Wnt Signaling and Histological Characteristics of Odontogenic Tumors
Received: 30-Dec-2019 / Accepted Date: 14-Jan-2020 / Published Date: 21-Jan-2020 DOI: 10.4172/2476-2024.1000160
Abstract
We examined Notch signaling in oral tumors; most of our reported findings are based on immunohistochemical studies of Notch expression in odontogenic tumors. In our investigation, we suggested that Notch signaling is involved in cell differentiation and epithelial-mesenchymal interaction in odontogenic tumors. In addition, Jagged is strongly expressed as a ligand of Notch in odontogenic tumors, and Jagged-Notch signaling may play an important role in odontogenic tumorigenesis. Wnt is expressed during tooth development and may be involved in odontogenic tumor cell differentiation and epithelial-mesenchymal interaction. Interestingly, Wnt and Notch expression sites are similar in odontogenic tumors, suggesting that Notch and Wnt signals cooperatively affect tumor formation.
Keywords: Wnt; Notch; Ameloblastoma; Ameloblastic fibroma; Odontogenic myxoma
Introduction
We have reviewed our studies on Notch signaling of oral neoplasms in the International Journal of Molecular Science [1]. We examined Notch signaling via immunohistochemistry in cases of ameloblastoma, ameloblastic carcinoma, calcifying epithelial odontogenic tumors, squamous odontogenic tumors, ameloblastic fibroma, odontogenic myxoma, and calcifying odontogenic cysts. In epithelial tumors, Notch-positive staining products were frequently detected in epithelial tumor nests, especially in differentiating cells. In mixed epithelial and mesenchymal tumors, Notch-positive products were present in both the epithelial and ectomesenchymal components. In mesenchymal tumors, Notch-positive products were not detected. In cases of odontogenic carcinoma, products strongly positive for Notch were detected in most neoplastic cells. Positive reactions tended to be strong in areas with high proliferating activity.
In our investigation, we suggest that Notch signaling plays a role in cytological differentiation, epithelial-mesenchymal interaction, formation of tumor stroma, and acquisition of tissue specific characteristics in neoplastic cells of tooth enamel organ-derived neoplasms, including benign and malignant neoplasms. Our previous studies demonstrated that Jagged is a major Notch ligand expressed on odontogenic tumors [2,3]. Therefore, Notch signaling in odontogenic tumorigenesis is thought to be triggered by Jagged-Notch signaling. Jagged-Notch signaling is involved in hard tissue formation in tooth development [4]. This signaling is also inherited in tumorigenesis and may be related to acquisition of the characteristics of odontogenic tumors.
In tooth development, the Bmp, Fgf, Shh, Wnt and Notch pathways act as major signaling pathways and are involved in imparting organspecific features [5-7]. Wnt signaling modulates diverse cellular processes during embryogenesis and histogenesis. In addition, deregulation of Wnt signaling has been implicated in tumorigenesis and progression. It is known that Wnt signaling plays fundamental roles in odontogenesis through epithelial-mesenchymal interactions; this interaction is thought to directly reflect the development of odontogenic neoplasms [6]. Our review also conveys that Wnt signaling may be involved in tumor cell differentiation of odontogenic tumors. Therefore, we additionally examined Wnt signaling molecules in odontogenic tumors.
Materials and Methods
10 cases of ameloblastic fibroma (as mixed epithelial and mesenchymal tumors), 12 cases of ameloblastoma (as epithelial tumors) and 10 cases of odontogenic myxoma (as mesenchymal tumors) from the archives of the Department of Oral Pathology and Medicine, Graduate School of Medicine, Dentistry and Pharmaceutical Sciences, Okayama University, were reassessed histopathologically based on the WHO classification. After histopathological examination, we examined the distribution of Wnt1 and β-catenin through double immunofluorescence staining. This study was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (Approval number: 1604-015).
Results
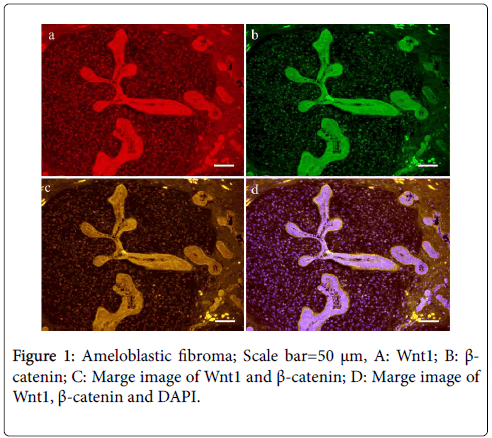
Histopathologically, ameloblastic fibroma was composed of cell-rich dental papilla-like mesenchymal tissue and enamel organ-like epithelial nests and strands. The peripheral layered cells of these cell nests were columnar or cuboidal and lined up in a palisade fashion. Ameloblastoma was composed of mature loose connective tissue stroma and enamel organ-like tumor nests. The central cells were loosely arranged, showing stellate reticulum. Odontogenic myxoma was composed of randomly oriented stellate, spindle-shaped, and/or round cells. Immunohistochemical analysis of ameloblastic fibroma revealed Wnt1 in almost epithelial and mesenchymal components. The peripheral columnar cells of epithelial tumor nests were strongly positive for Wnt1. Although β-catenin was clearly localized to the cell membrane of tumor cells, nuclear translocation was observed in stellate reticulum cells and several papilla-like mesenchymal cells (Figure 1).
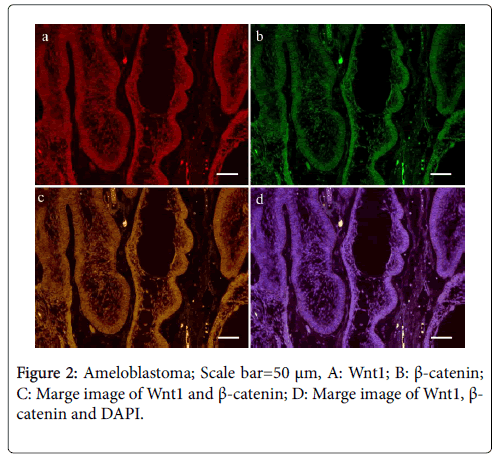
In the case of ameloblastoma, Wnt1 and β-catenin were present mainly in the tumor nests (Figure 2).
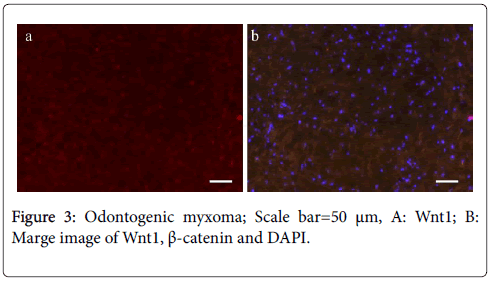
On the other hand, their expression in the stroma was restricted. In the case of odontogenic myxoma, almost no Wnt1- and β-cateninpositive tumor cells were detected (Figure 3); this finding is in contrast to that observed in the mesenchymal tissue of ameloblastic fibroma, which showed several Wnt1- and β-catenin-positive cells.
Discussion
Results of this examination suggest that Wnt signaling plays a role in the tumorigenesis of ameloblastic fibroma through odontogenic epithelium-ectomesenchymal tissue interaction. Interestingly, the immunohistochemical localization of Notch reported in our study is similar to the expression pattern of Wnt localization in odontogenic tumors. Recent studies have suggested a crosstalk between Notch signals and Wnt signals [8,9].
Conclusion
In general, Wnt signaling is responsible for the regulation of cell fate, morphogenesis, and/or development. Results of our additional examination suggest that Wnt signaling may be involved in cross-talk with Notch signaling, which affects the tumorigenesis of odontogenic tumors through odontogenic cell differentiation and epitheliumectomesenchymal tissue interaction. Signalings that regulate tooth development may be related to the acquisition of the characteristics of odontogenic tumors, and examination of these signaling factors may help in the diagnosis of odontogenic tumors.
Declarations
Ethics approval and consent to participate.
This study was approved by the Ethics Committee of Okayama University Graduate School of Medicine, Dentistry and Pharmaceutical Sciences (Approval number: 1604-015).
Consent for publication
Not applicable.
All data generated or analysed during this study are included in this published article.
Conflict of Interest
The authors declare that they have no competing interests.
Funding
This work was supported by JSPS KAKENHI Grant Numbers JP18K09789, JP18K17224, JP19K19160.
Author's contributions
KN, KT and HK selected appropriate cases of odontogenic tumors. KN, SY and HM performed the histological examination of odontogenic tumors. KN, TK and HN evaluated and analyzed the immunostaining results. KN was a major contributor in writing the manuscript. All authors read and approved the final manuscript.
Acknowledgements
Not applicable.
References
- Nakano K, Takabatake K, Kawai H, Yoshida S, Maeda H, et al. (2019) Notch signaling affects oral neoplasm cell differentiation and acquisition of tumor-specific characteristics. Int J Mol Sci 20: E1973.
- Siar CH, Kawakami T, Buery RR, Nakano K, Tomida M, et al. (2011) Notch signaling and ghost cell fate in the calcifying cystic odontogenic tumor. Eur J Med Res 16: 501-506.
- Siar CH, Nakano K, Ng KH, Tomida M, Nagatsuka H, et al. (2010) Squamous odontogenic tumor of the mandible: A case report demonstrating immunoexpession of notch1,3,4, jagged1 and delta1. Eur J Med Res 15: 180-184.
- Jheon AH, Prochazkova M, Meng B, Wen T, Lim YJ, et al. (2016) Inhibition of notch signaling during mouse incisor renewal leads to enamel defects. J Bone Miner Res 31: 152-162.
- Balic A, Thesleff I (2015) Tissue interactions regulating tooth development and renewal. Curr Top Dev Biol 115: 157-186.
- Liu F, Millar SE (2010) Wnt/beta-catenin signaling in oral tissue development and disease. J Dent Res 89: 318-330.
- Cai X, Gong P, Huang Y, Lin Y (2011) Notch signalling pathway in tooth development and adult dental cells. Cell Prolif 44: 495-507.
- Kay SK, Harrington HA, Shepherd S, Brennan K, Dale T, et al. (2017) The role of the Hes1 crosstalk hub in notch-wnt interactions of the intestinal crypt. PLoS Comput Biol 13: e1005400.
- Uribe-Etxebarria V, Luzuriaga J, GarcÃa-Gallastegui P, Agliano A, Unda F, et al. (2017) Notch/Wnt cross-signalling regulates stemness of dental pulp stem cells through expression of neural crest and core pluripotency factors. Eur Cell Mater 34: 249-270.
Citation: Nakano K, Takabatake K, Kawai H, Yoshida S, Maeda H, et al. (2020) Wnt Signaling and Histological Characteristics of Odontogenic Tumors. Diagn Pathol Open 5: 160. DOI: 10.4172/2476-2024.1000160
Copyright: © 2020 Nakano K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Open Access Journals
Article Tools
Article Usage
- Total views: 4968
- [From(publication date): 0-2020 - Dec 20, 2025]
- Breakdown by view type
- HTML page views: 4026
- PDF downloads: 942