Research Article Open Access
Tramadol vs. Buprenorphine for the Treatment of Opioid Dependence: A Comparative Study
Mehak Pahwa*, BS Sidhu, Rajnish Raj and Eish KumarDepartment of Psychiatry, Government Medical College, Patiala, India
- Corresponding Author:
- Dr. Mehak Pahwa (garg)
Junior Resident Psychiatry
Department of Psychiatry, Government Medical College, Patiala, India
Tel: 91 905 188 8070
E-mail: mehakgarg14@gmail.com
Received date: May 29, 2015; Accepted date: August 06, 2015; Published date: August 12, 2015
Citation: Pahwa M, Sidhu BS, Raj R, Kumar E (2015) Tramadol vs. Buprenorphine for the Treatment of Opioid Dependence: A Comparative Study. J Addict Res Ther 6:239. doi:10.4172/2155-6105.1000239
Copyright: © 2015 Pahwa M, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Background: Tramadol is an atypical centrally acting synthetic analgesic and its o-demethylated metabolite, (+)-o-demethyltramadol (known as m1), has greater affinity for the mu-opioid receptor which makes it a candidate for opiate withdrawal treatment. , it appears to have low abuse potential and is a non-scheduled analgesic.
Aim and Objective: to compare the effects and relative clinical utility of Tramadol and Buprenorphine in the treatment of heroine withdrawal in opioid dependant patients.
Methods: Ina randomized open label parallel group design study, 70 patients with opioid dependence with daily heroine use equal to 1-10 mg, 10-20 mg, >20 mg methadone using stratified proportionate sampling were assigned into two treatment groups i.e. Tramadol and Buprenorphine. Flexible dosing schedule was followed and drug was titrated using (COWS) Clinical Opiate Withdrawal Scale and (CGI) Clinical Global Impression
Results: There was statistically significant difference between the two groups in patients with moderate level of addiction with 78% of Tramadol group and 57% of the Buprenorphine group completed detoxification at the end of 12 weeks. Whereas patients with severe level of addiction had to be maintained on buprenorphine and detoxification could not be completed with high dropout rate in tramadol group
Discussion: Tramadol has good efficacy in detoxification and relapse prevention in patients with moderate level of opioid dependence as compared to buprenorphine, Whereas Buprenorphine is better for maintenance treatment and is of higher clinical utility in severe level of opioid dependence where maintenance therapy is required.
Introduction
Many medications have been used over the past thirty years for the treatment of opioid withdrawal, including methadone, LAAM, propoxyphene, clonidine, parenteral buprenorphine, and, more recently, sublingual buprenorphine. Each has been found to have clinical strengths and limitations. Tramadol is an atypical centrally acting synthetic analgesic. In its parent form, tramadol exists as a racemic mixture of two active enantiomers which undergo hepatic biotransformation to form N- and O-demethylated compounds. The O-demethylated metabolite, (+)-O-demethyltramadol (known as M1), has greater affinity for the mu-opioid receptor than the parent compound and is responsible for tramadol’s mu-opioid activity, and is primarily due to its binding to the micro receptor [1]. Despite this micro receptor activity, tramadol appears to have low abuse potential and was approved as an unscheduled analgesic in the USA in 1994 based largely on epidemiologic experience, and a number of animal and human [2-4] studies suggested, it had low abuse potential. The pharmacologic profile of tramadol makes it a candidate for opiate withdrawal treatment; Whereas Buprenorphine has high abuse potential.
Aims and objective
To compare the effects and relative clinical utility of tramadol [5-8] and buprenorphine [9,10] in the treatment of heroine withdrawal in opioid dependant patients.
Materials and Methods
• Randomized open label parallel group design study was done in a deaddiction unit of tertiary care hospital in India (Punjab)
• Inclusion criteria for this study were Male sex, heroin as drug of choice, current opioid physical dependence (i.e., withdrawal symptoms), and no current abuse of oral opioid analgesics.
• Exclusion criteria included female sex, major mental illness (e.g., schizophrenia), significant medical problem (e.g., history of seizure or hypertension), no other concurrent drug abuse, i.e. alcohol or benzodiazepine.
• 82 male Patient in the age group of 20 to 50 years that met inclusion criteria were categorized into three groups as mild, moderate and severe on the basis of amount of daily heroin use, i.e. <10 mg of methadone equivalent, 10-20mg methadone equivalent and >20 mg methadone equivalent.
• There were 35 patients who met the criteria for mild drug use, 32 for moderate and 15 that met the criteria for severe drug use. Among these patients were matched for sociodemographic characteristics and Proportionate Stratified Random sampling was done and patients were randomly assigned in two treatment groups (i.e. tramadol and buprenorphine) in each category.
• There were 12 dropouts leaving total no of patients to be 70: 30 in the mild group (15 each), 28 in the moderate group (14 each category), and 12 in severe group (6 in each category).
• Baseline investigations were done and after 8-12 hours of last heroine intake and treatment was initiated with 2 mg of buprenorphine and 100 mg of tramadol which was gradually increased as per patient’s withdrawal symptoms.
• Flexible dosing schedule was followed and dose was titrated on the basis of objective and subjective evaluation using Clinical Opiate Withdrawal Scale (COWS) [11] and Clinical Global Impression (CGI) [12].
• Patients were detoxified in inpatient setting and followed with intensive outpatient treatment. Measurements using COWS and CGI were taken at every alternate week and patients were followed up for 12 weeks.
Results
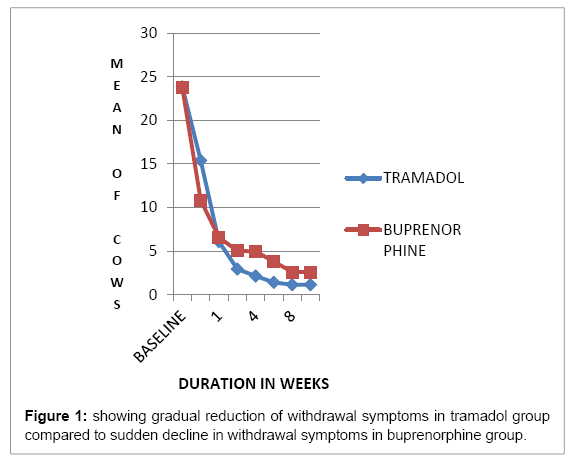
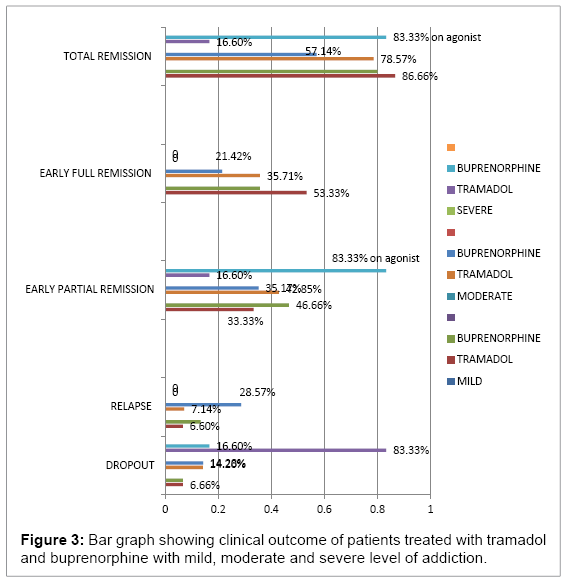
In the mild group (1-10 mg methadone equivalent): out of 15 patients in each category patients, 8(53.33%) patients achieved early full remission and 5(33.33%) early partial remission in tramadol group and 36%, 46.66% respectively in the buprenorphine group . Moderate (10- 20mg methadone equivalent): out of 14 patients in the tramadol group: early full remission was achieved by 5 (35.71) and partial remission by 6 (42.85%) summed up to 78.57%, whereas in buprenorphine group, 3 (21.42%) patients achieved full remission and 5(53.17%) partial remission amounting to total of 57.14% remission. Difference in the two was due to high relapse rate in buprenorphine group after detoxification i.e. 28.57%. Severe (>20 mg methadone) only 16.6% of patients could be sustained in tramadol group whereas 66.66% patients were maintained on buprenorphine at the end of 12 weeks. Tramadol-treated patients had higher average withdrawal symptoms when compared to the buprenorphine group and a greater reduction in withdrawal symptoms over time. In the tramadol group, average COWS maximum at week1 was 36 and in buprenorphine it was 24 (p=0.001) whereas at week 12 COWS max was 3 in tramadol and 8 in buprenorphine (p<0.05) (Figure 1) showing gradual reduction of withdrawal symptoms in tramadol group and no increase in withdrawal symptoms after drug cessation as compared to sudden decline in withdrawal symptoms in buprenorphine group which was followed by higher withdrawal symptoms on tapering the dose or after cessation of the drug (Figures 2 and 3) (Table 1).
| CGI | Tramadol | Buprenorphine | ||||
|---|---|---|---|---|---|---|
| Moderate group | MEAN | SD | MEAN | SD | t | P |
| BaselineCGI-S | 10.26 | 25.11 | 10.33 | 25.08 | -0.56 | 0.5 |
| 2weeks CGI-I | 9.20 | 25.40 | 8.66 | 25.55 | 3.22 | 0.006* |
| 12weeks CGI-I | 8.066 | 25.72 | 9.133 | 25.45 | -2.41 | 0.02* |
Table 1: T test: CGI scores of moderate level of addiction group.
Conclusion
• Tramadol appears to have comparable clinical efficacy as buprenorphine for treatment of patients with low levels of opioid dependence [5,13]
• Patients with moderate level of dependence; tramadol has more efficacy in detoxification and relapse prevention with minimum abuse potential.
• Patients with severe and persistent form of addiction are more likely to have co occurring psychiatric morbidity and typically require long term comprehensive treatment and in such patient’s induction and maintenance on buprenorphine may be more effective than detoxification for engaging and retaining patients in Comprehensive Outpatient addiction treatment.
• Detoxification with flexible dose schedule and tailoring the treatment according to individual has better outcomes as compared to fixed dose rapid or ultra-rapid detoxification [14].
Summary /Discussion
• Tramadol has good efficacy [5,6,13] in detoxification and relapse prevention in patients with moderate level of opioid dependence as compared to buprenorphine [15].
• Whereas Buprenorphine is better for maintenance treatment and is of higher clinical utility in severe level of opioid dependence where maintenance therapy is required [10,12].
• These findings, if reproduced in larger studies with stronger research designs, have potentially great implications for the management of opioid withdrawal in both the inpatient and outpatient setting.
References
- Raffa RB, Friderichs E, Reimann W, Shank RP, Codd EE, et al. (1993) Complementary and synergistic antinociceptive interaction between the enantiomers of tramadol. J Pharmacol Exp Ther 267: 331-340.
- Preston KL, Jasinski DR, Testa M (1991) Abuse potential and pharmacological comparison of tramadol and morphine. Drug Alcohol Depend 27: 7-17.
- Camí J, Lamas X, Farré M (1994) Acute effects of tramadol in methadone-maintained volunteers. Drugs 47 Suppl 1: 39-43.
- Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC (2010) Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology (Berl) 211: 457-466.
- Tamaskar R, Parran TV Jr, Heggi A, Brateanu A, Rabb M, et al. (2003) Tramadol versus buprenorphine for the treatment of opiate withdrawal: a retrospective cohort control study. J Addict Dis 22: 5-12.
- Lofwall MR, Walsh SL, Bigelow GE, Strain EC (2007) Modest opioid withdrawal suppression efficacy of oral tramadol in humans. Psychopharmacology (Berl) 194: 381-393.
- Wesson DR, Ling W (2003) The Clinical Opiate Withdrawal Scale (COWS). J Psychoactive Drugs 35: 253-259.
- Sobey PW, Parran TV Jr, Grey SF, Adelman CL, Yu J (2003) The use of tramadol for acute heroin withdrawal: a comparison to clonidine. J Addict Dis 22: 13-25.
- Seivewright N (2000) Community treatment of drug misuse: more than methadone. Cambridge University Press.
- RCGP (2004) Guidance for the use of buprenorphine for the treatment of opioid dependence in primary care.
- Guy W(2000) Clinical Global Impressions (CGI) Scale. Modified From: Rush J, et al: Psychiatric Measures, APA,Washington DC.
- Caldiero RM, Parran TV Jr, Adelman CL, Piche B (2006) Inpatient initiation of buprenorphine maintenance vs. detoxification: can retention of opioid-dependent patients in outpatient counseling be improved? Am J Addict 15: 1-7.
- Threlkeld M, Parran TV, Adelman CA, Grey SF, Yu J (2006) Tramadol versus buprenorphine for the management of acute heroin withdrawal: a retrospective matched cohort controlled study. Am J Addict 15: 186-191.
- Van den Brink W, Haasen C (2006) Evidenced-based treatment of opioid-dependent patients. Can J Psychiatry 51: 635-646.
- Lanier RK, Lofwall MR, Mintzer MZ, Bigelow GE, Strain EC (2010) Physical dependence potential of daily tramadol dosing in humans. Psychopharmacology (Berl) 211: 457-466.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 51970
- [From(publication date):
September-2015 - Sep 03, 2025] - Breakdown by view type
- HTML page views : 47160
- PDF downloads : 4810