The Role of FoxO4 in Post-Myocardial Infarction Left Ventricular Remodeling.
Received: 12-Oct-2015 / Accepted Date: 12-Nov-2015 / Published Date: 19-Nov-2015
Abstract
Inflammation in post-myocardial infarct (MI) is necessary for myocyte repair and wound healing. Unfortunately it is also a key component of adverse post-myocardial infarction (MI) left ventricular (LV) remodeling that can lead to heart failure. FoxO4 has pleiotropic cell-type and context-dependent functions involved in a variety of human diseases. Recently, we identified a novel function of FoxO4 in post-MI LV remodeling. FoxO4 promotes early post-MI inflammatory response via endothelial Arginase 1 (Arg1). FoxO4 can activate the transcription of endothelial Arg1 in response to ischemia, leading to decreased nitric oxide production and enhanced monocyte adhesion and transmigration through endothelial barrier. Inactivation of FoxO4 resulted in attenuated post-MI inflammation and better preserved cardiac function compared to WT mice. FoxO4 could be a potential therapeutic target in post-MI heart repair and regeneration.
Post-MI LV Remodeling
Myocardial infarction (MI), commonly known as heart attack, is a major public health problem [1]. MI can result in a maladaptive remodeling of left ventricles (LV) that leads to LV dysfunction and eventual heart failure [2]. The acute mortality of MI is decreasing due to improved managements and treatment strategies including early coronary reperfusion therapy. However, the prevalence of heart failure as a result of a maladaptive post-MI LV remodeling is still steadily increasing. The cardiovascular risk for patients with MI is still 10-fold higher than healthy human [3]. Consequently, the morbidity, mortality, and economic cost related to ischemic heart disease are rising worldwide.
Because the heart has limited regenerative capacity, it responds to MI injury by a spontaneous wound repair process which ultimately results in replacement of dead cardiomyocytes with a collagen-based scar [4]. Wound healing is closely intertwined with ventricular remodeling, a complex process that involves both the infarcted and non-infarcted myocardium, and leads to alteration in the size, shape, and physiology of the heart. The extent of post-MI remodeling is an important predictor for mortality of heart failure after infarction, and depends on the size of the infarct and on the mechanical and structural characteristics of the healing wound.
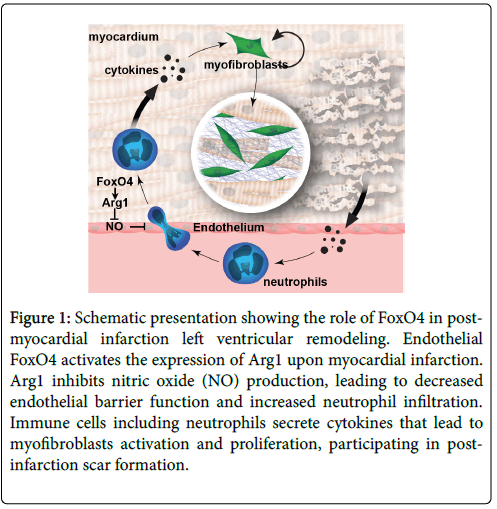
The temporal and spatial events after MI are well delineated and many of the cell types and molecular factors involved were identified [4-10]. The post-MI remodeling consists of three distinctive and overlapping stages: inflammation, proliferation, and maturation (Figure 1). The functions of inflammation are to breakdown the necrotic/apoptotic cardiomyocytes and surrounding matrix, to clean up the debris, and to activate downstream reparative pathways. Immune cells participating in the inflammatory response are mainly infiltrated neutrophils and macrophages from circulation. In the proliferative stage, activated cardiac fibroblasts or other cell lineages proliferate and differentiate into myofibroblasts that accumulate in the infarct area and produce large amounts of extracellular matrix (ECM) proteins, leading to collagen deposition in the wound area. In the final maturation stage, matrix proteins are cross-linked, forming a dense collagen-based scar to provide mechanical support to the infarcted heart [4-10]. Despite decades of research effort, the interplay among the cell types and molecular factors remain elusive. Because post-MI LV remodeling is at first an adaptive response and later becomes maladaptive, many of the molecules involved have pleiotropic functions. These functions are highly contextual, dependent on cell types and timing in the reparative pathway, and often blur the line between “good” and “bad”.
Figure 1: Schematic presentation showing the role of FoxO4 in postmyocardial infarction left ventricular remodeling. Endothelial FoxO4 activates the expression of Arg1 upon myocardial infarction. Arg1 inhibits nitric oxide (NO) production, leading to decreased endothelial barrier function and increased neutrophil infiltration. Immune cells including neutrophils secrete cytokines that lead to myofibroblasts activation and proliferation, participating in postinfarction scar formation.
Post-MI inflammatory response is part of cardiac repair pathways and plays a critical role in determining the size of the infarct and quality of the repair [6]. Global non-selective inhibition of inflammation can result in defective wound healing leading to smaller but weaker scar with tendency to rupture [11,12]. Uncontrolled excessive inflammation may activate proapoptotic pathways inducing further loss of cardiomyocytes, augment matrix degradation causing cardiac rupture, and impair collagen deposition leading to formation of a scar with reduced tensile strength, thus increasing chamber dilation [12]. There have been great efforts and many clinical trials in the past three decades to find an effective therapy that reduces the length and damage of the inflammatory reaction and at the same time does not interfere with the reparatory pathways [6,12]. However, no adequate therapy for the inflammatory response has yet emerged.
FoxO Proteins in Biological Processes
FoxO4 is a member of fork head transcription factor O subfamily that also includes FoxO1, O3, and O6. FoxO proteins regulate a variety of biological processes including oxidative stress response, metabolism, immunity, and apoptosis [13,14]. FoxO proteins are known to activate anti-oxidant genes Sod2 and Catalase as well as genes involved in cell survival pathways [15]. Combined deficiency of FoxO1 and FoxO3 in the adult cardiomyocytes resulted in an exacerbated oxidative damage and decreased myocardial function after acute ischemia/reperfusion (I/R) injury or myocardial infarct [16]. FoxO1 or FoxO3 in cardiomyocytes is shown to attenuate calcineurin activity and inhibits agonist-induced cardiomyocyte hypertrophy and inhibits transverse aortic constriction-induced cardiac hypertrophy in vivo [17]. On the other hand, deletion of FoxO1 in cardiomyocytes is also shown to protect mice against metabolic stress-induced diabetic cardiomyopathy [18]. These results suggest that FoxO proteins play both protective and pathological functions in the stressed heart depending on the pathological stress signals. Unlike FoxO1 and FoxO3, the role of FoxO4 in heart diseases is less well-studied. We have shown previously that FoxO4 modulates the phenotypes of smooth muscle cells (SMCs) by repressing Srf/Myocd-activated differentiation genes [19] and activating the transcription of MMP9 [20]. We have also shown that FoxO4 can inhibit NF-?B-activated gene transcription and inactivation of FoxO4 is associated with elevated susceptibility to chemical-induced colitis [21] and high-fat diet induced atherosclerosis [22].
FoxO4 in Post-MI LV Remodeling
Recently we demonstrated a role for FoxO4 in post-MI LV remodeling [23]. We induced MI in FoxO4-/- and WT littermates by permanent ligation of left anterior descending coronary artery for up to 5 weeks. FoxO4-/- mice had significantly higher survival rate without ventricular wall rupture, better-preserved cardiac functions, and reduced infarct size. Deletion of FoxO4 also prevented adverse LV remodeling including ventricular dilation and cardiac hypertrophy. Microarray gene profiling experiments revealed that FoxO4 KO mouse hearts had significantly reduced inflammatory gene expression at post- MI day 1. The number of neutrophils in the knockout mice is significantly decreased. To identify the underlying cellular mechanism, we systematically deleted FoxO4 in cardiomyocyte (cKO) using αMHC-cre, endothelial cells (ecKO) using Tie2-cre, and immune cells using bone marrow transplant. While no significant difference of post- MI cardiac phenotype was observed in FoxO4 deleted in cardiomyocytes or immune-cells compared to their respective control littermates, deletion of FoxO4 in endothelial cells protected mice against MI injury with better preserved cardiac function, reduced inflammation, and neutrophil infiltration. Further molecular and biochemical studies revealed that FoxO4 can activate the transcription of Arginase 1 (Arg1) in endothelial cells in response to ischemia.
The identification of Arg1 as a novel FoxO4 targeted gene is interesting and has significant clinical implication [24]. Arginase is a metabolic enzyme that converts L-arginine to L-ornithine and urea [25]. Arginase exists in two distinct isoforms, Arg1 and Arg2 that have different intracellular localization. Arg1 is a cytosolic protein whereas Arg2 is localized in the mitochondrion. As arginine is the sole substrate for all three nitric oxide synthase (NOS) isoforms, increased arginase activity may reduce the bioavailability of arginine for nitric oxide (NO) production. NO is a potent vasodilator, which allows for essential perfusion of injured myocardial tissue and has been shown to have beneficial effect on cardiovascular diseases under both physiological and pathological conditions [26]. Emerging evidence suggests that increased activity and expression of arginase are associated with several pathological cardiovascular conditions, including hypertension [27], pulmonary arterial hypertension [28], atherosclerosis [29], myocardial infarction [30], and vascular dysfunction in diabetes mellitus [31]. Many of the pathological effects of arginase on these cardiovascular diseases are attributed to its inhibition of NO production, which has been the basis for studies with arginase inhibitors in both animals and humans [24]. In our studies, we found that knockdown of FoxO4 in human aortic endothelial cells (HAECs) upregulated NO production in response to ischemia and inhibited adhesion of monocyte to endothelial cells, a potential mechanism for reduced neutrophil infiltration in post-MI FoxO4-/- mouse hearts. Arginase inhibitor administrated at time of MI also inhibited early post-MI inflammation and preserved post-MI cardiac function.
Summary
In summary, the post-MI inflammatory response is a part of cardiac repair pathways and unfortunately also a key component of subsequent heart failure pathology. We recently find that FoxO4 promotes the early inflammatory response by suppression of endothelial barrier function, a previously unrecognized function. We also made a novel connection between FoxO4 and Arginase 1 (Arg1) and showed that inhibition of arginase activity in MI can reduce post-MI inflammation and preserve post-MI cardiac function (Figure 1). Our results suggest that FoxO4 may be a potential therapeutic target for post-MI heart repair and regeneration.
References
- Valensi P, Lorgis L, Cottin Y (2011) Prevalence, incidence, predictive factors and prognosis of silent myocardial infarction: a review of the literature.Arch Cardiovasc Dis 104: 178-188.
- Gajarsa JJ, Kloner RA (2011) Left ventricular remodeling in the post-infarction heart: a review of cellular, molecular mechanisms, and therapeutic modalities.Heart Fail Rev 16: 13-21.
- Landmesser U, Wollert KC, Drexler H (2009) Potential novel pharmacological therapies for myocardial remodelling. Cardiovasc Res 81: 519-527.
- Dobaczewski M, Gonzalez-Quesada C, Frangogiannis NG (2010) The extracellular matrix as a modulator of the inflammatory and reparative response following myocardial infarction. J Mol Cell Cardiol 48: 504-511.
- Souders CA, Bowers SL, Baudino TA (2009) Cardiac fibroblast: the renaissance cell. Circ Res 105: 1164-1176.
- Saxena A, Russo I, Frangogiannis NG (2015) Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res .
- Davis J, Molkentin JD (2014) Myofibroblasts: trust your heart and let fate decide. J Mol Cell Cardiol 70: 9-18.
- Lajiness JD, Conway SJ (2014) Origin, development, and differentiation of cardiac fibroblasts. J Mol Cell Cardiol 70: 2-8.
- Chen W, Frangogiannis NG (2013) Fibroblasts in post-infarction inflammation and cardiac repair. BiochimBiophysActa 1833: 945-953.
- Ma Y, de Castro Brás LE, Toba H, Iyer RP, Hall ME, et al. (2014) Myofibroblasts and the extracellular matrix network in post-myocardial infarction cardiac remodeling. Pflugers Arch 466: 1113-1127.
- Roberts R, DeMello V, Sobel BE (1976) Deleterious effects of methylprednisolone in patients with myocardial infarction.Circulation 53: I204-206.
- Christia P, Frangogiannis NG (2013) Targeting inflammatory pathways in myocardial infarction. Eur J Clin Invest 43: 986-995.
- Eijkelenboom A, Burgering BM (2013) FOXOs: signalling integrators for homeostasis maintenance. Nat Rev Mol Cell Biol 14: 83-97.
- Wang Y, Zhou Y, Graves DT (2014) FOXO transcription factors: their clinical significance and regulation. Biomed Res Int 2014: 925350.
- Greer EL, Brunet A (2005) FOXO transcription factors at the interface between longevity and tumor suppression.Oncogene 24: 7410-7425.
- Sengupta A, Molkentin JD, Paik JH, DePinho RA, Yutzey KE (2011) FoxO transcription factors promote cardiomyocyte survival upon induction of oxidative stress. J BiolChem 286: 7468-7478.
- Ni YG, Berenji K, Wang N, Oh M, Sachan N, et al. (2006). Foxo transcription factors blunt cardiac hypertrophy by inhibiting calcineurin signaling. Circulation 114:1159-68.
- Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, et al. (2012) Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 122: 1109-1118.
- Liu ZP, Wang Z, Yanagisawa H, Olson EN (2005) Phenotypic modulation of smooth muscle cells through interaction of Foxo4 and myocardin.Dev Cell 9: 261-270.
- Li H, Liang J, Castrillon DH, DePinho RA, Olson EN, et al. (2007) FoxO4 regulates tumor necrosis factor alpha-directed smooth muscle cell migration by activating matrix metalloproteinase 9 gene transcription. Mol Cell Biol 27: 2676-2686.
- Zhou W, Cao Q, Peng Y, Zhang QJ, Castrillon DH, et al. (2009) FoxO4 inhibits NF-kappaB and protects mice against colonic injury and inflammation. Gastroenterology 137: 1403-1414.
- Zhu M, Zhang QJ, Wang L, Li H, Liu ZP (2011) FoxO4 inhibits atherosclerosis through its function in bone marrow derived cells. Atherosclerosis 219: 492-498.
- Zhu M, Goetsch SC, Wang Z, Luo R, Hill JA, et al. (2015) FoxO4 Promotes Early Inflammatory Response Upon Myocardial Infarction via Endothelial Arg1. Circ Res 117: 967-977.
- Pernow J, Jung C (2013) Arginase as a potential target in the treatment of cardiovascular disease: reversal of arginine steal? Cardiovasc Res 98: 334-343.
- Morris SM Jr (2012) Arginases and arginine deficiency syndromes. CurrOpinClinNutrMetab Care 15: 64-70.
- Lundberg JO, Gladwin MT, Weitzberg E (2015) Strategies to increase nitric oxide signalling in cardiovascular disease. Nat Rev Drug Discov 14: 623-641.
- Kuo L, Hein TW (2013) Vasomotor regulation of coronary microcirculation by oxidative stress: role of arginase. Front Immunol 4: 237.
- Morris CR, Gladwin MT, Kato GJ (2008) Nitric oxide and arginine dysregulation: a novel pathway to pulmonary hypertension in hemolytic disorders. CurrMol Med 8: 620-632.
- Xiong Y, Yu Y, Montani JP, Yang Z, Ming XF (2013) Arginase-II induces vascular smooth muscle cell senescence and apoptosis through p66Shc and p53 independently of its L-arginine urea hydrolase activity: implications for atherosclerotic plaque vulnerability. J Am Heart Assoc2:e000096.
- Jung C, Gonon AT, Sjöquist PO, Lundberg JO, Pernow J (2010) Arginase inhibition mediates cardioprotection during ischaemia-reperfusion. Cardiovasc Res 85: 147-154.
- Toque HA, Nunes KP, Yao L, Xu Z, Kondrikov D, et al. (2013) Akita spontaneously type 1 diabetic mice exhibit elevated vascular arginase and impaired vascular endothelial and nitrergic function.PLoS One 8: e72277.
Citation: Liu ZP (2015) The Role of FoxO4 in Post-Myocardial Infarction Left Ventricular Remodeling. Atheroscler open access 1: 101.
Copyright: © 2015 Liu ZP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Usage
- Total views: 15731
- [From(publication date): 2-2016 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 10970
- PDF downloads: 4761