Unexpected Macrophage Activity Alters the Inflammatory Process in Necrotizing Enterocolitis
Received: 13-May-2017 / Accepted Date: 31-May-2017 / Published Date: 06-Jun-2017 DOI: 10.4172/2576-3881.1000115
Abstract
Objective: Macrophages play a role in clearing bacteria from the gut in the initial stages of necrotizing enterocolitis (NEC). Macrophage Inflammatory Protein-1α (MIP-1α), also known as CCL3, is a chemokine produced by macrophages that enhances the immune response. It acts as both a recruiter of immune cells and a coactivator of other macrophages. Interleukin-12 (IL-12) is a pro-inflammatory chemotactant produced by several types of phagocytic cells such as dendritic cells and neutrophils, but primarily by macrophages. We hypothesized that the pro-inflammatory state associated with NEC pathophysiology would induce increased expression of MIP-1α and IL-12 that would be detectable in serum and intestinal tissue.
Methods: Timed pregnant Sprague-Dawley rats were randomized by litter. Controls were delivered vaginally and dam-fed. NEC pup groups were delivered 12 h prematurely via Cesarean section, formula fed, given a single oral dose of lipopolysaccharide, and subjected to intermittent cold and hypoxia as part of a proven NEC rat model protocol. Animals were sacrificed at 0, 12, 24, 48, 72, and 96 h of life and serum and intestinal tissue samples were collected. Samples were analysed via western blot using antibodies with affinity to MIP-1α and IL-12 respectively.
Results: Serum and ileal levels of MIP-1 alpha were increased from 48 to 72 h in NEC animals when compared with controls. Serum and ileal IL-12 was downregulated in NEC groups at 12 and 24 h compared to controls.
Conclusion: Macrophage function plays an important role in the first 48 h of NEC pathophysiology. Downregulation of IL-12 in the setting of increased MIP-1α expression may represent deranged or inhibited macrophage function in the context of NEC pathogenesis. Further work to elucidate the significance of these findings is warranted.
Keywords: Necrotizing enterocolitis; Macrophage; Cytokine; Chemokine; MIP-1 alpha; CCL3; IL-12
6732Introduction
Necrotizing enterocolitis (NEC) is a devastating gastrointestinal illness primarily affecting premature infants. NEC is characterized by epithelial destruction leading to gut barrier failure, and requires surgery in 20-40% of those affected. Of infants requiring operative intervention, 20-50% will die from their disease [1-3]. Despite years of research into the cause, prevention, and treatment of NEC, the mortality rate has remained the same over the last three decades, and no definitive cause has been found [4,5].
Macrophages have been shown to play an important role in the clearance of bacteria in the initial stages of NEC [6]. Activation of macrophages is characterized by the release of many intercellular mediators including cytokines, growth factors, and chemokines. Macrophage inflammatory protein 1-alpha (MIP-1α) (also known as CCL3) is a chemokine that is upregulated in macrophages in response to stressors such as exposure to bacterial lipopolysaccharide (LPS) [7,8].
Interleukin-12 (IL-12) is a pro-inflammatory cytokine produced by macrophages, dendritic cells, and B cells, and works to activate macrophages and recruit phagocytic cells to sites of infection. Mice that are deficient in IL-12 or its receptor are more susceptible to bacterial infections than their normal counterparts [9-11].
We hypothesized that the pro-inflammatory state associated with NEC would lead to an up-regulation of both MIP-1α and IL-12.
Materials and Methods
Approval for the study was granted by the Institutional Animal Care and Use Committee (IACUC), the Biohazard Safety Committee, and the Research and Development Committee of the Robley Rex Veteran’s Affairs Hospital in Louisville, KY. Timed pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN) were housed for one week prior to initiation of experimental protocol, and allowed water and rat chow ad lib.
Dams were then randomized to the Control or NEC group and their pups were assigned accordingly after birth. Control dams delivered their pups vaginally. Control pups were kept with their mothers and allowed to nurse on demand until the time of sacrifice for tissue and serum harvest. Time matched NEC dams underwent carbon dioxide anesthesia and decerebration prior to rapid cesarean delivery of their pups 12 h prematurely. NEC pups were revived and kept in a warmed incubator at 37.0 degrees centigrade.
A previously published protocol was used to induce NEC in the NEC pups [12,13]. Briefly, the pups were orally gavage fed formula consisting of 20 g Similac 60/40 (Ross Pediatrics, Columbus, OH) dissolved in 100 mL Esbilac puppy formula (Pet-Ag, New Hampshire, IL) every four hours and were given one oral dose of lipopolysaccharide (2 mg/kg Salmonella enterica serotype typhimurium; Sigma Chemical CO., St. Louis, MO) at 12 h of life. Feeds were started at 0.1 mL/feed at time 0 and advanced to 0.4 mL/ feed at 96 h for approximately 836.8 kJ/kg/day. Feeds and the oral dose of LPS were administered via a silastic 1.9 French single lumen catheter (Utah Medical Products, Inc., Midvale, UT) gently advanced into the stomach. The position was verified by the appearance of formula in the stomach during instillation of formula. NEC pups were also subjected to hypoxia (100% nitrogen gas for 60 sec) and hypothermia (4°C for ten minutes) every 12 h.
Control and NEC pups were sacrificed at 0, 12, 24, 48, 72, and 96 h of life. Both Control and NEC ileum samples were homogenized in RIPA lysis buffer (R0278 Sigma-Aldrich Chemical Co., St. Louis, MO) and a cocktail of phosphatase (P5726, Sigma-Aldrich) and protease inhibitors (P8340, Sigma-Aldrich), and protein concentrations were determined. Samples containing equal amounts of 35 μg of total protein were resolved by SDS-PAGE under reducing conditions. After electrophoresis, proteins were transferred to a polyvinylidene difluoride membrane and probed with antibodies against CCL-3 and IL-12 with a 1:1000 dilution in BSA. Each protein sample was standardized to its own β-actin level.
Ileum sections for Control and NEC groups from the 48 h time point were mounted in paraffin and stained with either H&E or Immunohistochemistry (IHC). The following scale was used to grade each sample: grade 0, normal and no damage; grade 1, epithelial cell lifting or separation; grade 2, sloughing of epithelial cells to mid villus level; grade 3, necrosis of the entire villus; and grade 4, transmural necrosis [14]. These samples were selected for IHC based on our prior studies showing a significant difference in intestinal blood flow at this place in the NEC protocol timeline [15]. IHC staining was performed using rabbit anti-rodent polyclonal antibodies for MIP-1α (CCL-3) and IL-12. The slides for IHC were graded into one of four different categories: (-) no significant staining, (+) slight positive, (++) moderately positive, and (+++) strongly positive.
Serum samples were taken at the time of sacrifice and prepared for analysis per the manufacturer’s instructions using a Luminex MagPix multianalyte cytokine array (Luminex, 23-plex cytokine panel specific for rat). Two-way analysis of variance (ANOVA) using SigmaPlot for Windows 11.1.0.102 (Systat Software, Inc., San Jose, CA) was used to determine the difference between groups and time points, and when a difference was found, it was confirmed with the Tukey-Kramer honestly significant difference test. The null hypothesis was rejected at p<0.05.
Results
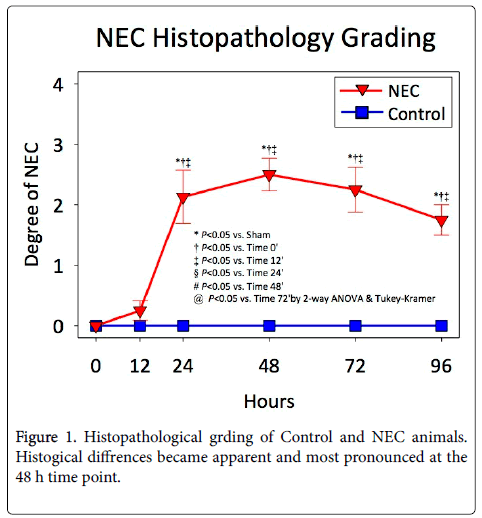
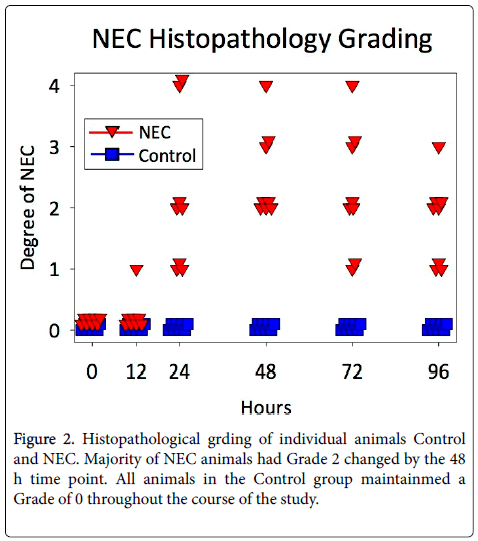
Histological signs of NEC became apparent in the NEC groups at 24 h, and were most pronounced at 48 h (Figure 1). All animals in the NEC group had at least Grade 1 histological signs of NEC by 24 h, and the majority of animals had Grade 2 changes by 48 h (Figure 2). All animals in the Control group maintained Grade 0 histology throughout the course of the study.
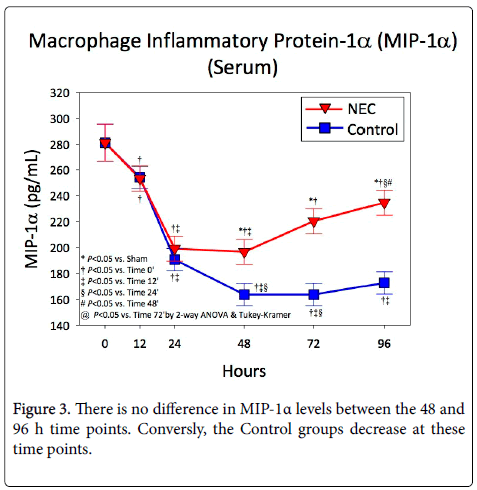
Serum levels of MIP-1α at 0, 12, 24, 48, 72, and 96 h are shown in Figure 3. There was no difference in the amount of MIP-1α present in NEC versus Control animals at the 0, 12, or 24 h time points. The level of MIP-1α started off somewhat high in both NEC and Control animals and experienced a significant drop between 12 and 24 h in both groups. After 24 h, the Control animals continued to experience a decrease in MIP-1α, whereas the NEC animals showed elevated levels.
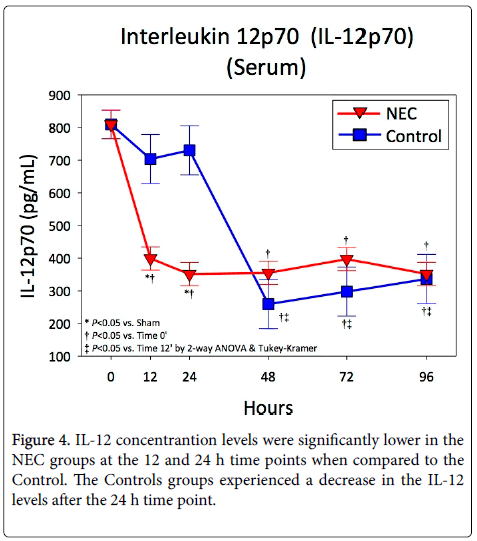
After an initial drop, Interleukin-12 levels were steady in NEC animals throughout the NEC protocol (Figure 4). Levels of IL-12 were significantly lower in NEC animals when compared to controls at 12 and 24 h. Following this, the Control animals experienced a drop in IL-12 while the NEC animals remained unchanged.
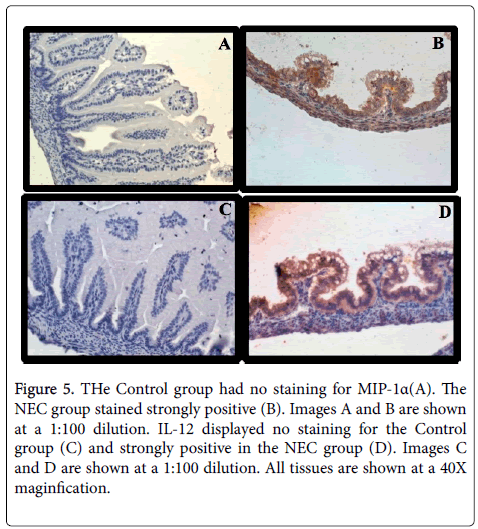
Immunohistochemistry of Control and NEC groups at the 48 h time point revealed similar results to serum levels at the same time point. The Control group (Figure 5A) showed no significant staining for MIP-1α while the NEC group stained strongly positive for MIP-1α (Figure 5B). Similarly for IL-12 the Control group displayed no significant staining (Figure 5C). The NEC group at the same time point stained strongly positive for IL-12 (Figure 5D).
Figure 5: THe Control group had no staining for MIP-1α(A). The NEC group stained strongly positive (B). Images A and B are shown at a 1:100 dilution. IL-12 displayed no staining for the Control group (C) and strongly positive in the NEC group (D). Images C and D are shown at a 1:100 dilution. All tissues are shown at a 40X maginfication.
Discussion
The exact pathophysiology of NEC continues to be unclear despite years of research into the cause. Many risk factors have been identified as contributing to the pathogenesis of NEC, including prematurity, enteral feeding, hypothermia, bacterial colonization, and hypoxia [16-23]. Many believe NEC to be secondary to a deranged or uncontrolled inflammatory response. Therefore, researchers have sought to elucidate the cascade of inflammatory cytokines found in NEC [24]. As a part of the innate immune system, the macrophage plays an important role in the recognition and clearance of bacteria from sites of infection. Present in tissues, macrophages are one of several cell types known as antigen presenting cells (APCs), capable of recognizing and engulfing foreign antigens for presentation to other immune cells to stimulate the clearance of infection.
Macrophage inflammatory proteins-1α, 1β, and 2 (MIPs) are heparin binding proteins with inflammatory and immunoregulatory activity [8]. The MIPs were initially characterized as proteins secreted from endotoxin-stimulated mouse macrophages, but further research demonstrated that neutrophils, fibroblasts, epithelial cells, and T cells are also MIP-secreting cells. The three MIPs have distinct characteristics, but share a few overlapping roles. All MIPs act primarily in a pro-inflammatory nature and are chemotactic, acting to recruit and activate other cells at the sites of inflammation or injury. MIP-1α acts with a broader specificity than MIP-1β in that it attracts B cells, cytotoxic T cells, and CD4+T cells, whereas MIP-1β works preferentially on CD4+ T cells [25]. The macrophage is thought to be the primary source for MIPs, but Sherry et al. demonstrated that T cells are either a major source of MIPs or that they act to markedly upregulate the production of MIPs by other cell types [7]. In that study, levels of MIP-1α were measured in mice in vitro after intraperitoneal injection of LPS, and were shown to peak at two hours, then fall to undetectable levels by eight hours.
In our study, there were similar amounts of MIP-1α at the 12 and 24 h time points in both the NEC and Control groups, and that level was trending downward. By 48 h, we saw a significantly different increase in MIP-1α in NEC animals compared to controls. This trend continued in the NEC animals and remained significant until 96 h-the end-point of our study. As one of the first responders to sites of infection and inflammation, it seems likely that the macrophage response to the induction of NEC would be nearly immediate, but we did not find that to be true. Pathological signs of NEC were first apparent at 24 h in NEC animals, but levels of MIP-1α in those animals did not increase significantly until 48 h. Additionally, instead of peaking and then becoming undetectable, we show a sustained increase in MIP-1α.
These findings are markedly different than those demonstrated in the prior work by Sherry et al. [7]. It is possible that the lag time between histopathological evidence of the disease compared to significantly elevated serum levels of MIP-1α in NEC animals demonstrates a slow reaction of macrophages in this NEC model. A delay in macrophage activation might perpetuate further damage to the intestines, worsening NEC. The sustained increase in MIP-1α in NEC animals demonstrates the continued inflammatory response as macrophages are attempting to fight off the intestinal inflammation.
IL-12, produced by macrophages, dendritic cells, and B cells, works synergistically with IL-18 to induce production of interferon-gamma (IFN-γ) from natural killer and B cells. Together, these proinflammatory factors can induce intestinal inflammation in mice [26]. Prior studies have demonstrated conflicting results regarding the production of IL-12 in rats undergoing a NEC-inducing protocol when compared to dam fed controls. While Nadler et al. showed decreased IL-12 mRNA expression in NEC animals [27], Halpern et al. showed an increase in IL-12 mRNA in NEC animals and a correlation between the number of IL-12 positive cells and the degree of tissue destruction [28]. The positive correlation between the number of IL-12 positive cells and the amount of tissue damage present led many to draw the conclusion that IL-12 is involved with the initial stages of NEC and may contribute to the intestinal necrosis associated with NEC [24].
Our findings (i.e., a relatively steady level of IL-12 in NEC animals compared to a marked elevation of IL-12 at 12 and 24 h in Control animals) suggest that macrophage function in NEC animals may be diminished. One might expect a reduced inflammatory reaction, but this is not confirmed histologically. There is clear progression of inflammation and necrosis of the intestine in the NEC animals despite the decreased amount of IL-12. Therefore, although other studies have demonstrated the involvement of IL-12 in tissue inflammation in the intestine, our findings of low IL-12 with markedly inflamed intestine indicate that further investigation into the activity of IL-12 in NEC animals is warranted.
Conclusion
Macrophages play a central role in the elimination of bacteria from the gut in the initial stages of NEC. Animals undergoing a NECinducing protocol experienced a delayed upregulation of MIP-1α and no upregulation of IL-12. This delay in the upregulation of the macrophage-secreted pro-inflammatory cytokine MIP-1α paired with a lack of response by IL-12 in NEC animals likely contributes to the pathophysiology of NEC. Enteral supplementation of IL-12, as we have previously done with Relaxin, may offer a mechanistic explanation of IL-12 and if this cytokine is contributing positively or negatively to the pathophysiology of NEC.
Acknowledgement
The authors would like to acknowledge the technical support of Amy J. Matheson and Samuel A. Matheson in the performance of these studies. The work is alos supported by Kosair Charities and the James R. Petersdorf Fund of Norton Healthcare.
References
- Petrosyan M, Guner YS, Williams M, Grishin A, Ford HR (2009) Current concepts regarding the pathogenesis of necrotizing enterocolitis. Pediatr Surg Int 25: 309-318.
- Blakely ML, Lally KP, McDonald S, Brown RL, Barnhart DC, et al. (2005) Postoperative outcomes of extremely low birth-weight infants with necrotizing enterocolitis or isolated intestinal perforation: a prospective cohort study by the NICHD Neonatal Research Network. Ann Surg 241: 984-989.
- Rowe MI, Reblock KK, Kurkchubasche AG, Healey PJ (1994) Necrotizing enterocolitis in the extremely low birth weight infant. J Pediatr Surg 29: 987-990.
- Berrington JE, Hearn RI, Bythell M, Wright C, Embleton ND (2012) Deaths in preterm infants: changing pathology over 2 decades. J Pediatr 160: 49-53.
- Horbar JD, Badger GJ, Carpenter JH, Fanaroff AA, Kilpatrick S, et al. (2002) Trends in mortality and morbidity for very low birth weight infants, 1991-1999. Pediatrics 110: 143-151.
- Emami CN, Mittal R, Wang L, Ford HR, Prasadarao NV (2012) Role of neutrophils and macrophages in the pathogenesis of necrotizing enterocolitis caused by Cronobacter sakazakii. J Surg Res 172: 18-28.
- Sherry B, Espinoza M, Manogue KR, Cerami A (1998) Induction of the chemokine beta peptides, MIP-1 alpha and MIP-1 beta, by lipopolysaccharide is differentially regulated by immunomodulatory cytokines gamma-IFN, IL-10, IL-4, and TGF-beta. Mol Med 4: 648-657.
- Driscoll KE (1994) Macrophage inflammatory proteins: biology and role in pulmonary inflammation. Exp Lung Res 20: 473-490.
- Alberts B, Johnson A, Lewis J, Raff M, Roberts K, et al. (2002) The adaptive immune system. (4th Edition), Garland Science, New York, USA.
- Göebel A, Kavanagh E, Lyons A, Saporoschetz IB, Soberg C, et al. (2000) Injury induces deficient interleukin-12 production, but interleukin-12 therapy after injury restores resistance to infection. Ann Surg 231: 253-261.
- O'Suilleabhain C, O'Sullivan ST, Kelly JL, Lederer J, Mannick JA, et al. (1996) Interleukin-12 treatment restores normal resistance to bacterial challenge after burn injury. Surgery 120: 290-296.
- Barlow B, Santulli TV (1975) Importance of multiple episodes of hypoxia or cold stress on the development of enterocolitis in an animal model. Surgery 77: 687-690.
- Barlow B, Santulli TV, Heird WC, Pitt J, Blanc WA, et al. (1974) An experimental study of acute neonatal enterocolitis-the importance of breast milk. J Pediatr Surg 9: 587-595.
- Caplan MS, Hedlund E, Adler L, Hsueh W (1994) Role of asphyxia and feeding in a neonatal rat model of necrotizing enterocolitis. Pediatr Pathol 14: 1017-1028.
- Walker SK, Matheson PJ, Schreiner MT, Smith JW, Garrison RN, et al. (2013) Intraperitoneal 1.5% Delflex improves intestinal blood flow in necrotizing enterocolitis. J Surg Res 184: 358-364.
- Downard CD, Grant SN, Matheson PJ, Guillaume AW, Debski R, et al. (2011) Altered intestinal microcirculation is the critical event in the development of necrotizing enterocolitis. J Pediatr Surg 46: 1023-1028.
- Lin PW, Stoll BJ (2006) Necrotising enterocolitis. Lancet 368: 1271-1283.
- Hsueh W, Caplan MS, Qu XW, Tan XD, De Plaen IG, et al. (2003) Neonatal necrotizing enterocolitis: clinical considerations and pathogenetic concepts. Pediatr Dev Pathol 6: 6-23.
- Kosloske AM (1994) Epidemiology of necrotizing enterocolitis. Acta Paediatr Suppl 396: 2-7.
- Neu J (1996) Necrotizing enterocolitis: the search for a unifying pathogenic theory leading to prevention. Pediatr Clin North Am 43: 409-432.
- Krediet TG, van Lelyveld N, Vijlbrief DC, Brouwers HA, Kramer WL, et al. (2003) Microbiological factors associated with neonatal necrotizing enterocolitis: protective effect of early antibiotic treatment. Acta paediatrica 92: 1180-1182.
- Kliegman RM (1990) Models of the pathogenesis of necrotizing enterocolitis. J Pediatr 117: S2-5.
- Martinez-Tallo E, Claure N, Bancalari E (1997) Necrotizing enterocolitis in full-term or near-term infants: risk factors. Biol Neonate 71: 292-298.
- Markel TA, Crisostomo PR, Wairiuko GM, Pitcher J, Tsai BM, et al. (2006) Cytokines in necrotizing enterocolitis. Shock 25: 329-337.
- Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV (1993) Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes. J Exp Med 177: 1821-1826.
- Chikano S, Sawada K, Shimoyama T, Kashiwamura SI, Sugihara A, et al. (2000) IL-18 and IL-12 induce intestinal inflammation and fatty liver in mice in an IFN-gamma dependent manner. Gut 47: 779-786.
- Nadler EP, Dickinson E, Knisely A, Zhang XR, Boyle P, et al. (2000) Expression of inducible nitric oxide synthase and interleukin-12 in experimental necrotizing enterocolitis. J Surg Res 92: 71-77.
- Halpern MD, Holubec H, Dominguez JA, Williams CS, Meza YG, et al. (2002) Up-regulation of IL-18 and IL-12 in the ileum of neonatal rats with necrotizing enterocolitis. Pediatr Res 51: 733-739.
Citation: Raque JL, Matheson PJ, Connolly K, Shepherd JA, Stamper EA, et al. (2017) Unexpected Macrophage Activity Alters the Inflammatory Process in Necrotizing Enterocolitis. J Cytokine Biol 2: 115. DOI: 10.4172/2576-3881.1000115
Copyright: © 2017 Raque Jl, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 4282
- [From(publication date): 0-2017 - Aug 29, 2025]
- Breakdown by view type
- HTML page views: 3277
- PDF downloads: 1005