, 95% CI=0.342-0.949, p=0.031) is associated with longer OS. While multivariate cox regression analysis showed that the effect of EPHA3 on OS did not reach statistical significance (HR=0.635, 95% CI=0.372-1.086, p=0.097) (Figures 2a-2d).
Figure 2: Association between EPHA3 mutation and prognosis of NSCLC patients. Note: a) Kaplan-Meier curves comparing OS of patients with or without EPHA3 mutation in the MSKCC cohort; b) Kaplan-Meier curves comparing OS of patients with or without EPHA3 mutation in the TCGA cohort; (c) Forest plot displaying the results of univariate cox proportional-hazard regression analysis of EPHA3 mutation and other common TKI-sensitive gene mutations in the MSKCC cohort; (d) Forest plot displaying the results of multivariate cox proportional-hazard regression analysis of EPHA3 mutation and other common TKI-
sensitive gene mutations in the MSKCC cohort.
EPHA3 mutation is relative to the enhancement of tumor immunogenicity and gene mutations in DDR pathways
Tumor immunogenicity is related to the efficacy of immunotherapy. To some degree, TMB and NAL could reflect the immunogenicity of tumor and are related to the curative effect of ICIs [17,18]. To find out the relationship between EPHA3 mutation and tumor immunogenicity, we investigated the differences of TMB and NAL between EPHA3-Mut group and EPHA3-Wt group. In both MSKCC cohort (p<0.001) and TCGA cohort (p<0.0001), TMB of EPHA3-Mut group was higher than EPHA3-Wt group. We also observed that EPHA3-Mut group shows significantly higher NAL compared to EPHA3-Wt group in TCGA cohort (p<0.0001).
Some reports showed that some mutations in DDR pathways could represent genomic instability and may be related to better outcomes of immunotherapy [19]. We compared mutations in 9 DDR pathways between EPHA3-Mut and EPHA3-Wt group in TCGA cohort. As expected, in EPHA3-Mut group, the mutation rates were higher in 7 DDR pathways, including Base Excision Repair (BER), Homologous Recombination (HR), Nucleotide Excision Repair (NER), Nonhomologous End-Joining (NHEJ), Mismatch Repair (MMR), Translesion DNA Synthesis (TLS) and Fanconi Anemia (FA) (Figures 3a-3d).
Figure 3: Association of EPHA3 mutation with tumor mutation burden, neoantigen load and mutations in DNA Damage Repair (DDR) pathways in NSCLC patients. Note: (a,b) EPHA3 mutated patients had a markedly higher TMB (number of mutations per Mb) in both the MSKCC-IO cohort and the TCGA cohort; (c) Comparison of NAL (number of neoantigen per Mb) between the EPHA3-Mut and EPHA3-Wt group tumors in the TCGA cohorts (Mann-Whitney U test); (d) Comparison of mutation rate in the DDR pathways between the EPHA3-Mut group and EPHA3-Wt group in the TCGA cohort (Chi-square test); *p<0.05; **p<0.01; ***p<0.001;****p<0.0001; ns: No significance.
These results indicated that EPHA3 mutation is correlated with the enhancement of tumor immunogenicity and more gene mutations in DDR pathways, which suggested a better prognosis of NSCLC patients receiving ICIs.
EPHA3 mutation is correlated with immune soakage
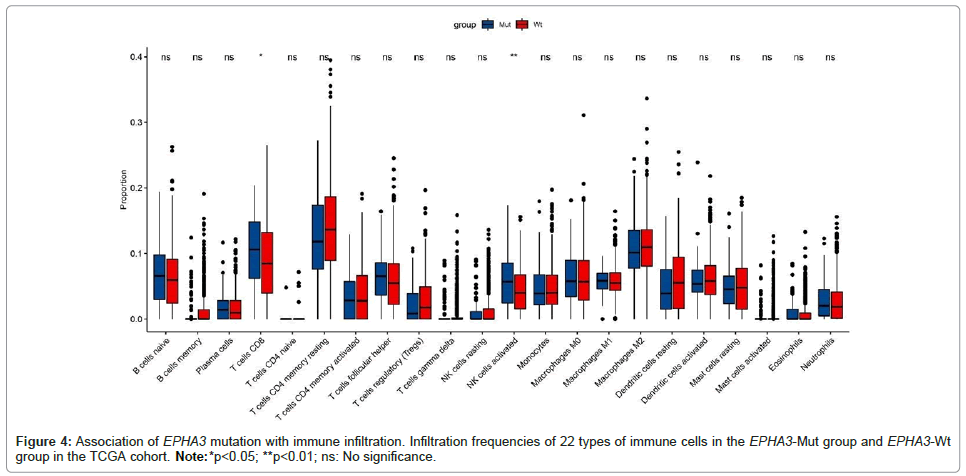
The immune status of the TIME also has effects on the efficacy of immunotherapy [20]. To investigate how the EPHA3 mutation affects TIME, we used the immune cell signature matrix to study the differences of soakage of immune cells between EPHA3-Mut group and EPHA3-Wt group in TCGA cohort. As expected, in EPHA3-Mut group, CD8+ T cells (p<0.05) as well as activated NK cells (p<0.01) were found obviously increased (Figure 4).
EPHA3 mutation is correlated with changes in some tumor- related biological pathways
In order to identify whether EPHA3 mutation acts on tumor- related biological pathways, we performed GSEA on TCGA cohort and compared the results of EPHA3-Mut group and EPHA3-Wt group. As shown in Supplementary Figure 1, a number of immune-related pathways were markedly up-regulated in the EPHA3-Mut group, such as activation of immune response pathway, B cell activation pathway, INF-γ response. In contrast, some pathways such as mTORC1 signaling pathway and MYC targets V1 pathway were down-regulated in the EPHA3-Mut group. These results showed that EPHA3 mutation has positive effect on immune soakage and immune response.
Discussion
Through univariate cox regression analysis and Kaplan-Meier survival analysis, we observed that EPHA3 mutation has a favorable effect on OS of NSCLC patients receiving ICIs, while no effect on patients who treated without ICIs. Although the multivariate cox regression analysis of the effect of EPHA3 on OS did not reach statistical significance, there is still an obvious tendency of EPHA3 to prolong OS of NSCLC patients treated with ICIs (HR=0.635), the reason for not reaching statistical significance (p=0.097) may be that the sample size was insufficient to detect the significance of the difference. Through gene interaction analysis, we found that EPHA3 co-mutated with several genes that may have potential effect for immunotherapy. Then, our investigation on tumor immunogenicity discovered that there are higher TMB, NAL, as well as more mutations in DDR pathways in EPHA3-Mut group. In addition, increased soakage of immune cells in Tumor Microenvironment (TME) were found in EPHA-Mut group. GSEA analysis showed that some tumor-related biological pathways, including immune response-related pathways were up-regulated in EPHA3-Mut group, while some pathways were down-regulated. These findings proved that EPHA3 may act as a potential prognostic biomarker for immunotherapy. Eph receptors are closely related to cellular repulsion, adhesion and other activities, EPH gene mutations are related to tumorigenesis, tumor immunity and tumor angiogenesis [21,22]. Recently, some studies indicated that Eph receptors could suppress the immune response by modulate innate and adaptive immunity in the TME [23]. Thus, researchers hypothesized that we may inhibit the function of these receptors to raise immune response and enhance the efficacy of ICIs. In recent studies, EPHA5 and EPHA7 have been found to be related to immunotherapy efficacy in patients with lung cancer [24,25]. Besides, researchers have found that EPHA3 was one of the most commonly mutated genes in lung cancer and may inhibit the formation of lung adenocarcinoma [26]. However, the relationship between EPHA3 mutation and ICIs has not been found out yet. Our study is the first to represent the association between the efficacy of ICIs and EPHA3 mutation in NSCLC patients, the result showed that among NSCLC patients receiving ICIs, those with EPHA3 mutation tend to get longer OS. Our study enriched the group of ICIs' prognostic biomarkers, strengthened the connection between Eph family and immunotherapy. In our study, EPHA3 tended to co-mutate with KRAS, ZFHX3, PTPRT and RBM10. ZFHX3 mutation and PTPRT mutation have been found as a protective biomarker for immunotherapy on NSCLC patients [27,28]. RBM10 deficiency was found been associated with higher TMB and PD-L1 expression in NSCLC patients, that means RBM10 may affect the prognosis of patients using ICIs. Several reports also showed that tumors with KRAS mutated showed higher TMB and patients with KRAS mutated tended to benefit more from PD-1 inhibitors [29-31]. These findings support our hypothesis that EPHA3 mutation may has a positive impact on the efficacy of immunotherapy in NSCLC patients. ICIs kill tumor cells based on their immunogenicity, which is primarily determined by tumor antigenicity and antigen presentation efficiency [32]. Antigens enable the immune system to distinguish body's own tissues and cancer cells. Neo-antigens are derived from about 10% of the non-synonymous somatic mutations and play an important role in antitumor response, for they are main targets of T-cell-mediated antitumor immunity [33,34]. In this study, EPHA3-Mut group showed higher TMB and NAL level, indicating that EPHA3 mutation may be associated with better prognosis of NSCLC patients receiving immunotherapy. Some studies reported that patients with mutations in DDR pathways tend to have higher TMB and NAL level, and might also result in high Microsatellite Instability (MSI-H), which means a higher immunogenicity of tumor [35]. It is also reported that mutations in DDR pathway in tumors directly affect the soakage of immune cells at the TIME, thus affecting the efficacy of ICIs [36-38]. Researchers found that gene mutations in DDR pathways may serve as a reliable biomarker to evaluate the efficacy of ICIs in clinical application [39]. In this study, we found that EPHA3-Mut group showed more mutations in DDR pathway, which means better prognosis in ICIs. The analysis of immune cell soakage in the TIME of NSCLC patients in the MSKCC cohort showed that CD8+ T cells and activated NK cells were significantly increased in the EPHA3-Mut group. On the one hand, tumor microenvironment can induce the expression of PD-1 in CD8 + T cells as well as NK cells, which can bind to PD-L1 on the surface of tumor cells, thus assisting tumor cells in immune escape, that makes CD8+ T cells and NK cells become the main target of PD-1 inhibitors [40,41]. On the other hand, activated CD8+ T lymphocytes cells are the primary effector cells of antitumor immunity in the body, NK cells also take a significant part in tumor clearance, deficit of NK cells leads to an increased risk of cancer development, thus, the degree of CD8+ T cells and NK cells’ soakage in the TIME has a significant impact on tumor immunotherapy [42,43]. In our study, GSEA analysis showed that some immune-related pathways were upregulated, while E2F targets, MYC targets V1 and mTORC1 signaling pathways are downregulated in EPHA3-Mut group. E2F is an important transcription factor involved in carcinogenesis [44]. There are researches reported that there is a higher expression of E2F in NSCLC tumors, besides, overexpression of E2F1 and E2F2 was observably related to poor prognosis of patients [45]. MYC is a transcription factor with a wide range of roles, which can regulate a variety of cell activities including cell differentiation and proliferation through a variety of pathways. Scholars found that overexpression of MYC obviously reduced the populations of CD3 + , CD8+ T and disabled T cell soakage in the TIME in Triple-negative breast cancer [46]. Mammalian Target of Rapamycin (mTOR) is an important regulator of cell growth and proliferation as well as a central regulator of immune response, mainly acting through two multi-protein complexes, mTORC1 and mTORC2 [47,48]. It has been reported that mTOR plays an important part in the modulation of immune responses, it could regulate a variety of functions of professional antigen- presenting cells and also play an important role in regulatory T cells and effector T cells [49]. Some scholars identified that the dysfunction of immune cells is related to down regulation of proliferative signals (MYC targets, E2F targets, mTORC1 signaling) [50]. These findings indicated that EPHA3 mutation has an effect on immune function and may further affect the efficacy of ICIs. The present study had several shortcomings. Firstly, this is a retrospective study, it may induce bias to this study. Then, the sample size of MSKCC cohort is limited, more cases and experiments are needed to confirm the results. Thirdly, due to the lack of relevant gene mutation information in clinical patients, the findings of this study lack clinical validation. Finally, due to the lack of detailed gene expression data in the MSKCC cohort, we were unable to verify the relationship of EPHA3 mutation and immune cell soakage of tumor immune microenvironment and the gene mutation rate in the DDR pathways in the MSKCC cohort.
Conclusion
As shown in this study, EPHA3 mutation may be a potential prognosis biomarker of NSCLC patients receiving ICIs. EPHA3 mutation had been proved to have relationship with longer OS, more abundant immune cell soakage and stronger tumor antigenicity in patients treated with ICIs. However, it is necessary to deepen the research on the molecular mechanism of how EPHA3 mutation works on immunotherapy and further preclinical and prospective clinical studies are needed to assess the clinical potential of EPHA3 mutation as a biomarker to predict the prognosis of NSCLC patients those who receiving ICIs.
Acknowledgements
I would like to express my heartfelt gratitude to my tutor and my senior sister Zhihui Wang, who have helped me a lot in the process of writing my paper, they have given me a lot of valuable advice on the topic selection and modification of my thesis. They are always very patient for my questions and encourage me when I have difficulties in writing my paper. The authors declare that they have no competing interests.
Funding Source Declarations
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of Data and Material
The datasets used and analyzed during the current study are available from the Memorial Sloan Kettering Cancer Center database and The Cancer Genome Atlas database.
Author Agreements
All authors have seen and approved the final version of the manuscript being submitted. We warrant that the article is the authors' original work, hasn't received prior publication and isn't under consideration for publication elsewhere.
Ethical Approval
This study has been approved by the Ethics Committee of the Second Affiliated Hospital of Harbin Medical University, including ethical approval, statement of informed consent and exemption of informed consent. (Ethical review approval number: YJSKY2022-526).
Publication Consent
In this study, no information was available to identify the subjects and informed consent could not be obtained.
Declaration of Interest Statement
We declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Author Contributions
Qin Qin: Sorted out the research ideas, responsible for statistical drawing, and the main writer of the paper. Di Wang: Provide important guidance in solving difficult or complex problems in an article. Meng Ma and Jing Ai: Responsible for data collection and correction of grammar. Lili Deng: The main designer of the study and also responsible for the final review of the paper. All authors read and approved the final manuscript.
References
- Gubin MM, Zhang X, Schuster H, Caron E, Ward JP, et al. (2014) Checkpoint blockade cancer immunotherapy targets tumour-specific mutant antigens. Nature 515:577-581.
[Crossref] [Google Scholar] [PubMed]
- Carbognin L, Pilotto S, Milella M, Vaccaro V, Brunelli M, et al. (2015) Differential activity of nivolumab, pembrolizumab and mpdl3280a according to the tumor expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity analysis of trials in melanoma, lung and genitourinary cancers. PLoS One 10:e0130142.
[Crossref] [Google Scholar] [PubMed]
- Keenan TE, Burke KP, van Allen EM (2019) Genomic correlates of response to immune checkpoint blockade. Nat Med 25:389-402.
- Carbone DP, Reck M, Paz-Ares L, Creelan B, Horn L, et al. (2017) First-line nivolumab in stage IV or recurrent non-small-cell lung cancer. N Engl J Med 376:2415-2426.
[Crossref] [Google Scholar] [PubMed]
- Akbay EA, Koyama S, Carretero J, Altabef A, Tchaicha JH, et al. (2013) Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov 3:1355-1363.
- Yi C, Chen L, Lin Z, Liu L, Shao W, et al. (2021) Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology 74:2544-2560.
[Crossref] [Google Scholar] [PubMed]
- Li P, Huang T, Zou Q, Liu D, Wang Y, et al. (2019) FGFR2 promotes expression of PD-L1 in colorectal cancer via the JAK/STAT3 signalling pathway. J Immunol 202:3065-3075.
[Crossref] [Google Scholar] [PubMed]
- Osborne N, Sundseth R, Burks J, Cao H, Liu X, et al. (2019) Gastrin vaccine improves response to immune checkpoint antibody in murine pancreatic cancer by altering the tumor microenvironment. Cancer Immunol Immunother 68:1635-1648.
[Crossref] [Google Scholar] [PubMed]
- Gale NW, Holland SJ, Valenzuela DM, Flenniken A, Pan L, et al. (1996) Eph receptors and ligands comprise two major specificity subclasses and are reciprocally compartmentalized during embryogenesis. Neuron 17:9-19.
[Crossref] [Google Scholar] [PubMed]
- Samstein RM, Lee CH, Shoushtari AN, Hellmann MD, Shen R, et al. (2019) Tumor mutational load predicts survival after immunotherapy across multiple cancer types. Nat Genet 51:202-206.
[Crossref] [Google Scholar] [PubMed]
- Karamitopoulou E, Andreou A, Wenning AS, Gloor B, Perren A, et al. (2022) High Tumor Mutational Burden (TMB) identifies a microsatellite stable pancreatic cancer subset with prolonged survival and strong anti-tumor immunity. Eur J Cancer 169:64-73.
[Crossref] [Google Scholar] [PubMed]
- Ready N, Hellmann MD, Awad MM, Otterson GA, Gutierrez M, et al. (2019) First-line nivolumab plus ipilimumab in advancednon-small-cell lung cancer (CheckMate 568): Outcomes by programmed death ligand 1 and tumor mutational burden as biomarkers. J Clin Oncol 37:992-1000.
[Crossref] [Google Scholar] [PubMed]
- Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, et al. (2018) The immune landscape of cancer. Immunity 48:812-830.
[Crossref] [Google Scholar] [PubMed]
- Knijnenburg TA, Wang L, Zimmermann MT, Chambwe N, Gao GF, et al. (2018) Genomic and molecular landscape of DNA damage repair deficiency across the cancer genome atlas. Cell Rep 23:239-254.
[Crossref] [Google Scholar] [PubMed]
- Newman AM, Liu CL, Green MR, Gentles AJ, Feng W, et al. (2015) Robust enumeration of cell subsets from tissue expression profiles. Nat Methods 12:453-457.
[Crossref] [Google Scholar] [PubMed]
- Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, et al. (2005) Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA 102:15545-15550.
[Crossref] [Google Scholar] [PubMed]
- Chan TA, Yarchoan M, Jaffee E, Swanton C, Quezada SA, et al. (2019) Development of tumor mutation burden as an immunotherapy biomarker: Utility for the oncology clinic. Ann Oncol 30:44-56.
[Crossref] [Google Scholar] [PubMed]
- Matsushita H, Sato Y, Karasaki T, Nakagawa T, Kume H, et al. (2016) Neoantigen load, antigen presentation machinery and immune signatures determine prognosis in clear cell renal cell carcinoma. Cancer Immunol Res 4:463-471.
[Crossref] [Google Scholar] [PubMed]
- Jiang M, Jia K, Wang L, Li W, Chen B, et al. (2021) Alterations of DNA damage response pathway: Biomarker and therapeutic strategy for cancer immunotherapy. Acta Pharm Sin B 11:2983-2994.
[Crossref] [Google Scholar] [PubMed]
- Zhao Y, Cao Y, Chen Y, Wu L, Hang H, et al. (2021) B2M gene expression shapes the immune landscape of lung adenocarcinoma and determines the response to immunotherapy. Immunology 164:507-523.
[Crossref] [Google Scholar] [PubMed]
- Brantley-Sieders D, Schmidt S, Parker M, Chen J (2004) Eph receptor tyrosine kinases in tumor and tumor microenvironment. Curr Pharm Des 10:3431-3442.
[Crossref] [Google Scholar] [PubMed]
- Pasquale EB (2010) Eph receptors and ephrins in cancer: Bidirectional signalling and beyond. Nat Rev Cancer 10:165-180.
[Crossref] [Google Scholar] [PubMed]
- Janes PW, vail ME, Ernst M, Scott AM (2021) Eph receptors in the immunosuppressive tumor microenvironment. Cancer Res 81:801-805.
[Crossref] [Google Scholar] [PubMed]
- Huang W, Lin A, Luo P, Liu Y, Xu W, et al. (2021) EPHA5 mutation predicts the durable clinical benefit of immune checkpoint inhibitors in patients with lung adenocarcinoma. Cancer Gene Ther 28:864-874.
[Crossref] [Google Scholar] [PubMed]
- Zhang Z, Wu HX, Lin WH, Wang ZX, Yang LP, et al. (2021) EPHA7 mutation as a predictive biomarker for immune checkpoint inhibitors in multiple cancers. BMC Med 19:26.
[Crossref] [Google Scholar] [PubMed]
- London M, Gallo E (2020) Critical role of EphA3 in cancer and current state of EphA3 drug therapeutics. Mol Biol Rep 47:5523-5533.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhou N, Lin A, Luo P, Chen X, et al. (2021) ZFHX3 mutation as a protective biomarker for immune checkpoint blockade in non-small cell lung cancer. Cancer Immunol Immunother 70:137-151.
[Crossref] [Google Scholar] [PubMed]
- Wang X, Wu B, Yan Z, Wang G, Chen S, et al. (2021) Association of PTPRD/PTPRT mutation with better clinical outcomes in NSCLC patients treated with immune checkpoint blockades. Front Oncol 11:650122.
[Crossref] [Google Scholar] [PubMed]
- Liu B, Wang Y, Wang H, Li Z, Yang L, et al. (2021) RBM10 deficiency is associated with increased immune activity in lung adenocarcinoma. Front Oncol 11:677826.
[Crossref] [Google Scholar] [PubMed]
- Dong ZY, Zhong WZ, Zhang XC, Su J, Xie Z, et al. (2017) Potential predictive value of TP53 and KRAS mutation status for response to PD-1 blockade immunotherapy in lung adenocarcinoma. Clin Cancer Res 23:3012-3024.
[Crossref] [Google Scholar] [PubMed]
- Landre T, Justeau G, Assié JB, Chouahnia K, Davoine C, et al. (2022) Anti-PD-(L)1 for KRAS-mutant advanced non-small-cell lung cancers: A meta-analysis of randomized-controlled trials. Cancer Immunol Immunother 71:719-726.
[Crossref] [Google Scholar] [PubMed]
- Wang S, He Z, Wang X, Li H, Liu XS, et al. (2019) Antigen presentation and tumor immunogenicity incancer immunotherapy response prediction. Elife 8:1.
[Crossref] [Google Scholar] [PubMed]
- Meraviglia-Crivelli D, Zheleva A, Barainka M, Moreno B, Villanueva H, et al. (2022) Therapeutic strategies to enhance tumor antigenicity: Making the tumor detectable by the immune system. Biomedicines 10:1.
[Crossref] [Google Scholar] [PubMed]
- Ma W, Pham B, Li T (2022) Cancer neoantigens as potential targets for immunotherapy. Clin Exp Metastasis 39:51-60.
[Crossref] [Google Scholar] [PubMed]
- Gong Z, Yang Y, Zhang J, Guo W (2021) Evaluation of 30 DNA damage response and 6 mismatch repair gene mutations as biomarkers for immunotherapy outcomes across multiple solid tumor types. Cancer Biol Med 18:1080-1091.
[Crossref] [Google Scholar] [PubMed]
- Peyraud F, Italiano A (2020) Combined PARP inhibition and immune checkpoint therapy in solid tumors. Cancers (Basel) 12:1.
[Crossref] [Google Scholar] [PubMed]
- Sun W, Zhang Q, Wang R, Li Y, Sun Y, et al. (2021) Targeting DNA damage repair for immune checkpoint inhibition: Mechanisms and potential clinical applications. Front Oncol 11:648687.
[Crossref] [Google Scholar] [PubMed]
- Shi C, Qin K, Lin A, Jiang A, Cheng Q, et al. (2022) The role of DNA Damage Repair (DDR) system in response to Immune Checkpoint Inhibitor (ICI) therapy. J Exp Clin Cancer Res 41:268.
[Crossref] [Google Scholar] [PubMed]
- Mouw KW, Goldberg MS, Konstantinopoulos PA, D'Andrea AD (2017) DNA damage and repair biomarkers of immunotherapy response. Cancer Discov 7:675-693.
[Crossref] [Google Scholar] [PubMed]
- Chen Y, Xu J, Wu X, Yao H, Yan Z, et al. (2021) CD147 regulates antitumor CD8 (+) T-cell responses to facilitate tumor-immune escape. Cell Mol Immunol 18:1995-2009.
[Crossref] [Google Scholar] [PubMed]
- Alvarez M, Simonetta F, Baker J, Morrison AR, Wenokur AS, et al. (2020) Indirect impact of PD-1/PD-L1 blockade on a murine model of NK cell exhaustion. Front Immunol 11:7.
[Crossref] [Google Scholar] [PubMed]
- Paul M, Ohashi PS (2020) The roles of CD8 (+) T cell subsets in antitumor immunity. Trends Cell Biol 30:695-704.
[Crossref] [Google Scholar] [PubMed]
- Wagner AK, Kadri N, Tibbitt C, van de Ven K, Bagawath-Singh S, et al. (2022) PD-1 expression on mouse intratumoral NK cells and its effects on NK cell phenotype. iScience 25:105137.
[Crossref] [Google Scholar] [PubMed]
- Gomez-Gutierrez JG, Garcia-Garcia A, Hao H, Rao XM, Montes de Oca-Luna R, et al. (2010) Adenovirus-mediated expression of truncated E2F-1 suppresses tumor growth in vitro and in vivo. Cancer 116:4420-4432.
[Crossref] [Google Scholar] [PubMed]
- Gao Z, Shi R, Yuan K, Wang Y (2016) Expression and prognostic value of E2F activators in NSCLC and subtypes: A research based on bioinformatics analysis. Tumour Biol 37:14979-14987.
[Crossref] [Google Scholar] [PubMed]
- Wu SY, Xiao Y, Wei JL, Xu XE, Jin X, et al. (2021) MYC suppresses STING-dependent innate immunity by transcriptionally upregulating DNMT1 in triple-negative breast cancer. J Immunother Cancer 9:1.
[Crossref] [Google Scholar] [PubMed]
- Kim LC, Cook RS, Chen J (2017) mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene 36:2191-2201.
[Crossref] [Google Scholar] [PubMed]
- Zeng H, Tong F, Bin Y, Peng L, Gao X, et al. (2022) The predictive value of pak7 mutation for immune checkpoint inhibitors therapy in non-small cell cancer. Front Immunol 13:834142.
[Crossref] [Google Scholar] [PubMed]
- Thomson AW, Turnquist HR, Raimondi G (2009) Immunoregulatory functions of mTOR inhibition. Nat Rev Immunol 9:324-337.
[Crossref] [Google Scholar] [PubMed]
- Huang W, Zhang Y, Chen S, Yin H, Liu G, et al. (2022) Personalized immune subtypes based on machine learning predict response to checkpoint blockade in gastric cancer. Brief Bioinform 24:1.
Citation: Qin Q, Wang D, Ma M, Ai J, Deng L (2024) The Role of EPHA3Mutation in the Prognosis of Non-Small Cell Lung Cancer Patients Receiving Immuno -thearpy. Diagnos Pathol Open 9:244
Copyright: © 2024 Qin Q, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 724
- [From(publication date): 0-0 - Dec 11, 2025]
- Breakdown by view type
- HTML page views: 442
- PDF downloads: 282