Research Article Open Access
The Value of HER2 neu and EphA2 expressions in Gastric Adenocarcinoma Prognosis
Ola A Harb1*, Hanaa A Atwa1, Rasha Haggag2, Shereen El-Shorbagy2, Lobna A Abdelaziz3, Safa A Balata3, Fady M Habib4 and Loay M Gertallah4
1Department of Pathology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
2Department of Medical Oncology, Faculty of Medicine, Zagazig University, Zagazig, Egypt
3Department of Clinical Oncology and nuclear medicine, Faculty of Medicine, Zagazig University, Zagazig, Egypt
4Department of General Surgery, Faculty of Medicine, Zagazig University, Zagazig, Egypt
- *Corresponding Author:
- Ola A Harb
Department of Pathology
Faculty of Medicine, Zagazig University
Zagazig, Egypt
Tel: 01224963123
E-mail: olaharb2015@gmail.com
Received date: March 12, 2017; Accepted date: March 31, 2017; Published date: April 07, 2017
Citation: Harb OA, Atwa HA, Haggag R, El-Shorbagy S, Abdelaziz LA, et al. (2016) The Value of HER2 neu and EphA2 expressions in Gastric Adenocarcinoma Prognosis. J Gastroint Dig Syst 7:498. doi: 10.4172/2161-069X.1000498
Copyright: © 2017 Harb OA, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Gastrointestinal & Digestive System
Abstract
Background: Gastric adenocarcinoma (GAC) is a serious disease with poor outcome. Discovering novel molecular targeted therapies is a recent point of research to improve prognosis. One of the newly discovered targets is the receptor tyrosine kinases (RTKs); it is a member of trans-membrane receptors which had important roles in proliferation and apoptosis. RTKs were found to have different expression patterns in several malignancies. HER2 neu which is a member of HER family is a proto-oncogene that is formed of four receptor tyrosine kinases. Erythropoietinproducing hepatocellular (Eph) molecules are major RTKs members and one of those molecules is EphA2 which has many different functions in cancer as tumor initiation, progression, angiogenesis and spread. We aimed to explore the expression patterns of both HER2 neu and EphA2 in GAC patients using immunohistochemistry, and to correlate their expressions with clinico-pathological factors and prognosis of our patients Methods: HER2 neu and EphA2 expressions were assessed in sections from forty blocks of paraffin which were diagnosed as GAC. Then we analyzed the correlations between their expressions and disease outcome of GAC patients. Results: HER2 neu and EphA2 positive expressions in GAC were positively correlated with tumor grade and stage (p<0.001 and p=0.002 respectively), inadequate response to therapy (p<0.001 and p=0.002 respectively), increase recurrence rate of GAC (p=0.002), and with poor survival (p<0.001). Conclusion: GAC patients with high expressions of both HER2 neu and EphA2 had unfavorable prognosis.
Keywords
Gastric adenocarcinoma; HER2 neu; EphA2; Prognosis
Introduction
Gastric adenocarcinoma (GAC) is the 4th common malignancy, the 2nd cause of fatality among all cancers worldwide [1], and the 11th among cancers of the gastrointestinal tract in Egypt [2]. GAC is a fatal disease with a very poor outcome when discovered in advanced and inoperable stage. Discovering novel molecular targets is a current point of research aiming to improve the prognosis. One of the newly discovered targets is the receptor tyrosine kinases (RTKs) that are member of trans-membrane receptors which have an important role in controlling the cellular proliferation and apoptosis. They have different expression patterns in cancers [3] HER2 neu which is a member of the human epidermal receptor family, is a proto-oncogene encoded on chromosome 17, and formed of four receptors tyrosine-kinases which transport signals that are outside the cells to inside cells so as to begin intracellular signaling pathways by many signal transducing agents. The major roles of HER2 neu in tissues like gastrointestinal tract, the breast, heart and kidneys are; increasing cell proliferation, decreasing apoptosis; which may facilitate uncontrolled cell growth, and also stimulating oncogenesis [4,5]. Studies addressing the association between HER2-neu expression and GAC patient’s prognosis were conflicting as some studies demonstrated positive correlation between HER2-neu expression and GAC patient’s prognosis while other studies failed to demonstrate similar results [6-8]. Erythropoietin-producing hepatocellular (Eph) molecules are major detected RTKs members that is detected at lower levels in non-neoplastic-epithelial cells [9], and it has many different functions in cancers as tumor initiation, progression, angiogenesis and spread [10,11]. EphA2 receptor is a RTK-Ras signaling component [12]. There are few studies that had analyzed the correlations between HER2 neu and EphA2 expressions and gastric carcinoma prognosis, and no previous studies assessed the expressions of both markers together in GAC. In this study, we aimed to explore the expressions of both markers in GAC patients using immunohistochemistry and correlate their expressions with clinicopathological markers and the prognosis.
Patients and Methods
This prospective study was carried out at Zagazig University Hospitals, the study protocol was approved by the Ethical Committee of Faculty of Medicine, Zagazig University, it comprised 40 diagnosed GAC, and we used the TNM-staging-system modified by AJCC-Cancer-Staging-Manual 7th edition for staging of gastric adenocarcinoma [13,14]. HER2 neu and EphA2 expressions were assessed in sections from forty blocks of paraffin which were diagnosed as GAC in Pathology-department, in the period between 2011 and 2015. Patient’s data and follow up was done at medical oncology department and clinical oncology and nuclear medicine department, faculty of medicine, Zagazig University. Correlations between the expression patterns of both markers and patient’s prognosis were analyzed.
Inclusion criteria: Patients with histologically confirmed GAC.
Exclusion criteria: Concurrent or history of other malignancy.
All patients were subjected to the following: Detailed history taking from patients, full physical-examinations, hematological and biochemical laboratory evaluation (complete blood count (CBC), liver functions and kidney functions tests), CT chest, abdomen and pelvis and bone scan if needed. Upper GI endoscopy was done and three to four biopsies were collected. Total gastrectomy and lymph node dissection was done for operable patients with a minimum of 15 lymph nodes removed. Fluorouracil (5-FU) based chemotherapy regimens were administered according to the tumor stage, radiotherapy is given if needed; response to treatment was evaluated by physicalexamination, CT abdomenand pelvis, CT chest. All patients were followed by clinical examination and radiological evaluation every 3-4 months for 2 years.
Immunohistochemical staining
Streptavidine-biotin technique was used for immune histochemical staining with primary monoclonal mouse anti- HER-2/neu Ab- 20 (L87+2ERB19) diluted 1/200 at 4°C overnight (Thermo Fisher Scientific, Lab Vision Corporation, Fremont, USA)and primary anti- EphA2 (D4A2) XP® Rabbit mAb at a dilution of 1:200 (Cell Signaling Technology) [15].
Evaluation of immunohistochemical expression of HER2 neu as used in TOGA trial
The staining was membranous and the degree of immunostaining was scored as followed: 0: absent-reactivity or only membranous reactivity in less than ten percent of cancer cells, one: Faint or barely perceptible membranous reactivity in more than or equal to ten percent of cancer cells; cells are reactive only in part of their membrane, two: Weak to moderate complete, basolateral or lateral membranous reactivity in more than or equal ten percent of cancer cells and Three: Strong complete, basolateral or lateral membranous reactivity in more than or equal ten percent of tumor cells [16].
Evaluation of immunohistochemical expression of EphA2
We consider only cytoplasmic staining as positive for EphA2, ten fields for all sections were selected randomly, assessed and graded then we evaluated the extent of stain and gave it scores 0, 1, 2 and 3 (0=0–5%; 1=6–25%; 2=26–50%; 3=more than 50%) and intensity of stain and gave it scores 0, 1, 2 and 3 (0=negative; one=weak intensity; two=moderate intensity; three=strong intensity), and summations of scores of both the intensity and extent of stain gave final scores from 0-6. We used score 3 as a cut off value above which was considered as over expression and below which was considered as low expression [9].
Statistical analysis
Our statistics were by using program of-SPSS 22.0, windows (USA, SPSS Inc., Chicago, IL,) and (Belgium, MedCalc Software bvba 13, Ostend,). Percent of categorical variables were compared using Pearson’s Chi-square or Fisher’s exact tests when any one of them was appropriate. DFS and OS were assessed using the method of Kaplan-Meier curve and log-rank test. A p-value <0.05 was considered significant.
Results
Patient-data
GAC patient’s data that were included in the study are summarized in Table 1. There were 28 (70%) males and 12 (30%) females with age ranged from (40-80) years (Mean: 59.97 ± 9.20 years), 36 (90%) cases were intestinal type and 4 (10%) cases were diffuse type adenocarcinoma.
| Characteristics | Number | % | Characteristics | Number | % |
| Age (year) | AJCC stage | ||||
| Mean ± SD | 59.97 ± 9.20 | Stage IB | 6 | 15% | |
| Median (Range) | 60 (40-80) | Stage IIA | 5 | 12.50% | |
| <60 years | 14 | 35% | Stage IIB | 6 | 15% |
| ≥60 years | 26 | 65% | Stage IIIA | 4 | 10% |
| Sex | Stage IIIB | 9 | 22.50% | ||
| Male | 28 | 70% | Stage IIIC | 10 | 25% |
| Female | 12 | 30% | EphA2 | ||
| Initial site | Negative | 18 | 45% | ||
| Proximal | 24 | 60% | Positive | 22 | 55% |
| Distal | 12 | 30% | HER2 neu | ||
| Diffuse | 4 | 10% | Negative | 20 | 50% |
| Size | Positive | 20 | 50% | ||
| <5 cm | 17 | 42.50% | EphA2 and HER2 neu | ||
| >5 cm | 23 | 57.50% | Negative/Negative | 17 | 42.50% |
| Histopathologicalsu type | Negative/Positive | 1 | 2.50% | ||
| Intestinal | 36 | 90% | Positive/Negative | 3 | 7.50% |
| Diffuse | 4 | 10% | Positive/Positive | 19 | 47.50% |
| Grade | Response to treatment | ||||
| Well differentiated | 12 | 30% | PD | 6 | 15% |
| Moderate differentiated | 20 | 50% | SD | 3 | 7.50% |
| Poor differentiated | 8 | 20% | PR | 3 | 7.50% |
| T | CR | 28 | 70% | ||
| T1b | 5 | 12.50% | NR | 9 | 22.50% |
| T2 | 7 | 17.50% | OAR | 31 | 77.50% |
| T3 | 6 | 15% | Follow-up | ||
| T4a | 11 | 27.50% | Mean ± SD | 26.02 ± 14.50 | |
| T4b | 11 | 27.50% | Median (Range) | 25 (6-58) | |
| N | Events | ||||
| N0 | 8 | 20% | Disease free | 12 | 30% |
| N1 | 11 | 27.50% | Relapse | 16 | 40% |
| N2 | 10 | 25% | Died | 21 | 52.50% |
| N3 | 11 | 27.50% | |||
Table 1: Clinicopathological features, immunohistochemical markers and disease outcome of our patients.
Immunohistochemical results (Tables 1-3 and Figures 1 and 2)
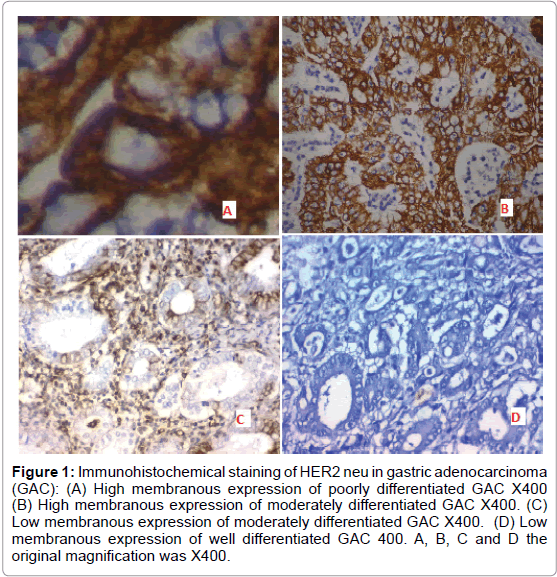
A) Positive expression of HER2 neu was detected in 20 out of 40 (50%) cases of adenocarcinoma of the stomach and it was significantly correlated with higher tumor grade, high incidence of L.N metastases and advanced stage of the tumor (p=0.002 and p<0.001 respectively) (Figure 1).
Figure 1: Immunohistochemical staining of HER2 neu in gastric adenocarcinoma (GAC): (A) High membranous expression of poorly differentiated GAC X400 (B) High membranous expression of moderately differentiated GAC X400. (C) Low membranous expression of moderately differentiated GAC X400. (D) Low membranous expression of well differentiated GAC 400. A, B, C and D the original magnification was X400.
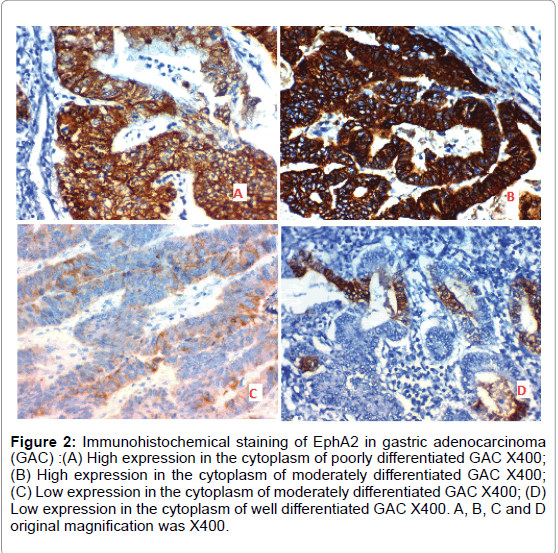
B) Positive expression of EphA2 was detected in 22 out of 40 (55%) cases of adenocarcinoma of the stomach and was significantly positively correlated with grade, L.N metastases and stage of the tumor (p=0.005, p=0.002 and p<0.001 respectively) (Figure 2).
Figure 2: Immunohistochemical staining of EphA2 in gastric adenocarcinoma (GAC) :(A) High expression in the cytoplasm of poorly differentiated GAC X400; (B) High expression in the cytoplasm of moderately differentiated GAC X400; (C) Low expression in the cytoplasm of moderately differentiated GAC X400; (D) Low expression in the cytoplasm of well differentiated GAC X400. A, B, C and D original magnification was X400.
C) The positive expression of both EphA2 and HER2 neu together in stomach adenocarcinoma was detected in 19 out of 40 cases and was significantly positively correlated with grade and stage of the tumor (p=0.002 and p<0.001 respectively) (Table 2).
| EphA2 | HER2/neu | p-value | |||||
| All (N=40) | Negative (N=18) |
Positive (N=22) | p-value | Negative (N=20) | Positive (N=20) |
||
| Characteristics | No. % | No. % | No. % | No. % | No. % | ||
| Age (years) | |||||||
| Mean ± SD | 59.97 ± 9.20 | 56.83 ± 9.20 | 62.54 ± 8.55 | 0.049* | 57 ± 8.64 | 62.95 ± 8.96 | 0.039* |
| Median (Range) | 60 (40-80) | 55 (40-75) | 63 (45-80) | ||||
| <60 years | 14 (35%) | 8 (57.1%) | 6 (42.9%) | 0.257‡ | 8 (57.1%) | 6 (42.9%) | 0.507‡ |
| ≥60 years | 26 (65%) | 10 (38.5%) | 16 (61.5%) | 12 (46.2%) | 14 (53.8%) | ||
| Sex | |||||||
| Male | 28 (70%) | 13 (46.4%) | 15 (53.6%) | 0.781‡ | 14 (50%) | 14 (50%) | 1.000‡ |
| Female | 12 (30%) | 5 (41.7%) | 7 (58.3%) | 6 (50%) | 6 (50%) | ||
| Initial site | |||||||
| Proximal | 24 (60%) | 11 (45.8%) | 13 (54.2%) | 0.126‡ | 13 (54.2%) | 11 (45.8%) | 0.105‡ |
| Distal | 12 (30%) | 7 (58.3%) | 5 (41.7%) | 7 (58.3%) | 5 (41.7%) | ||
| Diffuse | 4 (10%) | 0 (0%) | 4 (100%) | 0 (0%) | 4 (100%) | ||
| Size | |||||||
| <5 cm | 17 (42.5%) | 10 (58.8%) | 7 (41.2%) | 0.131‡ | 10 (58.8%) | 7 (41.2%) | 0.337‡ |
| Histopathological subtype | |||||||
| Intestinal | 36 (90%) | 18 (50%) | 18 (50%) | 0.114‡ | 20 (55.6%) | 16 (44.4%) | 0.106‡ |
| Diffuse | 4 (10%) | 0 (0%) | 4 (100%) | 0 (0%) | 4 (100%) | ||
| Grade | |||||||
| Well differentiated | 12 (30%) | 8 (66.7%) | 4 (33.3%) | 0.005§ | 9 (75%) | 3 (25%) | 0.002§ |
| Moderate differentiated | 20 (50%) | 10 (50%) | 10 (50%) | 11 (55%) | 9 (45%) | ||
| Poor differentiated | 8 (20%) | 0 (0%) | 8 (100%) | 0 (0%) | 8 (100%) | ||
| T | |||||||
| T1b | 5 (12.5%) | 5 (100%) | 0 (0%) | <0.001§ | 5 (100%) | 0 (0%) | <0.001§ |
| T2 | 7 (17.5%) | 6 (85.7%) | 1 (14.3%) | 7 (100%) | 0 (0%) | ||
| T3 | 6 (15%) | 5 (83.3%) | 1 (16.7%) | 5 (83.3%) | 1 (16.7%) | ||
| T4a | 11 (27.5%) | 2 (18.2%) | 9 (81.8%) | 3 (27.3%) | 8 (72.7%) | ||
| T4b | 11 (27.5%) | 0 (0%) | 11 (100%) | 0 (0%) | 11 (100%) | ||
| N | |||||||
| N0 | 8 (20%) | 5 (62.5%) | 3 (37.5%) | 0.002§ | 6 (75%) | 2 (25%) | 0.002§ |
| N1 | 11 (27.5%) | 9 (81.8%) | 2 (18.2%) | 8 (72.7%) | 3 (27.3%) | ||
| N2 | 10 (25%) | 3 (30%) | 7 (70%) | 5 (50%) | 5 (50%) | ||
| N3 | 11 (27.5%) |
1 (9.1%) |
10 (90.9%) |
1 (9.1%) | 10 (90.9%) | ||
| AJCC stage | |||||||
| Stage IB | 6 (15%) | 6 (100%) | 0 (0%) | <0.001§ | 6 (100%) | 0 (0%) | <0.001§ |
| Stage IIA | 5 (12.5%) | 5 (100%) | 0 (0%) | 5 (100%) | 0 (0%) | ||
| Stage IIB | 6 (15%) | 4 (66.7%) | 2 (33.3%) | 6 (100%) | 0 (0%) | ||
| Stage IIIA | 4 (10%) | 3 (75%) | 1 (25%) | 2 (50%) | 2 (50%) | ||
| Stage IIIB | 9 (22.5%) | 0 (0%) | 9 (100%) | 1 (11.1%) | 8 (88.9%) | ||
| Stage IIIC | 10 (25%) | 0 (0%) | 10 (100%) | 0 (0%) | 10 (100%) | ||
| EphA2 | |||||||
| Negative | 18 (45%) | 17 (85%) | 3 (15%) | <0.001‡ | - | - | |
| Positive | 22 (55%) | 1 (5%) | 19 (95%) | ||||
| HER2/neu | |||||||
| Negative | 20 (50%) | - | - | 17 (94.4%) | 1 (5.6%) | <0.001‡ | |
| Positive | 20 (50%) | - | - | 3 (13.6%) | 19 (86.4%) | ||
| Categorical variables were expressed as number (percentage), continuous variables were expressed as mean ± SD and median (range). *: Independent samples Student's t-test; ‡: Chi-square test; §: Chi-square test for trend; p<0.05 is significant. | |||||||
Table 2: Correlation between clinicopathological features and immunohistochemical markers of our patients.
D) Expressions of both markers were significantly positively correlated with each other (p<0.001).
E) No significant correlation was found between patient’s age or sex, histopathological subtype, initial site, or size of the tumor with markers expression.
Treatment response and survival analysis (Table 3 and Figure 3)
| Outcome | EphA2 | p-value | Her2/neu | p-value | |||
| All (N=40) | Negative (N=18) | Positive (N=22) | Negative (N=20) | Positive (N=20) | |||
| No. (%) | No. (%) | No. (%) | No. (%) | No. (%) | |||
| Response to treatment | |||||||
| PD | 6 (15%) | 0 (0%) | 6 (27.3%) | 0.003‡ | 0 (0%) | 6 (30%) | 0.001‡ |
| SD | 3 (7.5%) | 0 (0%) | 3 (13.6%) | 0 (0%) | 3 (15%) | ||
| PR | 3 (7.5%) | 0 (0%) | 3 (13.6%) | 0 (0%) | 3 (15%) | ||
| CR | 28 (70%) | 18 (100%) | 10 (45.5%) | 20 (100%) | 8 (40%) | ||
| NR | 9 (22.5%) | 0 (0%) | 9 (40.9%) | 0.002‡ | 0 (0%) | 9 (45%) | 0.001‡ |
| OAR | 31 (77.5%) | 18 (100%) | 13 (59.1%) | 20 (100%) | 11 (55%) | ||
| Relapse | |||||||
| Absent | 12 (30%) | 11 (61.1%) | 1 (4.6%) | 0.016‡ | 11 (55%) | 1 (5%) | 0.088‡ |
| Present | 16 (40%) | 7 (38.9%) | 9 (40.9%) | 9 (45%) | 8 (40%) | ||
| DFS | |||||||
| Mean (month) | 36.44 | 44.33 month | 20.67 month | <0.001† | 42.50 month | 19.14 month | <0.001† |
| (95% CI) | (26.64-43.25) | (36.37-52.30) | (18.85-22.49) | (34.95-50.05) | (17.98-20.31) | ||
| HR (95%CI) | - | 5.374 (1.814 – 15.922) | 12.547 (2.604 - 60.469) | ||||
| 12 month DFS (%) | 1 | 1 | 1 | 1 | 1 | ||
| 24 month DFS (%) | 0.629 | 0.833 | 0.222 | 0.85 | 0.714 | ||
| 36 month DFS (%) | 0.407 | 0.611 | - | 0.55 | - | ||
| 48 month DFS (%) | 0.407 | 0.611 | - | 0.55 | - | ||
| Mortality | |||||||
| Absent | 19 (47.5%) | 16 (88.9%) | 3 (13.6%) | <0.001‡ | 18 (90%) | 1 (5%) | <0.001‡ |
| Present | 21 | 2 (11.1%) | 19 (86.4%) | 2 (10%) | 19 (95%) | ||
| OS | |||||||
| Mean (month) | 35.25 | 54.13 month | 17.15 month | <0.001† | 54.71 month | 15.38 month | <0.001† |
| (95%CI) | (28.46-42.04) | (49.05-59.20) | (13.37-20.93) | (50.42-58.99) | (11.95-18.82) | ||
| HR (95%CI) | - | 19.821 (4.422-88.844) | 244.569 (3.925-15241.186) | ||||
| 12 month OS (%) | 0.75 | 1 | 0.545 | 1 | 0.5 | ||
| 24 month OS (%) | 0.592 | 0.944 | 0.298 | 1 | 0.167 | ||
| 36 month OS (%) | 0.446 | 0.882 | - | 0.882 | - | ||
| 48 month OS (%) | 0.446 | 0.882 | - | 0.882 | - | ||
| Categorical variables were expressed as number (percentage); ‡: Chi-square test; †: Log rank test; HR: Hazards Ratio; 95% CI: 95% Confidence Interval; p<0.05 is significant. | |||||||
Table 3: Correlation between immunohistochemical markers and disease outcome of our patients.
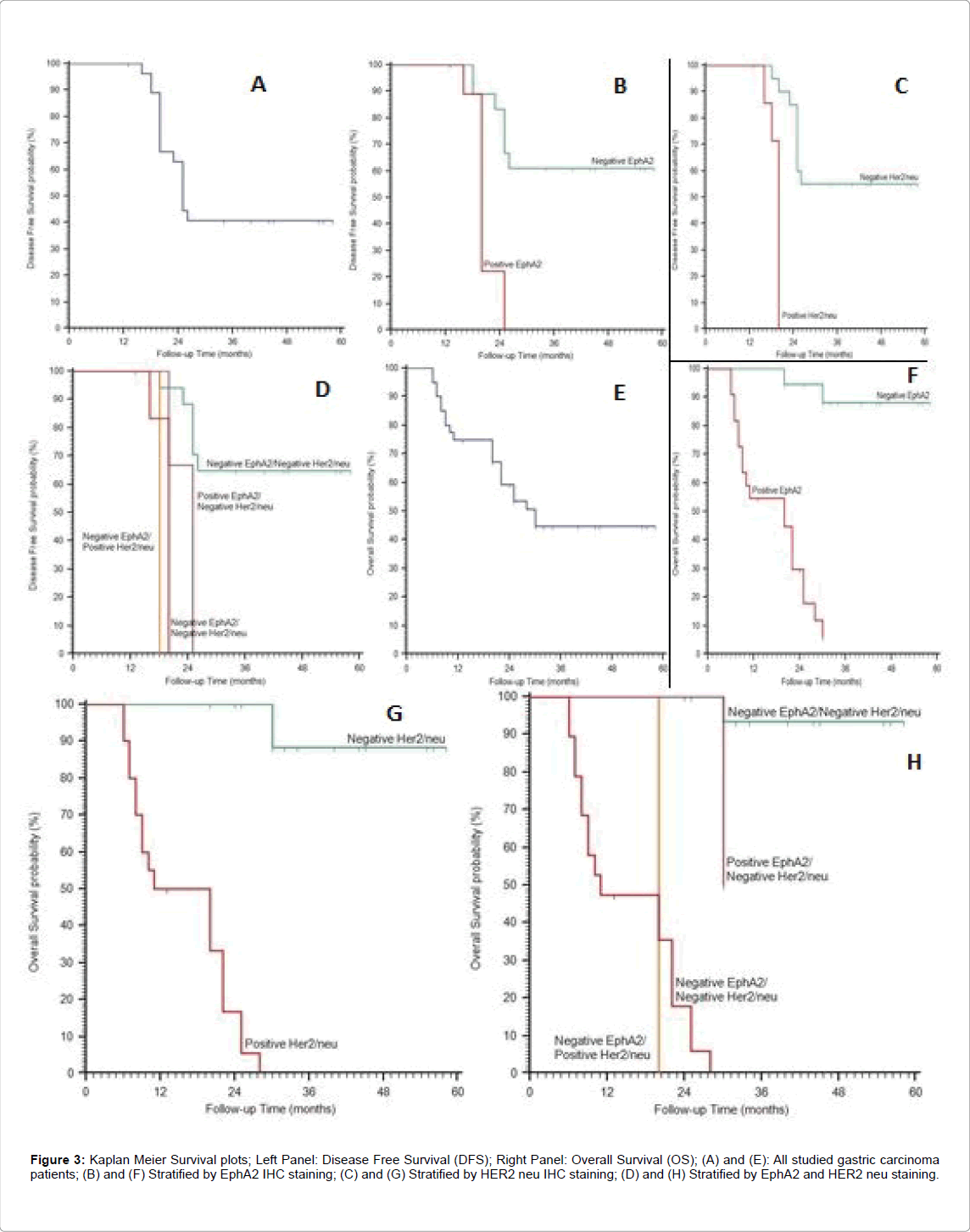
Figure 3: Kaplan Meier Survival plots; Left Panel: Disease Free Survival (DFS); Right Panel: Overall Survival (OS); (A) and (E): All studied gastric carcinoma patients; (B) and (F) Stratified by EphA2 IHC staining; (C) and (G) Stratified by HER2 neu IHC staining; (D) and (H) Stratified by EphA2 and HER2 neu staining.
Therapy response: Negative expression of EphA2 and HER2 neu were significantly associated with better response to therapy (p=0.002 p<0.001 respectively).
Tumor Recurrence: Positive expression of EphA2 was significantly associated with increase the incidence of tumor recurrence (p=0.002), but no significant relation was found between expression pattern of HER2 neu and tumor recurrence.
Survival analysis: Positive expressions of EphA2 and HER2 neu were significantly associated with shortened 2-year disease free survival (DFS) and 2-year overall survival (OS) (p<0.001).
Discussion
Many researchers had investigated the prognostic role of HER2 expression in cancers of many organs [11]. Previous studies have reported that the frequency of IHC detection of HER2 overexpression in GC varies from 10% to 22.1% [17], while positive expression of HER2 neu was detected in 50% of our cases and this high percentage of positive expression may be due to small patients number or due to inclusion of early as well as advanced stages in our study.
In our research we found that positive expression of HER2 neu was correlated with higher tumor grade, high incidence of L.N metastases and advanced stage of the cancer. Our results were close to Leni et al. [18] who reported that HER2 overexpression was more frequent in advanced GAC with high grade and advanced stage, and that was significantly associated with high disease recurrence and poor prognosis. Our results were consistent with Jia et al. who reported that HER2 over-expression was correlated with increased depth of invasion (P=0.045), lymph-nodes metastasis (P=0.026), and elevated clinical stage (P=0.026) but was not significantly associated with patient age, gender or cancer location [17]. Our results may be explained by that HER2 overexpression leads to increase cellular proliferation and inhibits apoptosis resulting in uncontrolled and excessive growth and spread of cancer.
Other different results were detected by Park et al. and Oh et al. [19,20] who stated that gastric tumor with HER2 neu amplification was only associated with old age and tumor size but it had no relation to prognosis. This discrepancy may be due the use of different immunohistochemical clones, the number of examined cases or the selection criteria that implied further study on a larger scale. Tessa and Raghuveer [21] assessed the expression HER-2 in cervical cancer and proved that it was positively associated with increasing the grade of cancer, presence of lymph node metastases and parametrial spread which was in agree with our results. Our results were also compatible with Park et al. [22] who studied Her2 amplification in colon cancer and reported that it was associated with higher rates of nodal metastasis and decreased patient survival. Hence, HER2 neu overexpression was found to be a prognostic factor for GAC and was negatively correlated with survival rates that were similar to results of Zhang et al. [23] and Park et al. [19] however, Jeung et al. [24] found no significant relation between HER2 neu expression and grade or stage of GAC; such difference that may be related to the nature of studied group and their number.
Also we found that positive expression of EphA2 was positively correlated with tumor grade, L.N metastases and tumor stage (p=0.005, p=0.002 and p<0.001 respectively). The results were similar to Huang et al. [7] and may be explained by that EphA2 stimulates proliferation, migration and spread of GAC cells mainly by increasing the expression of the epithelial mesenchymal transition markers like snail, N-cadherin, b-catenin, stimulating the Wnt/b-catenin pathway and by inhibition of E-cadherin in GAC cells. EPHA2 is overexpressed in a wide range of cancers and is associated with poor prognosis [25]. Many recent studies investigated the RTKs such as EphA2 and reported them as targets for molecular therapy for GAC [26-29], and also proved that EphA2 overexpression was positively correlated with factors that controlled angiogenesis and invasion in cancer cells because EphA2 receptor activation allowed vascular endothelial growth factor (VEGF)-dependent endothelial cell transport, sprouting, survival and expression of metalloproteinase, and these may be the causes of the poor clinical outcome of cancer patients with EphA2 overexpression, moreover the EphA2-EphrinA1 signaling axis regulates many steps that are essential for carcinogenesis and stimulation of downstream molecules like the phosphatidyl inositol 3’ kinases (PI3K), mitogen activated protein kinases (MAPK) and integrins along with epidermal growth factor receptor(EGFR) that regulate cell adhesion, cancer cell growth, metastases and development of vascular network.
In our study, positive expression of both EphA2 and HER2 neu in GAC was significantly positively correlated with each other (p<0.001), and negative expression of EphA2 and HER2 neu was significantly associated with better response to therapy. In addition we found that positive expression of EphA2 was significantly associated with increased incidence of tumor recurrence (p=0.002), but no statistically significant differences was found between expression of Her2 neu positive expression and tumor recurrence [30]. Moreover, positive expressions of Eph A2 and HER2 neu were significantly associated with shortened 2-year disease free survival and 2-year overall survival (p<0.001). Our results are in agreement with Otsu et al. [31] who reported that recurrence-free survival was worse in HER2-positive cases (p=0.045). When the analysis was conducted with intestinal types of cancer, RFS was considerably worse in the HER2-positive group (p=0.011), and Jia et al. who proved a significant association between HER2 overexpression, poor clinical outcome, and therapeutic drug resistance [17]. Whereas, Baykara et al. [32] found that HER2 positive expression had no significant effect on median OS.
Our results also go with Miyazaki et al. [29] who found that high expression of EphA2 was significantly correlated with variables related to tumor progression and poorer disease-specific survival. It has been reported that membrane type-1 matrix metalloproteinase (MT1-MMP) on tumor cells cleaves EPHA2 at the extracellular domain and the resultant truncated and membrane-anchoring forms of EPHA2 promote oncogenic signaling [33]. These findings suggest that tumor cells contain truncated forms of EPHA2 on their cell surface which are important for EPHA2-targeting cancer therapy using monoclonal antibodies (mAbs) that mediate antibody-dependent cellular cytotoxicity (ADCC) [25].
Summary and Conclusion
Positive expressions of EphA2 and HER2 neu in gastric adenocarcinoma were positively correlated with worse clinicopathological parameters and poor prognosis of our patients, furthermore expressions of both markers were positively correlated with each other, significantly (p<0.001). We also proved that positive expressions of EphA2 and HER2 were significantly associated with shortened 2-year DFS and 2-year OS (p<0.001).
Recommendations
On the basis of our findings, we recommend that all patients with gastric cancer either early or advanced to get the chance to be tested for EphA2 and HER2 neu status at the time of initial diagnosis as that may detect and classify patients with poor prognosis and those who will be resistant to the currently used therapy, so they may be included and benefit from trials on recent molecular targeted therapy. Also further studies on large scale of cases of different types of cancers would add benefits to the results of our research.
References
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917
- El Bolkainy MN, Nouh MA, Farahat IG, El Bolkainy TN, Badawy OM (2013) Gastrointestinal cancer in pathology of cancer. The National Cancer Institute, Cairo University (4th edn) pp: 147-230.
- Boku N (2014) HER2-positive gastric cancer. Gastric Cancer 17: 1-12
- Olayioye MA (2001) Update on HER-2 as a target for cancer therapy: Intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res 3: 385-389.
- Menard S, Pupa SM, Campiglio M, Tagliabue E (2003) Biologic and therapeutic role of HER2 in cancer. Oncogene 22: 6570-6578.
- Janjigian YY, Werner D, Pauligk C, Steinmetz K, Kelsen DP, et al. (2012) Prognosis of metastatic gastric and gastroesophageal junction cancer by HER2 status: A European and USA International collaborative analysis. Ann Oncol 10: 2656-2662.
- Jorgensen JT, Hersom M (2012) HER2 as a prognostic marker in gastric cancer: A systematic analysis of data from the literature. J Cancer 3: 137-144.
- Kim JW, Im SA, Kim M, Cha Y, Lee KH (2012) The prognostic significance of HER2 positivity for advanced gastric cancer patients undergoing first-line modified FOLFOX-6 regimen. Anticancer Res 32: 1547-1553.
- Yuan WJ, Ge J, Chen ZK,Wu SB, Shen H, et al. (2009) Over-expression of EphA2 and EphrinA-1 in human gastric adenocarcinoma and its prognostic value for postoperative patients. Dig Dis Sci 54: 2410-2417
- Udayakumar D, Zhang G, Ji Z, Njauw CN, Mroz P, et al. (2011) EphA2 is a critical oncogene in melanoma. Oncogene 30: 4921-4929.
- Tandon M, Vemula SV, Mittal SK (2011) Emerging strategies for EphA2 receptor targeting for cancer therapeutics. Expert OpinTherap Targets 15: 31-51
- Huang D, Xiao D, Li G, Ma J, Chen P, et al. (2014) EphA2 promotes epithelial-mesenchymal transition through the Wnt/b-catenin pathway in gastric cancer cells. Oncogene 33: 2737-2747
- Washington K (2010) 7th Edition of the AJCC Cancer Staging Manual: Stomach. Ann SurgOncol 17: 3077-3079.
- Edge SB, Compton CC (2010) The American Joint Committee on Cancer: The 7th edition of the AJCC cancer staging manual and the future of TNM. Ann SurgOncol 17: 1471-1474.
- Hsu SM, Raine L, Fanger H (1981) Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: A comparison between ABC and unlabeled antibody (PAP) procedures. J HistochemCytochem 29: 577-580.
- Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, et al. (2010) Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive. The Lancet 376: 687-697.
- Jia YX, Li TF, Zhang DD, Fan ZM, Fan HJ, et al. (2016) The co-expression and prognostic significance of c-MET, fibroblast growth factor receptor 2, and human epidermal growth factor receptor 2 in resected gastric cancer: A retrospective study. Onco Targets Ther 9: 5919-5929.
- Ieni A, Barresi V, Rigoli L, Caruso RA, Tuccari G (2015) HER2 status in premalignant, early, and advanced neoplastic lesions of the stomach. Disease Markers 234851: 1-10.
- Park DI, Yun JW, Park JH, Oh SJ, Kim HJ, et al. (2006) HER- 2/neu amplification is an independent prognostic factor in gastric cancer. Dig Dis Sci 51: 1371-1379.
- Oh HS, Eom DW, Kang GH, Ahn YC, Lee SJ, et al. (2014) Prognostic implications of EGFR and HER-2 alteration assessed by immunohistochemistry and silver in situ hybridization in gastric cancer patients following curative resection. Gastric Cancer 17: 402-411.
- Tessa Joseph, Raghuveer VC (2015) HER-2/neu expression in cervical intraepithelial neoplasia and cervical carcinoma. Int J Biomed Adv Res 6: 47-52.
- Park DI , Oh SJ , Park SH , Yun JW, Kim HJ, et al. (2004) Clinical significance of HER-2/neu expression in colon cancer. Korean J Gastroenterol 44: 147-152.
- Zhang XL, Yang YS, Xu DP, QU JH, Guo MZ, et al. (2009) Comparative study on overexpression of HER2/neu and HER3 in gastric cancer. World J Surg 33: 2112-2118.
- Jeung J, Patel R, Vila L, Wakefield D, Liu C (2012) Quantitation of HER2/neu expression in primary gastroesophageal adenocarcinomas using conventional light microscopy and quantitative image analysis. Arch Pathol Lab Med 136: 610-617.
- Hasegawaa J, Suea M, Yamatoa M, Ichikawa J, Ishida S, et al. (2016) Novel anti-EPHA2 antibody, DS-8895a for cancer treatment. Cancer BiolTher 17: 1158-1167.
- Yuan W, Chen Z, Chen Z, Wu S, Guo J, et al. (2012) Silencing of EphA2 inhibits invasion of human gastric cancer SGC-7901 cells in vitro and in vivo. Neoplasma 59: 105-113.
- Hou F, Yuan W, Huang J, Qian L, Chen Z, et al. (2012) Overexpression of EphA2 correlates with epithelial-mesenchymal transition-related proteins in gastric cancer and their prognostic importance for postoperative patients. Med Oncol 29: 2691-2700
- Miyazaki K, Inokuchi M, Takagi Y, Kato K, Kojima K, et al. (2013) EphA4 is a prognostic factor in gastric cancer. BMC ClinPathol 13: 19-27.
- Walker-Daniels J, Hess AR, Hendrix MJ, Kinch MS (2003) Differential regulation of EphA2 in normal and malignant cells. Am J Pathol 162: 1037-1042
- Yvonne L, Liz Y, Aparna A, William M, Charles N, et al. (2007) EphA2 overexpression is associated with angiogenesis in ovarian cancer. Cancer 109: 332-340
- Otsu H, Oki E, Ikawa-Yoshida A, Kawano H, Ando K, et al. (2015) Correlation of HER2 expression with clinicopathological characteristics and prognosis in resectable gastric cancer 35: 2441-2446.
- Baykara M, Benekli M, Ekinci O, Irkkan SC, Karaca H, et al. (2015) Clinical Significance of HER2 Overexpression in Gastric and Gastroesophageal Junction Cancers. J Gastrointest Surg 19: 1565-1571.
- Koshikawa N, Hoshino D, Taniguchi H, Minegishi T, Tomari T, et al. (2015) Proteolysis of EphA2 converts it from a tumor suppressor to an oncoprotein. Cancer Res 75: 3327-3339.
Relevant Topics
- Constipation
- Digestive Enzymes
- Endoscopy
- Epigastric Pain
- Gall Bladder
- Gastric Cancer
- Gastrointestinal Bleeding
- Gastrointestinal Hormones
- Gastrointestinal Infections
- Gastrointestinal Inflammation
- Gastrointestinal Pathology
- Gastrointestinal Pharmacology
- Gastrointestinal Radiology
- Gastrointestinal Surgery
- Gastrointestinal Tuberculosis
- GIST Sarcoma
- Intestinal Blockage
- Pancreas
- Salivary Glands
- Stomach Bloating
- Stomach Cramps
- Stomach Disorders
- Stomach Ulcer
Recommended Journals
Article Tools
Article Usage
- Total views: 4299
- [From(publication date):
April-2017 - Aug 29, 2025] - Breakdown by view type
- HTML page views : 3306
- PDF downloads : 993