Research Article Open Access
Two Case Reports on Refractory Periodontitis: Systemic Implications and a Potential New Therapeutic Strategy
Michael B Goldberg1*, Lorne M Golub2, Hsi-ming Lee3 and Howard C Tenenbaum1,2,3,4
1Department of Periodontics, Faculty of Dentistry, University of Toronto, Toronto, Ontario, Canada.
2Department of Oral Biology and Pathology, School of Dental Medicine, Stony Brook University, State University of New York (S.U.N.Y.), Stony Brook, NY, USA.
3Maurice and Gabriella Goldschleger School of Dental Medicine, Tel Aviv University, Tel Aviv, Israel.
4Department of Laboratory Medicine and Pathology, Faculty of Medicine, University of Toronto, Toronto, Ont., Canada.
- *Corresponding Author:
- Dr. Michael B Goldberg
University of Toronto Faculty of Dentistry
Room 349A, 124 Edward Street
Toronto, Ontario, Canada
Tel: (416) 979- 4928-4502
E-mail: m.goldberg@dentistry.utoronto.ca
Received Date: October 08, 2013; Accepted Date: November 14, 2013; Published Date: November 18, 2013
Citation: Goldberg MB, Golub LM, Hsi-ming L, Tenenbaum HC (2013) Two Case Reports on Refractory Periodontitis: Systemic Implications and a Potential New Therapeutic Strategy. J Interdiscipl Med Dent Sci 1:101. doi: 10.4172/2376-032X.1000101
Copyright: © 2013 Goldberg MB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at JBR Journal of Interdisciplinary Medicine and Dental Science
Abstract
Two cases of Refractory Periodontitis (RP) were studied over an 8-month time period. Both received a novel regimen of “combination” Host-Modulation Therapy (HMT) (subantimicrobial-dose doxycycline plus low-dose flurbiprofen adjunctive to traditional non-surgical periodontal therapy. Prior to beginning “combination” HMT, Case #1 was characterized by progressive periodontal deterioration; dramatically elevated levels of inflammatory mediators (IL-1β) and tissue-destructive enzymes (collagenase activity) in periodontal pockets (GCF); and evidence of systemic inflammation (high serum levels of hs-C-reactive protein), and mildly elevated LDL and total levels of cholesterol, indicating an increased risk for cardiovascular disease (CVD). In contrast, Case #2 exhibited undetectable levels of local biomarkers of inflammation and tissue destruction in their periodontal pockets (GCF), and much lower levels of both serum hs-CRP and cholesterol indicating lower risk for CVD. Both cases, however, demonstrated the characteristics of RP including progressive loss of bone and attachment despite repeated periodontal therapy. During the 12-month regimen of, Case #1, who exhibited much higher levels of local and systemic biomarkers, responded much more dramatically to the “combination” therapy than Case #2. We suggest that this novel “combination” therapy may be particularly useful in a sub-set of RP patients who exhibit evidence of systemic inflammation in their serum (or plasma) samples indicating increased risk for CVD; in these patients “combination” therapy may dramatically reduce both periodontal disease activity and CVD risk. In order to test this hypothesis, which proposes the development of more individualized therapeutic strategies, it clearly must be validated in future randomized clinical trials.
Introduction
Of the different categories of inflammatory periodontal diseases, Refractory Periodontitis (RP) is the most enigmatic and difficult to treat. Fortunately it is relatively rare but still remains a significant clinical challenge. RP is characterized by progressive breakdown of the periodontal supporting tissues despite ongoing sanative/debridement, surgical, and/or pharmacological treatments [1-3] i.e., conventional therapy. A number of mechanisms have been proposed to explain the unique features of this condition, especially in contrast to the much more common chronic periodontitis which usually responds well to routine treatment. Some investigators have suggested that the persistence of a unique bacterial species might account for the inexorable progression of this condition or that it has simply been treated inadequately [4,5]. However, based on observations at a specialized university clinic dedicated to the diagnosis, management and investigation of RP (SCRP), Bhide et al. [6] proposed: (i) that bacterial infection may not play the pivotal role that has been traditionally proposed for this nonresponsive group of patients and; (ii) that patients with RP might be hyper-responsive, from an inflammatory perspective, even to bacteria that would ordinarily be considered non-pathogenic. In this regard, elevated levels of CD4 + and CD8+T-cell receptor type, as well as hyper-excitable PMN leukocytes, may play a role in the abnormal host response which characterizes this condition [7].
Various inflammatory mediators generated by the host tissues including, but not limited to, cytokines such as IL-1, TNFα, IL-6, and tissue-destructive enzymes, the matrix metalloproteinases such as collagenases (MMP-8, MMP-13), gelatinases (MMP-2, MMP- 9) and other neutral proteinases, e.g., neutrophil elastase, are widely considered to be key participants in periodontal breakdown [8-12]. Importantly, a link between local inflammatory periodontal disease and systemic inflammation, particularly as risks for Cardiovascular Disease (CVD), has been highlighted, increasingly, within the context of the impact of this common oral inflammatory disease on overall health. In this regard, elevated levels of acute phase reactants in serum, particularly C-reactive protein (CRP) but also others such as haptoglobin and fibrinogen, are recognized as important risk factors (likely even mediators) for CVD [13-15] and, in several clinical trials, have been associated with severe periodontitis as well [16-19].
With this background in mind, we have begun to assess the therapeutic potential of a novel “combination” therapy in patients with RP. This “combination” therapy consists of an orally (i.e., systemically) administered MMP-inhibitor (sub antimicrobial-dose doxycycline ; SDD) [11,12,19,20]), FDA-approved as an adjunct to scaling and root planning for the management of chronic periodontitis, combined with another host-modulating drug, the NSAID flurbiprofen, also administered orally and also in low doses [11]. As background, Lee et al. [11] demonstrated that pathologically-excessive matrix metalloproteinase (MMP) activity in gingival tissues was reduced significantly when patients with Chronic Periodontitis (CP), who required periodontal surgery, were placed on a regimen of SDD 3-weeks prior to the procedure. In a second group of patients in the same study, the administration of low-dose flurbiprofen, by itself, had no effect on these gingival tissue-destructive enzymes. However, when a 3rd group of patients with CP were administered a “combination” of SDD plus low-dose flurbiprofen 3-weeks prior to surgery, a dramatic synergistic benefit was seen-the excessive activity of collagenase, gelatinase and even PMN elastase were all reduced synergistically in extracts of the surgically-excised gingival tissues. This clinical study was carried out as a result of earlier studies testing this combination approach for management of experimentally-induced rheumatoid arthritis in rats where it was shown that this novel treatment resulted in a synergistic inhibition of MMPs extracted from arthritic joints, as well as reduced joint destruction based on radiographic analysis [21-24]. The mechanism appeared to be related to the ability of the NSAID to significantly increase the local uptake of the tetracycline compound by the inflamed tissues of the joints, independent of the blood levels of the drug which were unaffected by the NSAID [23,24]. Accordingly, we now compare the clinical and biomarker responses of two RP patients - one with evidence of systemic inflammation, the other without - to a 12-month regimen of “combination” therapy as an adjunct to traditional mechanical debridement procedures.
Case Report 1 and 2
Case report 1, characterized by elevated serum hs-CRP (i e., systemic inflammation)
Clinical characteristics and clinical response to therapy: Patient E (case #1) is a 42year-old male who originally presented to the University of Toronto Specialized Refractory Periodontitis Clinic (SCRP) in June 2007. Prior to his presentation, Mr. E’s dentist noted a gradual increase in attachment loss and in bleeding-on-probing despite regular non-surgical periodontal maintenance therapy. At the initial assessment at the SCRP, profuse bleeding on probing was noted in over 50% of the sites examined. However, there was minimal evidence for the presence of pathogenic bacteria based on immunofluorescence testing. In addition to more intensive sanative therapy, Patient E was placed on a course of SDD months before the current study. This treatment dramatically improved the clinical measurements however the benefits began to plateau within 18 months. Patient E then entered the clinical trial described in this paper. Prior to the start of the trial, Patient E had a mean of 3.68 mm attachment loss with a range of 2 mm to 7 mm. Bleeding on probing was noted at 2.49 sites /tooth at baseline. This dramatically improved during the 8-month regimen of “combination” therapy adjunctive to scaling and root planning reflecting no further deterioration in attachment level. In fact, there was a small increase in attachment levels (mean attachment loss 2.69 ± 0.58 mm) noted at the 8 month time period. Additionally, there was a significant reduction in bleeding on probing (down to 0.82 sites/ tooth at 8 months) noted at the end of the trial compared to baseline values.
Case report #2, serum hs-CRP within normal limits
Clinical characteristics and clinical response to therapy: Patient W (case #2) is a 47 year old female who presented to the SCRP in 2007 for management of the continuous deterioration of her periodontal condition despite appropriate suitable periodontal therapy, consisting of scaling and root planning, followed by open flap debridement in all four posterior sextants. She was then placed on a 3-4-month maintenance recall appointments, alternating between her periodontist and general dentist. Upon presentation to the SCRP, Patient W had a mean attachment loss of 4.33 mm with a range of 2 mm to 8 mm, with 2.68 sites/tooth showing bleeding on probing. Minimal counts of pathogenic bacteria were noted based on the same assays noted for case #1.Initial treatment in our clinic included 3-4-month recalls at her two dentists, as well as a regimen of Periostat®, 20 mg every 12 hours. Slight improvements were noted with the use of this regimen. It was at this time that Patient W. was entered into our clinical study.
Patient W was placed on the combination of SDD plus low-dose flurbiprofen for a period of 12 months. There was a dramatic decrease in bleeding on probing compared to baseline values. The initial response appears to be the most dramatic but overall the effects appear to be have been maintained throughout the study. Also noteworthy - - there appeared to be a slight increase in the level of bleeding on probing at the first post-study follow-up appointment 4 months after the cessation of drug therapy.
Clinical chemistry response to therapy: Local (gingival crevicular fluid/GCF) and systemic (serum) biomarkers for case #1 and case #2:
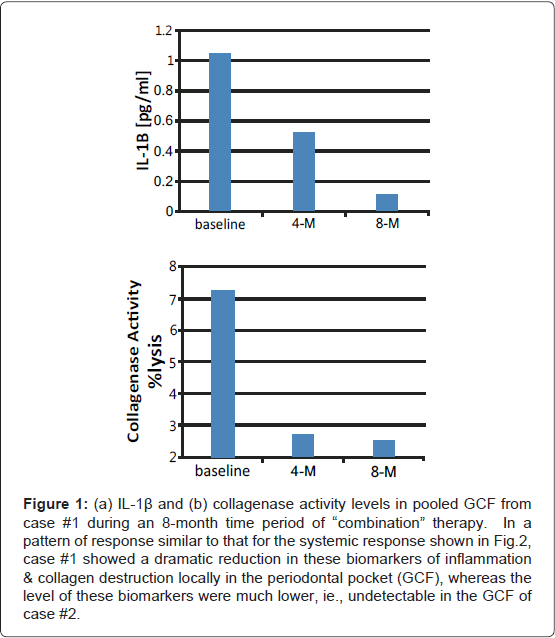
Local response: GCF samples were collected on filter paper strips inserted (10 seconds) into 4 pockets in each of the two patients. The samples were pooled, extracted, then analyzed for the inflammatory cytokine, Interleukin-1β (IL-1β), and for collagenase activity as described by us in detail previously [11,12,25]. The GCF samples (and blood samples; see below) were collected at the baseline appointment and at the 4 and 8 month appointments while the two patients were prescribed the “combination” therapy (see above); note that the patients were also assessed clinically (but not for biomarkers) at the 12-month and additional post-study appointments. In brief, Case #1 showed a progressive (and, ultimately, an 85%) reduction in GCF collagenase activity during the 8-months of combination treatment (Figure 1b) which was not seen in Case #2 because collagenase activity in this patient was undetectable at time 0, 4 and 8 months. In addition, Case #1 showed a >95% reduction in GCF IL-1B levels over the 8-month treatment regimen (Figure 1a); again,in case #2 the levels of this biomarker were too low to detect.
Figure 1: (a) IL-1β and (b) collagenase activity levels in pooled GCF from case #1 during an 8-month time period of “combination” therapy. In a pattern of response similar to that for the systemic response shown in Fig.2, case #1 showed a dramatic reduction in these biomarkers of inflammation & collagen destruction locally in the periodontal pocket (GCF), whereas the level of these biomarkers were much lower, ie., undetectable in the GCF of case #2.
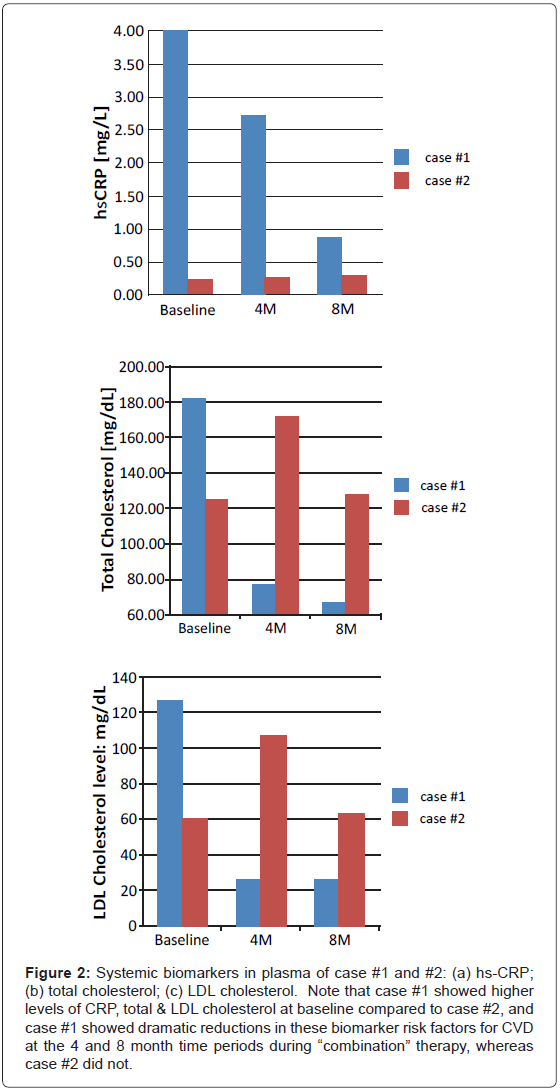
Systemic response: Blood samples were collected from the two patients at baseline, 4 and 8 month time periods, and biomarkers of systemic inflammation (high-sensitivity C-reactive protein and IL-6) and total, LDL and HDL cholesterol were measured as described by us previously [12,17,19-28]. On examination of systemic biomarkers in the plasma samples of the two patients, a pattern of change similar to that described for GCF biomarkers over the 8-month regimen of combination therapy was seen. At the baseline appointment, Case #1 exhibited a high level of hs-CRP in the plasma (4 mg/L) which corresponds to a high risk for cardiovascular disease (CVD) [13-15], and this systemic biomarker was progressively reduced at the 4 and 8-month appointments, while on combination therapy, to a plasma level of <1 mg/L which reflects a low risk for CVD (Figure 2a). In contrast, case #2 showed “low-risk” values for this biomarker of systemic inflammation, i.e., 0.5 mg/L, at all three appointments during the 8-month treatment protocol (Figure 2a). Interleukin-6 (IL-6), a long-lived inflammatory cytokine known to stimulate the production of acute phase proteins by the liver, was not detected in any of the plasma samples from these two RP patients. Regarding lipid profiles in the plasma samples, which (like hs-CRP) are important risk factors for CVD, both patients had values for total cholesterol, LDL (Figures 2b and c), and HDL cholesterol (the latter, a cardio-protective lipoprotein; not shown) within “normal” limits, although case #1 showed higher values for total cholesterol (180 mg/dl) and LDL cholesterol (130 mg/ dl) than case #2 (120 mg/dl and 62 mg/dl, respectively) at the baseline appointment, and both of these lipid fractions were reduced for total and LDL cholesterol during the 8-month period of treatment in case #1 but not in case #2 (Figures 2b and c).
Figure 2: Systemic biomarkers in plasma of case #1 and #2: (a) hs-CRP; (b) total cholesterol; (c) LDL cholesterol. Note that case #1 showed higher levels of CRP, total & LDL cholesterol at baseline compared to case #2, and case #1 showed dramatic reductions in these biomarker risk factors for CVD at the 4 and 8 month time periods during “combination” therapy, whereas case #2 did not.
Discussion
As observed by us prior to this study, the use of SDD as an adjunct to scaling and root planning reduces clinical measures of periodontal inflammation in patients with RP. However, as described in the current case-study report, a novel combination of SDD plus low-dose flurbiprofen appeared to result in an even greater improvement in clinical parameters, compared to SDD alone, in this poorly-understood category of periodontal disease.
Despite an indication of positive findings, it is important to emphasize the primary limitation of this report, namely that it involves only two case studies. However, the fact one of the subjects had evidence of systemic inflammation based on elevated serum levels of C-reactive protein (which large randomized clinical trials by Ridker et al. [13-15] have repeatedly linked to increased risk for CVD), whereas the second RP case showed normal values for this serum biomarker, makes the contrast in response to the combination therapy, for these two cases, intriguing for reasons that are described below.
Based on the Special Clinic’s experience, patients with RP are typically in a treatment phase for many years yet still exhibit signs and symptoms of severe and progressive periodontal disease leading to continual loss of periodontal attachment. Hence, the beneficial effects of adjunctive combination therapy in these cases can be viewed as a favorable response in that the progression of disease appeared to have been arrested, at the least. In this regard, by definition, patients with RP are expected to show progressive loss of attachment and periodontal breakdown despite vigorous traditional non-surgical and surgical therapy. Yet during the current protocol, case #1 actually exhibited an increase in clinical attachment (regeneration?) and case #2 also benefited from the adjunctive combination therapy by showing arrested attachment loss (disease stability). However, once this novel 12-month therapy was stopped, progressive deterioration of the periodontal breakdown appeared to activate again based on an increase in bleeding-on-probing. Regarding possible mechanisms, previous research by the SCRP has indicated that patients with refractory periodontitis (compared to those with chronic periodontitis) are characterized by elevated T-cell levels, particularly CD4+ cells, which suggests that these individuals are “hyper-immune” [6]. Consistent with this view, others [29] have noted that elevated CD4+ levels may be associated with clonal expansion of both B-cells and T-cells which could perpetuate the inflammatory state and prevent its “resolution”. Ultimately, this could lead to chronically-elevated levels of inflammatory biomarkers in the bloodstream, including such markers as C-reactive protein and other acute-phase reactants produced by the liver. In addition, Lee et al. [26] have demonstrated that levels of inflammatory mediators (e.g., cytokines such as IL-1β, IL-6) are elevated locally in the gingival crevicular fluid derived from patients with RP. In the current preliminary study, the combination of SDD plus low-dose flurbiprofen appeared to reduce local levels of IL- 1β and MMPs (i.e., collagenase activity) in the periodontal pockets of RP patients (which has also been seen in the gingival tissues of patients with CP [11]), as well as reducing abnormally-elevated levels of hs-CRP (and other biomarkers associated with CVD), namely total and LDL cholesterol within the circulation compared to pre-treatment values. Similar improvements in the levels of local and systemic biomarker of inflammation that occurred as a result of host-modulation therapy (i.e., SDD either with or without low-dose NSAID) have been observed during the treatment of patients with CP including (but not limited to) post-menopausal women who are vulnerable to CVD [11,19,25]. Thus, these benefits potentially could now be extended to the category of patients who are considered refractory to traditional mechanical debridement and surgical therapeutic procedures.
Of particular interest are the systemic implications of the observations in the current case-report study. In this regard, one of the two RP patients (case #1) exhibited abnormally high levels of hs-CRP in the circulation (4 mg/L), prior to initiating combination therapy, and these levels have been interpreted, based on previous extensive clinical trials, as a “high risk” for CVD [13-15,19]. The other RP patient (case #2) exhibited much lower baseline hs-CRP levels, 0.5 mg/L, indicating a “low-CVD risk”. A similar CVD risk pattern was observed for the blood cholesterol values - - case #1 showed substantially higher level of both total and LDL cholesterol than case #2 prior to initiating combination therapy. Also of interest, case #1, with the higher levels of CVD risk factors, responded to combination therapy with dramatic reductions in serum levels of both CRP and total and LDL cholesterol (as well as reductions, locally, in clinical and biomarkers of periodontal disease severity) during the current study. In contrast, case #2, who showed much lower levels of serum biomarkers of CVD, including CRP and cholesterol, did not respond as dramatically to “combination” therapy. Given the controversy that currently exists regarding the relationship between periodontal disease and cardiovascular disease risk, the current (albeit preliminary) report suggests the following intriguing possibility: namely, that a sub-set of refractory (or other categories of) periodontitis patients who are characterized by elevated levels of circulating high-sensitivity C-reactive protein (or by high levels of other biomarkers of systemic inflammation) may be those periodontitis patients who (i) are at high risk for CVD, and (ii) would benefit, both locally and systemically, from a “combination” of SDD and low-dose NSAID adjunctive to traditional non-surgical periodontal therapy - - whereas those RP patients with normal levels of systemic biomarkers may not be more susceptible to CVD, and aggressive (or other) periodontal therapy would be of value locally but not systemically. It should be noted that findings, consistent with our preliminary observations, were recently reported in a major NIH-funded clinical trial testing long-term administration of SDD adjunctive to periodontal maintenance therapy, in osteopenic postmenopausal women with chronic periodontitis, a category of patients considered vulnerable to cardiovascular disease [19].
It is also noteworthy that the patients who participated in this limited case-study purchased their own medications, with no financial assistance, indicating a high level of motivation (as well as their determination to keep their own teeth), the availability of implant (or other restorative) treatment notwithstanding. Additionally, no side-effects were noted with the combination therapy and, based on repeated interviews; the patients were substantially compliant with daily use of the medication. Clearly, the proposals presented here are only preliminary and require extensive testing by future randomized placebo-controlled clinical trials.
Summary: A novel combination therapy (subantimicrobial-dose doxycycline plus low-dose flurbiprofen) adjunctive to repeated scaling and root planning may produce systemic as well as local benefits at least in a cardiovascular disease-susceptible subset of refractory periodontitis patients.
Summary
A novel combination therapy (subantimicrobial-dose doxycycline plus lowdose flurbiprofen) adjunctive to repeated scaling and root planning may produce systemic as well as local benefits at least in a cardiovascular disease-susceptible subset of refractory periodontitis patients.
Key Findings
We suggest that a novel “combination” therapy may be particularly useful in a sub-set of refractory periodontal disease patients who exhibit evidence of systemic inflammation in their serum samples indicating increased risk for CVD; in these patients “combination” therapy may dramatically reduce both periodontal disease activity and CVD risk.
References
- Parameter on "refractory" periodontitis. American Academy of Periodontology (2000). J Periodontol 71: 859-860.
- Hirschfeld L, Wasserman B (1978) A long-term survey of tooth loss in 600 treated periodontal patients. J Periodontol 49: 225-237.
- Löe H, Anerud A, Boysen H, Smith M (1978) The natural history of periodontal disease in man. The rate of periodontal destruction before 40 years of age. J Periodontol 49: 607-620.
- Slots J, Rams TE, Listgarten MA (1988) Yeasts, enteric rods and pseudomonads in the subgingival flora of severe adult periodontitis. Oral Microbiol Immunol 3: 47-52.
- Colombo AP, Haffajee AD, Dewhirst FE, Paster BJ, Smith CM, et al. (1998) Clinical and microbiological features of refractory periodontitis subjects. J Clin Periodontol 25: 169-180.
- Bhide VM, Tenenbaum HC, Goldberg MB (2006) Characterization of patients presenting for treatment to a university refractory periodontal diseases unit: three case reports. J Periodontol 77: 316-322.
- Johnstone AM, Koh A, Goldberg MB, Glogauer M (2007) A hyperactive neutrophil phenotype in patients with refractory periodontitis. J Periodontol 78: 1788-1794.
- Wahl LM, Corcoran ML (1993) Regulation of monocyte/macrophage metalloproteinase production by cytokines. J Periodontol 64: 467-473.
- Lamster IB (1992) The host response in gingival crevicular fluid: potential applications in periodontitis clinical trials. J Periodontol 63: 1117-1123.
- Kjeldsen M, Holmstrup P, Lindemann RA, Bendtzen K (1995) Bacterial-stimulated cytokine production of peripheral mononuclear cells from patients of various periodontitis categories. J Periodontol 66: 139-144.
- Lee HM, Ciancio SG, Tüter G, Ryan ME, Komaroff E, et al. (2004) Subantimicrobial dose doxycycline efficacy as a matrix metalloproteinase inhibitor in chronic periodontitis patients is enhanced when combined with a non-steroidal anti-inflammatory drug. J Periodontol 75: 453-463.
- Reinhardt RA, Stoner JA, Golub LM, Lee HM, Nummikoski PV, et al. (2010) Association of gingival crevicular fluid biomarkers during periodontal maintenance with subsequent progressive periodontitis. J Periodontol 81: 251-259.
- Ridker PM, Danielson E, Fonseca FAH et al. (2008) Rosuvastatin to Prevent Vascular Events in Men and Women with Elevated C-Reactive Protein Levels. N Engl J Med 359: 2195-2207.
- Ridker PM, Hennekens CH, Buring JE, Rifai N (2000) C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med 342: 836-843.
- Ridker PM, Rifai N, Rose L, Buring JE, Cook NR (2002) Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med 347: 1557-1565.
- Salzberg TN, Overstreet BT, Rogers JD, Califano JV, Best AM, et al. (2006) C-reactive protein levels in patients with aggressive periodontitis. J Periodontol 77: 933-939.
- Kinane DF (1998) Periodontal diseases' contributions to cardiovascular disease: an overview of potential mechanisms. Ann Periodontol 3: 142-150.
- Craig RG, Yip JK, So MK, Boylan RJ, Socransky SS, et al. (2003) Relationship of destructive periodontal disease to the acute-phase response. J Periodontol 74: 1007-1016.
- Payne JB, Golub LM, Stoner JA, Lee HM, Reinhardt RA, et al. (2011) The effect of subantimicrobial-dose-doxycycline periodontal therapy on serum biomarkers of systemic inflammation: a randomized, double-masked, placebo-controlled clinical trial. JADA Dent Assoc 142: 262-273.
- Caton J, Ryan ME (2011) Clinical studies on the management of periodontal diseases utilizing subantimicrobial dose doxycycline (SDD). Pharmacol Res 63: 114-120.
- Greenwald RA, Moak SA, Ramamurthy NS, Golub LM (1992) Tetracyclines suppress matrix metalloproteinase activity in adjuvant arthritis and in combination with flurbiprofen, ameliorate bone damage. J Rheumatol 19: 927-938.
- Ramamurthy N, Greenwald R, Moak S, Scuibba J, Goren A, et al. (1994) CMT/Tenidap treatment inhibits temporomandibular joint destruction in adjuvant arthritic rats. Ann N Y Acad Sci 732: 427-430.
- Ramamurthy N, Leung M, Moak S, Greenwald R, Golub L (1993) CMT/NSAID combination increases bone CMT uptake and inhibits bone resorption. Ann N Y Acad Sci 696: 420-421.
- Leung MK, Greenwald RA, Ramamurthy NS, Moak SA, Koszulinski R, et al. (1995) Tenidap and flurbiprofen enhance uptake of matrix metalloproteinase inhibitor 4-dedimethylaminotetracycline in inflamed joints of adjuvant arthritic rats. J Rheumatol 22: 1726-1731.
- Golub LM, Lee HM, Stoner JA, Sorsa T, Reinhardt RA, et al. (2008) Subantimicrobial-dose doxycycline modulates gingival crevicular fluid biomarkers of periodontitis in postmenopausal osteopenic women. J Periodontol 79: 1409-1418.
- Lee HJ, Kang IK, Chung CP, Choi SM (1995) The subgingival microflora and gingival crevicular fluid cytokines in refractory periodontitis. J Clin Periodontol 22: 885-890.
- Beck JD, Offenbacher S (2005) Systemic effects of periodontitis: epidemiology of periodontal disease and cardiovascular disease. J Periodontol 76: 2089-2100.
- Brown DL, Desai K, Vakili .A, Nouneh C, Lee HM, et al. (2004) Clinical and biochemical results of the Metalloproteinase Inhibition with Subantimicrobial Doses of Doxycycline to Prevent Acute Coronary Syndromes (MIDAS) Pilot Trial. Arterioscler Thromb Vasc Biol 24: 733-738.
- Taubman MA, Kawai T (2001) Involvement of T-lymphocytes in periodontal disease and in direct and indirect induction of bone resorption. Crit Rev Oral Biol Med 12: 125-135.
Relevant Topics
- Cementogenesis
- Coronal Fractures
- Dental Debonding
- Dental Fear
- Dental Implant
- Dental Malocclusion
- Dental Pulp Capping
- Dental Radiography
- Dental Science
- Dental Surgery
- Dental Trauma
- Dentistry
- Emergency Dental Care
- Forensic Dentistry
- Laser Dentistry
- Leukoplakia
- Occlusion
- Oral Cancer
- Oral Precancer
- Osseointegration
- Pulpotomy
- Tooth Replantation
Recommended Journals
Article Tools
Article Usage
- Total views: 15481
- [From(publication date):
December-2013 - Aug 30, 2025] - Breakdown by view type
- HTML page views : 10750
- PDF downloads : 4731