What Promises the CJD Diagnosis in a Case of Rapidly Progressive Dementia?
Received: 02-Oct-2018 / Accepted Date: 26-Oct-2018 / Published Date: 30-Oct-2018 DOI: 10.4172/2161-0460.1000452
Keywords: Creutzfeldt-Jakob disease; Dementia; Diagnosis; Prion disease
Abbreviations
AD: Alzheimer Dementia; CJD: Creutzfeldt-Jakob Disease; CSF: Cerebrospinal Fluid; EEG: Electroencephalogram; LBD: Lewy Body Dementia; MMSE: Mini-Mental State Examination; MRI: Magnetic Resonance Imaging; RT-QuIC: Real-Time Quaking-Induced Conversion; WHO: World Health Organization.
Case Report
A 75-year-old, right-handed, white woman with a history of migraines presented to a tertiary care neurological centre for rapidly progressing cognitive decline. The previous year, she had sought care at a community clinic after several months of progressive memory changes noted by her family. At that time, she had difficulty finding her parked car, concentrating, and performing calculations. She reported having unformed auditory hallucinations and delusions. Her Mini-Mental State Examination (MMSE) score was 25. Two months after her evaluation at the community clinic, she became increasingly disoriented. She developed intermittent tangential speech and resting and action tremors. Laboratory test results were unremarkable for B12, folate, thyroid-stimulating hormone, antithyroperoxidase antibodies, complete blood count, and comprehensive metabolic panel. Apolipoprotein E testing was negative. Imaging reports of T2-weighted fluid-attenuated inversion recovery Magnetic Resonance Imaging (MRI) without contrast was reported as moderate to severe scattered hyper intensities consistent with small-vessel ischemia by the local radiological read. Cerebrospinal Fluid (CSF) studies revealed a normal basic profile, a negative infectious encephalitis panel, and absent 14-3-3 protein levels. Positron Emission Tomography (PET) has been done 6 months into her disease course revealed hypo metabolism of bilateral posterior temporal and parietal lobes and bilateral frontal lobes, consistent with Alzheimer Dementia (AD). A repeat MRI at 9 months was reportedly unchanged. A routine Electroencephalogram (EEG) showed left temporal slowing, mild diffuse background slowing, and no periodic waves. Over the next month, the patient’s disorientation significantly worsened, preventing further MMSE testing. About 1 year after the onset of symptoms, the patient could only communicate with “yes” and “no.”
The patient’s declining mental function and inability to perform activities of daily living required her to be placed in an assisted living facility. She developed severe cognitive impairment, mild right facial and leg weakness, and pseudobulbar affect unresponsive to dextromethorphan and quinidine. She presented to our facility 13 months after her initial evaluation. Her family history was notable for a brother with an unspecified tremor and a father with Parkinson disease, which he developed at age 80. She did not smoke or use recreational drugs, she consumed alcohol 1-2 times a month, and she consumed 1 cup of coffee daily. She had not traveled before symptom onset.
Our patient had a history of resting tremor and asymmetric Parkinsonism, but no myoclonus or startle myoclonus initially, which placed LBD in the differential diagnosis. Other potential causes, such as stroke or lymphoma, could have explained our patient’s physiological symptoms, but, when all factors are taken into account, such pathologies are improbable. Although MRI and 14-3-3 protein in CSF were was not interpreted as consistent to indicate prion disease at work-up, prion disease remained a possibility. Our patient’s history did not suggest seizures, and CSF studies were normal. However, autoimmune encephalitis has various presentations, including memory loss and behavioral changes, and CSF studies can be unremarkable. Central nervous system vasculitis was also possible but less likely in the absence of inflammatory markers and CSF pleocytosis or protein elevation.
At the time of our evaluation, the patient was primarily nonverbal. She was wheelchair bound and fully dependent on others for all activities of daily living. She frequently choked on food, had visual and auditory hallucinations, and was easily and frequently startled. On occasion, her mental status would wax and wane. Neurological examination demonstrated a paucity of movement, rigidity, and asymmetric cog wheeling. The patient did not follow commands and demonstrated pseudobulbar affect. No startle myoclonus was elicited. The severity of her cognitive deficits precluded administering an MMSE. We considered the differential diagnosis and diagnostic approach next.
Examinations
Rapidly progressive dementia often progresses to death within 1-2 years of onset. Prion diseases are rare, with an incidence of 1.2 in 1 million, but should be considered in the differential diagnosis [1]. However, data from major prion referral centers indicate that several other conditions are often misdiagnosed as prion disease [2,3]. In the National Prion Disease Pathology Surveillance Center cohort, 352 of 1,106 brain autopsies revealed non-prion pathology [3]. Of the 304 autopsies with adequate tissue for pathologic analysis, 51% (n=154) had AD, and 23% (n=71) had potentially reversible conditions. In a University of California, San Francisco (UCSF) cohort, 44% of evaluated patients (n=622) with rapidly progressive dementia were diagnosed with non-prion disease [4]. Atypical manifestations of AD (n=19) or Lewy Body Dementia (n=12) (LBD) in the brains of patients with suspected Creutzfeldt-Jakob Disease (CJD) have been reported [5]. The patients had rapidly progressive neurological symptoms or sharp wave complexes on EEG or both. Most AD and LBD patients also met CJD criteria. All patients had limb rigidity and myoclonus. Patients with a longer disease course were more likely to have AD, whereas those with Parkinsonism and fluctuations were more likely to have LBD.
Discussion
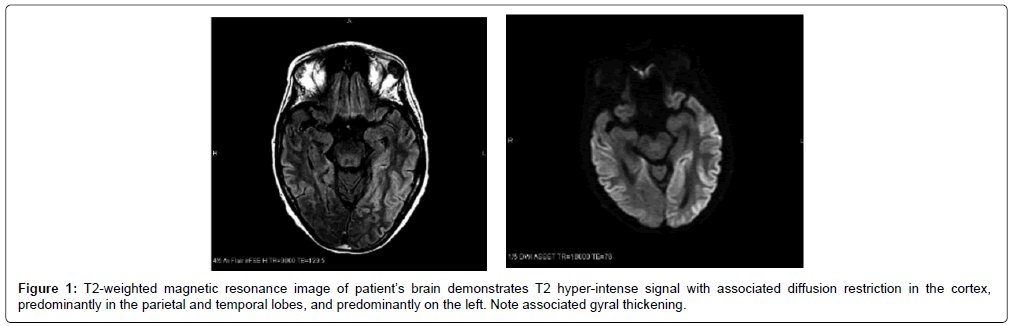
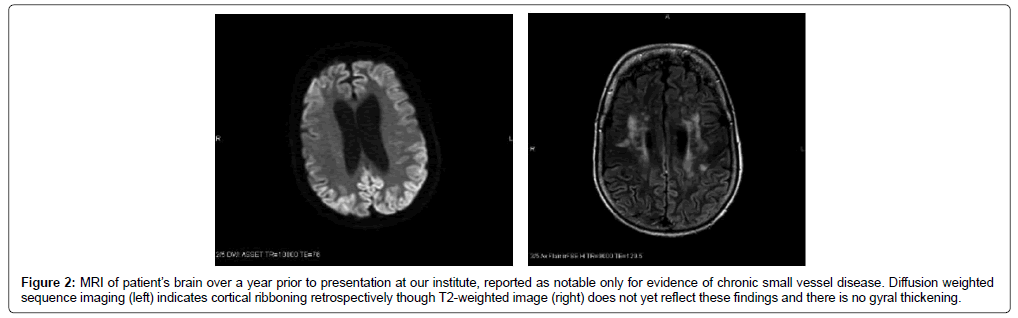
The findings of dementia, exaggerated startle, and parkinsonian features narrowed the differential diagnosis to prion disease or LBD. Although the initial MRI reports were unremarkable, the actual MRIs could not be obtained for evaluation initially. Repeat EEG showed only a moderately organized background, and occasional bilateral independent and generalized periodic discharges. The patient was admitted to the hospital after examination in our clinic. A repeat brain MRI showed bilateral cortical ribboning not reported on previous imaging (Figure 1). A brain biopsy confirmed a diagnosis of spongiform encephalopathy, and further pathologic testing revealed sporadic CJD. Of note, prior MRI images were not received until after diagnostic workup, including repeat imaging and brain biopsy. While the documented imaging reports did not report CJDassociated MRI findings, subsequent review of the images indicated that our patient had evidence of cortical ribboning on early imaging done at initial evaluation (Figure 2).
Figure 2: MRI of patient’s brain over a year prior to presentation at our institute, reported as notable only for evidence of chronic small vessel disease. Diffusion weighted sequence imaging (left) indicates cortical ribboning retrospectively though T2-weighted image (right) does not yet reflect these findings and there is no gyral thickening.
This case illustrates the reliability of the clinical diagnosis, symptoms, progression, and brain biopsy over that of an EEG, CSF, and MRI for the diagnosis of CJD. The characteristic MRI findings were identified in our patient late in the disease progression. Sporadic CJD is the most common prion disease, with a mean survival of about 6 months and mean age of onset of 64 years [6,7]. The most common initial symptom in 114 of 400 patients with definite or probable sporadic CJD was cognitive dysfunction (specifically memory, followed by aphasia and executive dysfunction). Cerebellar, constitutional, and behavioral symptoms were the next most common and occurred with similar frequency. Of the patients with cerebellar features (n=22), most (84%) had gait abnormalities; vertigo or dizziness comprised 41% of the constitutional symptoms [8-10].
Our patient met CJD clinical criteria and had progressed to advanced disease, but her history of reported unremarkable MRI and CSF findings mandated consideration of alternative diagnoses. We also considered repeat imaging to re-evaluate for changes.
Conclusion
The major diagnostic criteria for CJD are the 2007 UCSF criteria and the 2009 European MRI-CJD Consortium criteria, both of which are based on the revised World Health Organization (WHO) criteria from 1998 [9,10]. The WHO criteria are not sensitive for diagnosis early in disease as they rely on late symptoms for classification, whereas the UCSF and European MRI-CJD criteria include imaging findings. Restricted diffusion in cortical or deep gray matter, specifically caudate and putamen, has 92-96% sensitivity and 93-94% specificity for sporadic CJD [e-1]. EEG was the earliest available diagnostic test for sporadic CJD with characteristic findings of 1-2–Hz periodic sharp wave complexes. Typical EEG changes are not seen until late in the disease course [e-2]. CSF studies are usually normal or with slightly elevated protein. [e-3]. However, CSF, EEG, and MRI testing are not always adequate. In a 2016 study delineating 4 stages of CJD for earlystage detection, researchers found mostly negative results for 14-3-3 proteins in stage 1, a yield of around 80% by stage 2, and diminished sensitivity by stage 4 [e-4]. The same study found only a 30% sensitivity for EEG by stage 3 (Supplementary Table e-1) [e-4]. Various case reports have described insignificant EEG findings and MRIs without typical characteristics in confirmed CJD [e 5-6]. Additionally, a case report in 2008 described CJD without significant MRI or EEG findings, and negative 14-3-3 protein [e-7]. Our analysis of our patient relied on records reporting unremarkable MRIs. In retrospective review of the source images, cortical ribboning was evident. This is not an uncommon error the National Prion Clinic retrospectively found that 83 of 91 images in confirmed CJD cases had CJD-associated changes (91% sensitivity), but this discrepancy was reported in only 43 cases (47% sensitivity) [e-8]. Considering the inadequate sensitivity of prior testing methods, real-time quaking-induced conversion (RT-QuIC) seems most promising for CJD diagnosis. By amplifying amyloid fibrils and allowing detection of abnormal prion protein in CSF, RTQuIC has ≤ 92% sensitivity and 98-100% specificity [e-9]. RT-QuIC analysis provides early, quantitative results which are easier to obtain than brain biopsies. Furthermore, limiting brain biopsies limits the risk of iatrogenic cross-contamination. Results can take 5 days to return and can still miss 11-13% of CJD [e-10]. Newer generations of the RT-QuIC analysis have been reported to provide results in 4 h-14 h with a higher sensitivity [e-10]. Considering that brain biopsies have a 20% - 65% sensitivity, RT-QuIC should be the preferred diagnostic tool for rapidly progressing encephalopathy to rule out CJD because of its comparatively non-invasive and ante mortem nature [e-11].
Acknowledgments
The authors thank the staff of Neuroscience Publications at Barrow Neurological Institute for assistance with manuscript preparation.
Financial Support
National Institute on Aging (5P20GM109025 and Keep Memory Alive Foundation).
References
- Holman RC, Belay ED, Christensen KY, Maddox RA, Minino Am, et al. (2010) Human prion diseases in the United States. PLoS One 5: e8521.
- Poser S, Mollenhauer B, Kraubeta A, Zerr I, Steinhoff BJ, et al. (1999) How to improve the clinical diagnosis of Creutzfeldt-Jakob disease. Brain 122: 2345-2351.
- Chitravas N, Jung RS, Kofskey DM, Blevins JE, Gambetti P, et al. (2011) Treatable neurological disorders misdiagnosed as Creutzfeldt-Jakob disease. Ann Neurol 70: 437-444.
- Geschwind MD (2016) Rapidly Progressive Dementia. Continuum (Minneap Minn) 22: 510-537.
- Tschampa HJ, Neumann M, Zerr I, Henkel K, Schröter A, et al. (2001) Patients with Alzheimer’s disease and dementia with Lewy bodies mistaken for Creutzfeldt-Jakob disease. J Neurol Neurosurg Psychiatry 71: 33-39.
- Tuzun E, Dalmau J (2007) Limbic encephalitis and variants: Classification, diagnosis and treatment. Neurologist 13: 261-271.
- Geschwind MD (2015) Prion diseases. Continuum (Minneap Minn) 21: 1612-1638.
- Rabinovici GD, Wang PN, Levin J, Cook L, Pravdin M, et al. (2006) First symptom in sporadic Creutzfeldt-Jakob disease. Neurology 66: 286-287.
- World Health Organization (1998) Global surveillance, diagnosis and therapy of human transmissible spongiform encephalopathies: Report of a WHO consultation Geneva, Switzerland.
- Zerr I, Kallenberg K, Summers DM, Romero C, Taratuto A, et al. (2009) Updated clinical diagnostic criteria for sporadic Creutzfeldt-Jakob disease. Brain 132: 2659-2668.
Citation: Aslam S, Fritz MA, Cordes L, Sabbagh MN (2018) What Promises the CJD Diagnosis in a Case of Rapidly Progressive Dementia? J Alzheimers Dis Parkinsonism 8: 452. DOI: 10.4172/2161-0460.1000452
Copyright: © 2018 Aslam S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 5202
- [From(publication date): 0-2018 - Nov 11, 2025]
- Breakdown by view type
- HTML page views: 4311
- PDF downloads: 891