Therapeutic Applications and Benefits from Postsurgical Use of the Phytotherapeutic Bromelain in Otorhinolaryngology: A Non- Interventional Study
Received: 24-Nov-2017 / Accepted Date: 05-Dec-2017 / Published Date: 12-Dec-2017 DOI: 10.4172/2161-119X.1000337
Abstract
Background: Bromelain is a phytotherapeutic drug that is well established in medicinal use for the treatment of injuries and postoperative swelling. It is frequently recommended in otorhinolaryngological indications. However, there is lack of recent clinical data on its use in this setting.
Methods: We conducted a non-interventional study including 102 patients to evaluate postsurgical clinical (pain, edema, hematoma, signs of inflammation) and specific nasal symptoms (hindrance of nasal breathing, impairment of sleep, impairment of food intake, impairment of smell, impairment of taste). The participating physicians furthermore assessed the potential additional benefits of bromelain therapy.
Results: Clinical and specific nasal symptoms improved significantly during postoperative recovery and an additional benefit was assigned by the physicians given ratings in more than fifty percent of the documented cases. According to the physicians, the consumption of analgesics was “low” in seventy percent of the patients during adjunctive therapy with bromelain.
Conclusion: Given the good tolerability of bromelain treatment, this study substantiates its safe and efficacious use in otorhinolaryngological practice.
Keywords: Bromelain; Surgery; Otorhinolaryngology; Nose; Sinuses; Clinical trial
Introduction
Bromelain is a crude, aqueous extract isolated from the stem of the pineapple plant (Ananas comosus L. , Bromeliaceae), constituting a complex mixture of proteases, enzyme inhibitors, further protein compounds as well as low-molecular-weight substances, among others. The phytotherapeutic drug has been used since decades for the treatment of inflammatory diseases, injuries and postsurgical conditions [1-3]. The mode of action of bromelain has yet to be clarified in detail [4], even though various biological activities have been described that may contribute to or may be considered as, a consequence of the anti-inflammatory action, the reduction in pain and edema-protective, edema-reducing properties [5-12]. In particular due to its anti-edematous and pain-reducing properties, bromelain is used in order to enhance postoperative recovery and thereby may contribute to a decrease in the use of non-steroidal anti-inflammatory drugs (NSAIDs).
Objective assessment of the anti-edematous effects of drugs remains a challenge. As an investigation in patients after wisdom teeth extraction [13]. Such sensitive test methods are not available for all clinical settings, however. In particular with regard to indications associated with edema and pain sensation, some authors argue that varying active pharmaceutical ingredients and formulations may exhibit differing potencies depending on the underlying type of pain and specific surgical procedures [14,15]. Exception, a beneficial effect of bromelain treatment on postoperative swelling could be persuasively demonstrated by use of a validated 3D face scanning method in a recent clinical
We therefore initiated a multicentric, prospective, noninterventional study in order to obtain further insight into the therapeutic applications and benefits of bromelain and to assess the efficiency of this phytotherapeutic after various surgical interventions within the scope of routine care in an otorhinolaryngological outpatient setting. There were no further instructions with regard to surgical technique or type of underlying disease, nor any prespecified exclusion criteria beyond the information given in the Summary of Product Characteristics.
Materials and Methods
Trial design
This trial is a prospective, multicentric, non-interventional study in patients who underwent otorhinolaryngological surgery and were prescribed bromelain following surgery. The study was carried out in 16 trial centers throughout Germany during the period February- December 2016. Patients included in the study were supposed to be above the age of 12. Known hypersensitivity to the active substance bromelain, to pineapple fruit or any of the excipients of the product represented a contraindication to the intake of study medication. Due to the non-interventional character of the trial, no further inclusion or exclusion criteria were defined beyond the information given in the Summary of Product Characteristics. However, the decision to prescribe bromelain to the respective patient must have been made by the physician before the inclusion of the patient in the trial and patients were enrolled only after they had been given sufficient information and had provided written consent concerning their participation in the trial. Each patient enrolled was subject to an initial, a follow-up and a final examination. Due to the non-interventional character of the study, the chronological sequence of the visits was not prespecified.
The trial was performed according to the German Drug Law, registered at the German Federal Institute for Drugs and Medical Devices (NIS study no. 6694) and in the German Clinical Trial Register (DRKS-ID: DRKS00010112) and was approved by the Institutional Review Board of the Freiburg Ethics Commission International (Code: 015/1616).
Trial medication
Medication used in this trial was manufactured at Ursapharm Arzneimittel GmbH, Saarbrücken, Germany. Bromelain tablets (Bromelaintabletten hysan®) were standardized to an enzymatic activity of 500 F.I.P. (Fédération Internationale Pharmaceutique) units per tablet. Generally, patients were advised to take one bromelain tablet twice daily, in the morning and the evening before meals. However, due to the non-interventional principle of the study, varying dosing schemes could be recommended by the physicians as well.
Efficacy and safety measurements
All data for this study were recorded on a single case report form (CRF) that was filled in by the physician. Operative indication and surgical technique were documented at the initial examination and symptoms and clinical signs were subsequently documented at each visit (initial, follow-up and final examinations). Pain, edema, hematoma and signs of inflammation (e.g. erythema) were assessed on an ordinal scale of 1-5 (1=none, 2=mild, 3=moderate, 4=strong, 5=very strong) and the individual values were added to obtain an average score for each symptom and each visit. Additionally, physicians could document the intensity of pain using a visual analogue scale (VAS) or a numerical rating scale (NRS: 0-10). Average scores for all symptoms were added in order to obtain the Clinical sum score for each visit.
In order to evaluate the clinical benefit of bromelain therapy, the physicians involved were also asked to assess the extent of pain intensity, edema and hematoma during bromelain therapy as compared to the expected extent (less-equivalent-increased- not assessable).
Nasal postsurgical symptoms (hindrance of nasal breathing, impairment of sleep, impairment of food intake, impairment of smell, impairment of taste) were assessed at each visit by use of the abovementioned scale (1=none, 2=mild, 3=moderate, 4=strong, 5=very strong) and individual values were added to obtain an average score for each symptom and each visit. Addition of average scores for all symptoms yielded the Nasal sum score.
During the follow-up and final examinations, the physicians involved evaluated the average intake of analgesics (non-steroidal antiinflammatory drugs) as: low-normal-increased or not assessable.
Postsurgical wound-healing in general was assessed by the physician at the follow-up and final examinations (very good-good-satisfactorybad), whether wound healing was observed within the expected temporal course (yes-no) and how the physician assessed wound healing with bromelain as compared to a therapy without bromelain (better-equivalent-worse or not assessable).
During the final examination, the patients evaluated treatment satisfaction with bromelain (very good-good-satisfactory-bad) and whether they would use it again under similar conditions (yes-no). Physicians assessed treatment success of bromelain (very good-goodsatisfactory- bad), the compliance of the patient (very good-goodsatisfactory- bad), whether treatment with bromelain should be continued (yes-no), whether they would prescribe bromelain again under similar conditions (yes-possibly-no) and how they assessed the period until the resumption of activities of daily living as compared to treatment without bromelain (significantly shorter-shorter-no difference-longer-significantly longer).
Tolerability of the product was evaluated by the physicians at the final examination using the ratings “flawless”, “acceptable” and “not acceptable”. Adverse events and severe adverse events had to be documented in the case report form at every visit by the investigator. The occurrence of severe adverse events had to be reported to the sponsor of the study within 24 hours.
Sample size and statistical analysis
In this observational study without a specific primary endpoint, no a priori sample size calculation was performed. To get valid and reliable results regarding safety and efficacy, a maximum of 120 patients were planned to be included in the trial between February and December 2016.
Data are presented as mean ± standard deviation (SD) for continuous variables or as relative frequencies for categorical variables. Occasionally, percentages did not add up to 100% due to rounding. Symptom scores of each time point were compared using a repeated measures ANOVA. A p-value of<0.05 was considered as statistically significant. All analyses were performed using SAS 9.4 (SAS Institute, Cary, NC).
Altogether 16 medical practices participated in this noninterventional trial and a total of 102 patients (49 males, 53 females) were included in the study and received bromelain treatment (first patient in: 04.02.2016; last patient out: 06.12.2016). One patient was lost to follow-up at last visit, thus the ITT population included n=102 patients at baseline and first follow-up and n=101 at last follow-up visit. The safety population included all patients receiving bromelain (n=102). On average, first follow-up took place 8.18 (± 5.69) days after surgery and the last visit 20.87 (± 8.86) days after surgery.
Overall, compliance was assessed by the physicians as “very good” in 48%, as “good” in 47%, as “satisfactory” in 2% and as “bad” in 1% of the patients (2% missing values).
Demographic data, surgical indications and recommended bromelain dosages are given in Tables 1 and 2. No clinically relevant differences were observed with regard to average bromelain dosage when different surgical indications were checked against each other.
| N | Mean (± st. dev.) | Median (min-max) | |
|---|---|---|---|
| Sex | |||
| Male | 49 | ||
| Female | 53 | ||
| Age (years) | 102 | 39.30 ± 14.95 | 37.16 (14.21-80.44) |
| Height (cm) | 101 | 172.96 ± 8.05 | 172 (159-96) |
| Weight (kg) | 102 | 78.28 ± 16.62 | 76 (53-150) |
| BMI (kg/m2) | 101 | 26.17 ± 4.77 | 24.91 (17.83-44.79) |
Table 1: Baseline characteristics of included patients.
| N | |
|---|---|
| Surgical indication* | |
| Chronic rhinosinusitis | 37 |
| Maxillary sinus | 30 |
| Frontal sinus | 11 |
| Sphenoidal sinus | 13 |
| Ethmoidal air cells | 16 |
| Polyposis | 25 |
| Nose | 13 |
| Sinuses | 16 |
| Correction of the inner nose | 62 |
| Nasal septum | 41 |
| Nasal concha | 54 |
| Correction of the outer nose | 8 |
| Crooked nose | 1 |
| Hump nose | 3 |
| Tension nose | 1 |
| Nose base/nostril correction | 3 |
| Hindrance of nasal breathing | 72 |
| Tonsillectomy | 2 |
| Hyposmia | 2 |
| Snoring | 1 |
| Dosage recommendation | |
| 2 × 1 tablet | 59 |
| 3 × 1 tablet | 33 |
| 1 × 1 tablet | 9 |
| 1 × 2 tablets | 1 |
Table 2: Surgical indications and dosage recommendations, *multiple answers possible.
Efficacy endpoints
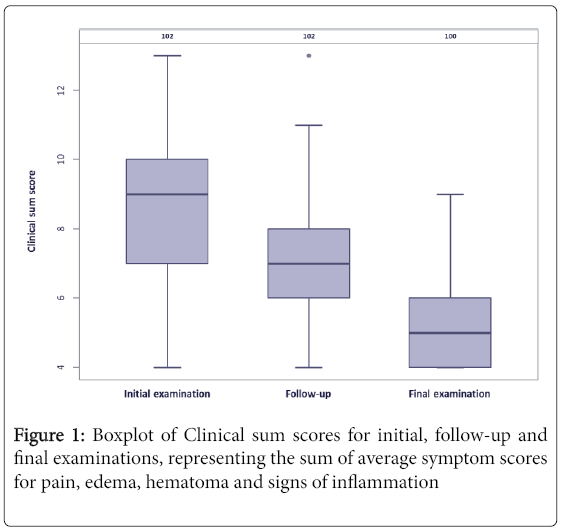
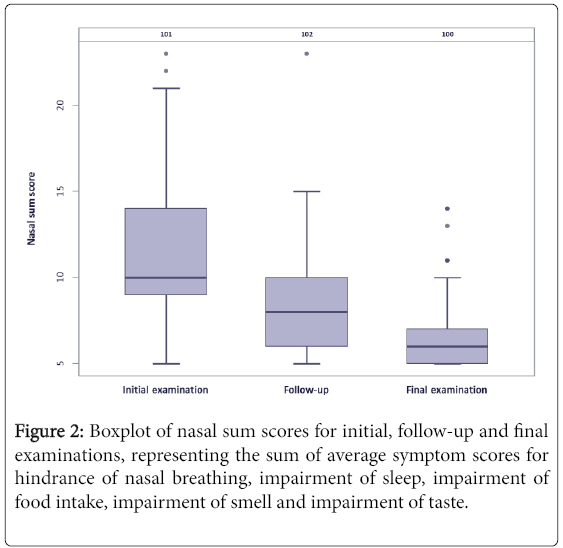
Clinical sum scores (sum of average symptom scores for pain, edema, hematoma and signs of inflammation) and Nasal sum scores (sum of average symptom scores for hindrance of nasal breathing, impairment of sleep, impairment of food intake, impairment of smell and impairment of taste) for the initial, follow-up and final examinations are given in Figures 1 and 2.
The average scores for each clinical symptom decreased from 2.44 ± 0.9 (initial examination) over 1.77 ± 0.67 (follow-up) to 1.3 ± 0.48 (final examination) for pain (p<0.001), from 2.68 ± 0.94 over 2.02 ± 0.72 to 1.52 ± 0.59 for edema (p<0.001), from 1.57 ± 0.74 over 1.31 ± 0.53 to 1.08 ± 0.27 for hematoma (p<0.001) and from 1.87 ± 0.83 over 1.64 ± 0.76 to 1.23 ± 0.55 for signs of inflammation (p<0.001), respectively.
No physician used a visual analogue scale for the assessment of pain and only a few assessments using numerical rating scale were obtained (initial examination: 5.27 ± 2.60, n=15; follow-up: 2.40 ± 0.97, n=10; final examination: 1.67 ± 1.50, n=9).
At the final examination, physicians were furthermore asked to assess the extent of pain intensity, edema and hematoma during bromelain therapy as compared to the expected extent of the respective symptom. 46% of the physicians assessed the level of pain as “less” as compared to treatment without bromelain, 23% as “equivalent” and none as “increased” (11%: not assessable; 21%: information missing). Similarly, 69% of the physicians assessed edema as “less”, 26% as “equivalent” and 1% as “increased” (2%: not assessable; 2%: missing) and finally the physicians evaluated hematoma in 44% of the cases as “less pronounced than expected”, in 18% as “equivalent”, none as “increased” (15%: not assessable; 24%: missing).
Wound healing was assessed as “improved” (“better”) compared to treatment without bromelain in 66% of the patients, as “equivalent” in 25% and as “worse” in 2% of the cases (5%: not assessable; 2%: missing). Overall wound healing was observed as “very good” and “good” in 92% of the patients (6%: satisfactory; 2%: missing) and as being within the expected temporal course (94%: yes; 3%: no; 3%: missing).
At the final examination, physicians judged the use of analgesics as “low” in 68% of their patients. Patients receiving analgesics were prescribed diclofenac (2 patients), acetaminophen (5 patients), metamizole sodium (7 patients) or ibuprofen (19 patients).
Average scores for nasal postsurgical symptoms were 2.99 ± 0.95 (initial examination), 2.09 ± 0.85 (follow-up) and 1.6 ± 0.67 (final examination) for hindrance of nasal breathing, 2.71 ± 1.05, 1.75 ± 0.88 and 1.29 ± 0.61 for impairment of sleep, 1.67 ± 0.93, 1.27 ± 0.6 and 1.05 ± 0.3 for impairment of food intake, 2.45 ± 1.28, 1.83 ± 0.91 and 1.43 ± 0.79 for impairment of smell and 1.76 ± 1.04, 1.41 ± 0.76 and 1.16 ± 0.51 for impairment of taste.
30% of the patients assessed the treatment with bromelain as “very good” at their final examination, 58% as “good”, 8% as “satisfactory” and 1% as “bad” (with 3% missing values), which is consistent with the physicians’ evaluation (very good: 40%; good: 49%; satisfactory: 7%; bad: 2%; missing values: 2%). 88% of the patients would use bromelain again under similar conditions, 8% would rather not use it again, 4% missing values. Physicians would prescribe bromelain again in patients with identical diagnoses in 89% of the cases, would not prescribe it in 4% and would possibly prescribe it in 5% of the cases (2% missing values).
In 11% of the patients, the physicians appraised the period until the resumption of activities of daily living as compared to treatment without bromelain as “significantly shorter”, in 67% of the cases as “shorter”, in 20% no difference was observed and in 1% the period was assessed as “significantly longer” (2% missing values).
Safety evaluation
Tolerability was assessed as “flawless” in 79% of the patients, as “acceptable” in 16% and as “not acceptable” in 4% of the applications (1% missing values). Six adverse events were reported, of which four (twice: impairment of wound healing, pimples, gastritis) were assessed by the physicians as being “possibly related”, one (impairment of wound healing) as “probably related” and one (diarrhea) as “certainly related” to the intake of bromelain. One patient suffering from impairment of wound healing was lost to follow-up due to his not attending his last visit.
Discussion
The anti-edematous action of bromelain is presumably the most important therapeutic benefit during the postoperative convalescence period. This pharmacodynamic effect is well described in various experimental and clinical studies [10,11,13,16,17] even though the mechanism on which this effect is based is not yet entirely clear [4]. The proteolytic activity of bromelain, however, is essential [17,18] and a lowering of the kininogen concentration in the plasma following treatment with bromelain also appears to play a role [19]. Furthermore, an increase of tissue permeability by fibrinolysis and promotion of reabsorption of edema fluid into blood circulation have been discussed [20]. Such a concert of activities that give the overall effect tends to be the rule rather than the exception for complex phytotherapeutic compositions and also contributes to other effects, such as pain relief and reduction in hematoma as observed for bromelain.
The major aim of this study was to investigate the routine use of bromelain in postsurgical otorhinolaryngological outpatient care. The most common indications for surgical interventions in this study were corrections of the inner nose (62 times), hindrance of nasal breathing (72), polyposis (25) and chronic rhinosinusitis (37), whereby multiple answers were possible for each patient due to comorbidity of disorders.
During the observation period, intensity of pain declined on average by 47% from the initial to the final examination, edema by 43%, hematoma by 31% and signs of inflammation by 34%. Similarly, specific nasal symptoms improved significantly during the postsurgical convalescence period. Obviously, these results must be reflected against the background of, on the one hand, self-limitation of symptoms due to the postsurgical wound healing process and remedy of cause of the complaints (e.g. hindrance of nasal breathing) and, on the other hand, the uncontrolled study design with a limited number of patients included. We therefore asked the physicians about their experience and expectations regarding the manifestation of symptoms in the respective patient against the effect observed with bromelain therapy. Physicians giving ratings for pain, edema, hematoma and wound healing substantiated the efficiency of bromelain by consistently assigning an additional benefit in more than fifty percent of the patients for each of these symptoms. Furthermore, physicians rated cumulative intake of analgesics as “low” in 68% of the patients. Tolerability of bromelain can be assessed as good overall.
Only relatively old reports on randomized, placebo-controlled trials on the postsurgical use of bromelain in otorhinolaryngology are available [21] and these were confirmed by the present observational study, allowing for an additional estimate of the therapeutic effect. Non-interventional studies are generally advantageous with regard to a more realistic depiction of everyday clinical practice, which is also reflected in the demographic distribution of the patients included in this trial (Table 1).
Conclusion
Thus, taking together the observed benefits of bromelain within this study and with more recent studies confirming the efficacious and safe use of this drug in other surgical indications of the face, e.g. wisdom teeth extraction [13], this non-interventional study supports the sensible use of this phytotherapeutic as an adjunctive therapy in present daily otorhinolaryngological routine.
Acknowledgement
The authors would like to acknowledge and express thanks to all physicians involved in the BROHNO-2015 study consortium for their participation.
Funding
The trial was funded by URSAPHARM Arzneimittel GmbH. Peter Meiser is employed at URSAPHARM Arzneimittel GmbH.
Conflict of Interest
The authors have declared that there is no further conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
References
- Shing C, Chong S, Driller M, Fell J (2016) Acute protease supplementation effects on muscle damage and recovery across consecutive days of cycle racing. Eur J Sport Sci 16: 206-212.
- Maurer HR (2001) Bromelain: Biochemistry, pharmacology and medical use. Cell Mol Life Sci 58: 1234-1245.
- Pavan R, Jain S, Shraddja, Kumar A (2012) Properties and therapeutic application of bromelain: A review. Biotechnol Res Int 2012: 1-6.
- Meiser P, Xu Z, Kirsch G, Jacob C (2014) Systemic enzyme therapy: Fact or fiction? A Review with focus on bromelains, proteolytic enzymes from the pineapple plant. In: Recent Advances in Redox Active Plant and Microbial Products. Springer, pp: 449-467.
- Yuan G, Wahlqvist ML, He G, Yang M, Li D (2006) Natural products and anti-inflammatory activity. Asia Pac J Clin Nutr 15: 143-152.
- Secor jr E, Shah S, Guernsey L, Schramm C, Thrall R (2012) Bromelain limits airway inflammation in an ovalbumin-induced murine model of established asthma. Altern Ther Health Med 18: 9-17.
- Müller S, März R, Schmolz M, Drewelow B, Eschmann K, et al. (2012) Placebo-controlled randomised clinical trial on the immunomodulating activities of low and high dose bromelain after oral administration-new evidence on the anti-inflammatory mode of action of bromelain. Phytother Res 27: 199-204.
- Bhui K, Prasad S, George j, Shukla Y (2009) Bromelain inhibits COX-2 expression by blocking the activation of MAPK regulated NF-kappa B against skin tumor-initiation triggering mitochondrial death pathway. Cancer Lett 282: 167-176.
- Bhui K, Tyagi S, Srivastava A, Singh M, Roy P, et al. (2012) Bromelain inhibits nuclear factor kappa-B translocation, driving human epidermoid carcinoma A431 and melanoma A375 cells through G(2)/M arrest to apoptosis. Mol Carcinog 51: 231-243.
- Gaspani L, Limiroli E, Ferrario P, Bianchi M (2002) In vivo and in vitro effects of bromelain on PGE(2) and SP concentrations in the inflammatory exudate in rats. Pharmacology 65: 83-86.
- Uhlig G Seifert J (1981) The effect of proteolytic enzymes (traumanase) on posttraumatic edema. Fortschr Med 99: 554-556.
- Netti C, Bandi G, Pecile A (1972) Anti-inflammatory action of proteolytic enzymes of animal bacterial origin administered orally compared with antiphlogistic compounds. Farmaco Prat 27: 453-466.
- Bormann KH, Weber K, Kloppenburg H, Koch A, Meiser P, et al. (2016) Perioperative bromelain therapy after wisdom teeth extraction - A randomized, placebo-controlled, double-blinded, three-armed, cross-over dose-finding study. Phytother Res 30: 2012-2019.
- Gray A, Kehlet H, Bonnet F, Rawal N (2005) Predicting postoperative analgesia outcomes: NNT league tables or procedure-specific evidence? Br J Anaesth 94: 710-714.
- Ong CK, Lirk P, Tan CH, Seymour RA (2007) An evidence-based update on nonsteroidal anti-inflammatory drugs. Clin Med Res 5: 19-34.
- Moss JN, Frazier CV, Martin GJ (1963) Bromelains. The pharmacology of the enzymes. Arch Int Pharmacodyn Ther 145: 166-189.
- Enomoto T, Mineshita S, Shigei T (1968) Protective effect of stem bromelain against adrenaline pulmonary edema and its dependence on the proteolytic activity. Jpn J Pharmacol 18: 260-265.
- Shigei T, Sakuma A, Nishiwaki T (1967) A study on the protective effect on bromelain, crude pineapple proteases, against adrenaline pulmonary edema in rats. Jpn Heart J 8: 718-720.
- Oh-ishi S, Uchida Y, Ueno A, Katori M (1979) Bromelain, a thiolprotease from pineapple stem, depletes high molecular weight kininogen by activation of hageman factor. Thromb Res 14: 665-672.
- Smyth R, Brennan R, Martin GJ (1962) Systemic biochemical changes following the oral administration of a proteolytic enzyme, bromelain. Arch Int Pharmacodyn Ther 136: 230-237.
- Seltzer A (1964) A double-blind study of bromelains in the treatment of edema and ecchymoses following surgical and non-surgical trauma to the face. Eye Ear Nose Throat Mon 43: 54-57.
Citation: Matschke R, Zeman F, Huppertz G, Koller M, Meiser P (2017) Therapeutic Applications and Benefits from Postsurgical Use of the Phytotherapeutic Bromelain in Otorhinolaryngology: A Non-Interventional Study. Otolaryngol (Sunnyvale) 7:337. DOI: 10.4172/2161-119X.1000337
Copyright: © 2017 Matschke R, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 7418
- [From(publication date): 0-2017 - Dec 23, 2025]
- Breakdown by view type
- HTML page views: 6362
- PDF downloads: 1056