Research Article Open Access
Preparation and Characterization of Microglia-Like Cells Derived from Rat, Mouse, and Human Bone Marrow Cells for Therapeutic Strategy of Alzheimer's Disease
Kazuyuki Takata1*, Tetsuya Takada1, Hironori Tatsuda1, Tomomi Tsuruno1, Kaneyasu Nishimura2, Shun Shimohama3 and Yoshihisa Kitamura11Department of Neurobiology, Kyoto Pharmaceutical University, Misasagi, Yamashina-ku, Kyoto 607-8414, Japan
2Department of Biological Repair, Institute for Frontier Medical Sciences and Department of Cell Growth and Differentiation, Center for iPS Cell Research and Application (CiRA) Kyoto University, 53 Kawahara-cho, Shogoin, Sakyo-ku, Kyoto 606-8507, Japan.
3Department of Neurology, Sapporo Medical University, School of Medicine, S1W16, Chuo-ku, Sapporo 060-8543, Japan
- *Corresponding Author:
- Kazuyuki Takata, Ph.D
Department of Neurobiology
Kyoto Pharmaceutical University, Misasagi
Yamashina-ku, Kyoto 607-8414, Japan
Tel: +81-75-595-4706
Fax: +81-75-595-4796
E-mail: kaz@mb.kyoto-phu. ac.jp
Received October 02, 2011; Accepted December 16, 2011; Published December 21, 2011
Citation: Takata K, Takada T, Tatsuda H, Tsuruno T, Nishimura K, et al. (2011) Preparation and Characterization of Microglia-Like Cells Derived from Rat, Mouse, and Human Bone Marrow Cells for Therapeutic Strategy of Alzheimer’s Disease. J Addict Res Ther S5:001. doi: 10.4172/2155-6105.S5-001
Copyright: © 2011 Takata K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Addiction Research & Therapy
Abstract
Dementia-associated neuropsychiatric symptoms, such as depression and apathy, are core features of Alzheimer’s disease (AD). Pathological hallmarks of AD include senile plaques, neurofibrillary tangles (NFTs), and neurodegeneration. Senile plaques are composed of amyloid-β (Aβ) and are surrounded by microglia, an immune effector cells in brains. Studies on responsible genes of familial AD have suggested that Aβ accumulation is a primary event that influences other AD pathologies, and the reduction of brain Aβ has been proposed as a therapeutic target for AD. On the other hand, microglial phagocytosis has been noted as an Aβ clearance system in the brain. In this context, the transplantation of microglia prepared from bone marrow cells may contribute to the clearance of Aβ in vivo brain. As a first step for this cell therapeutic strategy, we examined the preparation of microglia-like cells from mouse, rat, and human bone marrow cells by the treatment with macrophage colony-stimulating factor (M-CSF) and analyzed their phagocytic ability. In the cultivation of bone marrow cells collected from mouse, rat, and human, adherent cells on the culture dish were markedly increased by the treatment with human M-CSF. In addition, the adherent cells from mouse bone marrow cells were more sensitive to human M-CSF than that from rat bone marrow cells and more effectively expressed microglial makers, such as cluster of differentiation (CD) 11b, Iba1, and CD68. Furthermore, we demonstrated that a part of adherent cells derived from mouse and human bone marrow cells have phagocytic abilities of iron particles and Aβ peptides, and the treatment with human M-CSF significantly increased the number of phagocytic cells. Thus, we positively suggest that the microglia-like cells prepared from bone marrow cells by the treatment with human M-CSF could be a source for the cell therapeutic strategy for AD.
Keywords
Alzheimer’s disease; Microglia; Amyloid-β; Phagocytosis; Bone-marrow stem cell; Macrophage colony-stimulating factor; Differentiation
Abbreviations
Aβ: Amyloid-β, Aβ42, Aβ1-42; AD: Alzheimer’s disease; ANOVA: Analysis of Variance; APP: Amyloid Precursor Protein; βIII-T: Neuronal Class III Β-Tubulin; BBB: Blood-Brain Barrier; CD: Cluster Of Differentiation; EDTA: Ethylenediaminetetraacetic Acid; ES: Embryonic Stem; FCS: Fetal Calf Serum; Fisher’s PLSD: Fisher’s Protected Least Significant Difference; GM-CSF: Granulocyte- Macrophage Colony-Stimulating Factor; GFAP: Glial Fibrillary Acidic Protein; Iba1: Ionized Calcium Binding Adaptor Molecule 1; IMDM: Iscove’s Modified Dulbecco’s Medium; iPS: Induced Pluripotent Stem; Mac-1: Mouse CD11b; M-CSF: Macrophage Colony-Stimulating Factor; MEM: Minimum Essential Medium; NFTs: Neurofibrillary Tangles; NG2: Neuron/Glial 2 Chondroitin Proteoglycan; ODC: Oligodendrocytes; PBS: Phosphate Buffered-Saline; PS: Presenilin
Introduction
Alzheimer’s disease (AD) is characterized by progressive cognitive impairment as a consequence of extensive neuronal loss [1]. Almost all patients diagnosed with AD develop dementiaassociated neuropsychiatric symptoms at some stage during their disease [2]. Principal pathological features of AD are extracellular deposition of fibrillar amyloid-β (Aβ) and formation of intraneuronal neurofibrillary tangles (NFTs) composed of hyperphosphorylated tau. It is also well known that microglia markedly accumulate on the Aβ depositions (senile plaques) in AD brain [3]. Microglia are primary immune effector cells distributed ubiquitously throughout the central nervous system and have an ability as phagocytes. Although some studies have demonstrated that in some specific condition, microglia can be detrimental in the AD brain [4,5], many others have supported the theory that they are actually beneficial [6,7].
Aβ peptides are produced from amyloid precursor protein (APP) by combination cleavages with β-secretase and γ-secretase. Aβ is composed of 37-43 amino acid residues because γ-secretase, which is a protein complex containing presenilin (PS), generates the C-terminal of Aβ with different length [8]. In studies of familial AD, mutations in genes of APP, PS1, and PS2 have been found, and transgenic mice models carrying these familial AD-linked mutations enhance the Aβ production in their brains [9-11]. These findings strongly suggest that the accumulation of Aβ in brain is a primary event driving other AD pathogenesis, such as NFTs formations and neuronal loss (amyloid cascade hypothesis) [12].
A number of experimental studies have been performed to test the integrity of the amyloid cascade hypothesis. The studies of Aβ immunotherapy are principal one. Immunization with Aβ peptide in transgenic mouse models of AD significantly reduced the formation and deposition of Aβ and restored cognitive function [13-16]. In the experimental studies in animal models of AD, the phagocytic activity of microglia is proposed as an Aβ clearance system in the brain [17]. In a case report of the clinical trial of Aβ immunization also suggests microglial contribution in the clearance of Aβ in the human AD brain [18].
We previously transplanted with primary cultured rat microglia into the lateral ventricle in the Aβ-injected rats and demonstrated that exogenous microglia accumulated on Aβ deposit and increased Aβ clearance [19]. Thus, we speculate that the transplantation with freshly prepared exogenous human microglia may be effective for the Aβ clearance in human brain, and it positively suggests the cell therapeutic strategies for AD. In the clinical application of this cell therapeutic strategy for human AD, preparation of exogenous human microglia is a first and important step. However, ethical and technical difficulties in the primary-culture of human microglia preclude the preparation. Recent study demonstrated that the mouse bone marrow cells could be differentiated to microglia-like cells by the treatment with macrophage-colony stimulating factor (M-CSF) [20].
In this study, we cultured bone marrow cells collected from mouse, rat, and human in the presence of human M-CSF and characterized their abilities such as proliferation and Aβ phagocytosis. Thus, we evaluated a possibility of bone marrow-derived cells as a source for the cell therapeutic strategy for AD.
Materials and Methods
Preparation of rat and mouse bone marrow-derived cells and cell count
All animal experiments were carried out in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, and the protocols were approved by the Committee for Animal Research at Kyoto Pharmaceutical University. Bone marrow cells were harvested from male C57BL/6 mice or Sprague-Dawley rats by flushing the femurs and tibias with Minimum Essential Medium (MEM)-α (Wako, Osaka, Japan). Cells were centrifuged at 300 g for 5 min and gently resuspended with lysing buffer, which contains 150 mM NH4Cl, 1.0 mM KHCO3, and 0.1 mM ethylenediaminetetraacetic acid (EDTA), to remove red blood cells. After the filtration through 70-μm-diameter nylon mesh (BD Bioscience, Billerica, MA, USA), cells were centrifuged again at 300 g for 5 min. Cells collected were resuspended with MEM-α supplemented with 10% fetal calf serum (FCS), plated into 60-mm-diameter dishes at concentration of 2.5 x 106 cells/mL and then incubated at 37Ã?°C in 5% CO2/95% air in the presence or absence of 2.0 x 104 units/mL human M-CSF (Leukoprol®, Kyowa Hakko, Tokyo, Japan).
At three, seven, and fourteen days after the cultivation, non- adherent cells were discarded by gentle rinse with phosphate buffered- saline (PBS), whereas adherent cells in triplicate wells were collected with PBS containing 0.25% trypsin and 0.05% EDTA, and then cells were counted with improved Neubauer-type cell counter plate (PHJapan, Hiroshima, Japan).
Human bone marrow-derived cell culture
Human bone marrow cells expressing cluster of differentiation (CD)34 were purchased from Lonza (Basel, Switzerland) and were seeded at concentration of 1.5 x 105 cells/mL with Iscove’s modified Dulbecco’s medium (IMDM; Lonza) supplemented with 15% FCS at 37Ã?°C in 5% CO2/95% air in the presence or absence of 2.0 x 104 units/mL human M-CSF. At three, seven, and fourteen days after the cultivation, non-adherent cells were discarded by gentle rinse, and adherent cells in three views of 430 x 580-μm2 field from triplicate wells were counted under a light microscope (IX70; Olympus, Tokyo, Japan).
Flow cytometry
To investigate the differentiation state, flow cytometric method was employed. Adherent cells collected (1.0 x 105 cells) were incubated at 37Ã?°C for 2 hrs with antibodies against mouse CD11b (Mac-1; dilution 1:100, Invitrogen, Carlsbad, CA, USA), rat CD11b (clone OX42; 1:500, Harlan Sera-Lab, Leicestershire, UK), mouse CD68 (clone FA-11; 1:500, Serotec, Oxford, UK), rat CD68 (clone ED-1; 1:500 Serotec), ionized calcium binding adaptor molecule 1 (Iba1; 1:1,000, WAKO), glial fibrillary acidic protein (GFAP; 1:1,000, Dako, Glostrup, Denmark), oligodendrocytes (ODC; 1:1,000, Millipore, Billerica, MA, USA), neuron/glial 2 chondroitin proteoglycan (NG2; 1:500, Millipore), or neuronal class III β-tubulin (βIII-T; clone TUJ1; 1:1,000, Covance, Princeton, NJ, USA). After washes with PBS, cells were subsequently incubated at 37Ã?°C for 2 hrs with Alexa Fluor® 633-conjugated secondary antibodies against mouse immunoglobulin (Ig) G, rat IgG, or rabbit IgG, appropriately (each 1:500, Invitrogen). Cells labeled with secondary antibodies were sorted by a fluorescence- activated cell sorter (FACSCalibur, Becton Dickinson, San Jose, CA), and data were analyzed with CellQuest software (Becton Dickinson).
Assay for phagocytosis
To assess phagocytic ability, adherent cells were treated with super-paramagnetic iron particles (Resovist®; 10 μg/mL, Nihon Schering K.K.,Osaka, Japan) or synthetic human Aβ1-42 peptide (Aβ42; 1 μM, AnaSpec, San Jose, CA, USA) for 12 hrs. The Aβ42 peptide used in this study is hydrochloride form and self-aggregates into oligomeric and fibrillar Aβ [21]. The smear patterns of this Aβ42 peptide in Western blot analysis were shown in our previous study [22]. After several rinses, cells were then fixed with PBS containing 4% paraformaldehyde. To visualize iron particles phagocytosed by adherent cells, Prussian blue method was utilized by the incubation of fixed cells with PBS containing 1% potassium ferrocyanide and 1% hydrogen chloride at 4Ã?°C for 30 min. The number of blue-colored cells in five or three views of 450 x 580-μm2 field from quintuplicate wells for mouse cells or triplicate wells for human cells was counted, respectively.
Aβ phagocytosis was investigated by laser confocal microscopic study. Fixed cells originated from mouse bone marrow cells were incubated with anti-mouse CD11b antibody (Mac-1; 1:100, Invitrogen) and anti-Aβ antibody (clone 82E1; 1:1,000, Immuno-Biological Laboratories Co., Ltd., Gunma, Japan) at 4Ã?°C for 4 days. Human cells were incubated with anti-Iba1 antibody (1:1,000, WAKO) and anti-Aβ antibody (clone 82E1; 1:1,000). Cells were then appropriately followed by the incubation with Alexa Fluor® 488-congugated anti-mouse IgG antibody and Alexa Fluor® 546-conjugated anti-rat IgG antibody or Alexa Fluor® 546-conjugated anti-rabbit IgG antibody (each 1:500, Invitrogen) at room temperature for 2 hrs. For the counter staining of nuclei, Hoechst 33258 (1:5,000, Invitrogen) was added on this step. Subsequently, fluorescent was detected by a laser confocal microscope (LSM510META; Carl Zeiss, Jena, Germany). For image analysis in mouse bone marrow-derived cells in triplicate wells, intensity of Aβ immunoreactivity in the intracellular space was determined after background subtraction using the fixed cut-off, <95 in 0-255 levels (WinRoof, Mitani, Fukui, Japan). In the studies using human bone marrow-derived cells, the number of cells in three views of 450 x 580- μm2 field from triplicate wells was counted.
Statistical analysis
All of the data represent the means ± S.E.M. Statistical significance of differences between the treatments at each time point was determined by Student’s t-test (StatView, Abacus Concepts, Berkeley, CA, USA). Data was further analyzed by two-way analysis of variance (ANOVA) followed by Fisher’s protected least significant difference (Fisher’s PLSD) post-hoc tests with treatment and culture days as sources of variation.
Results
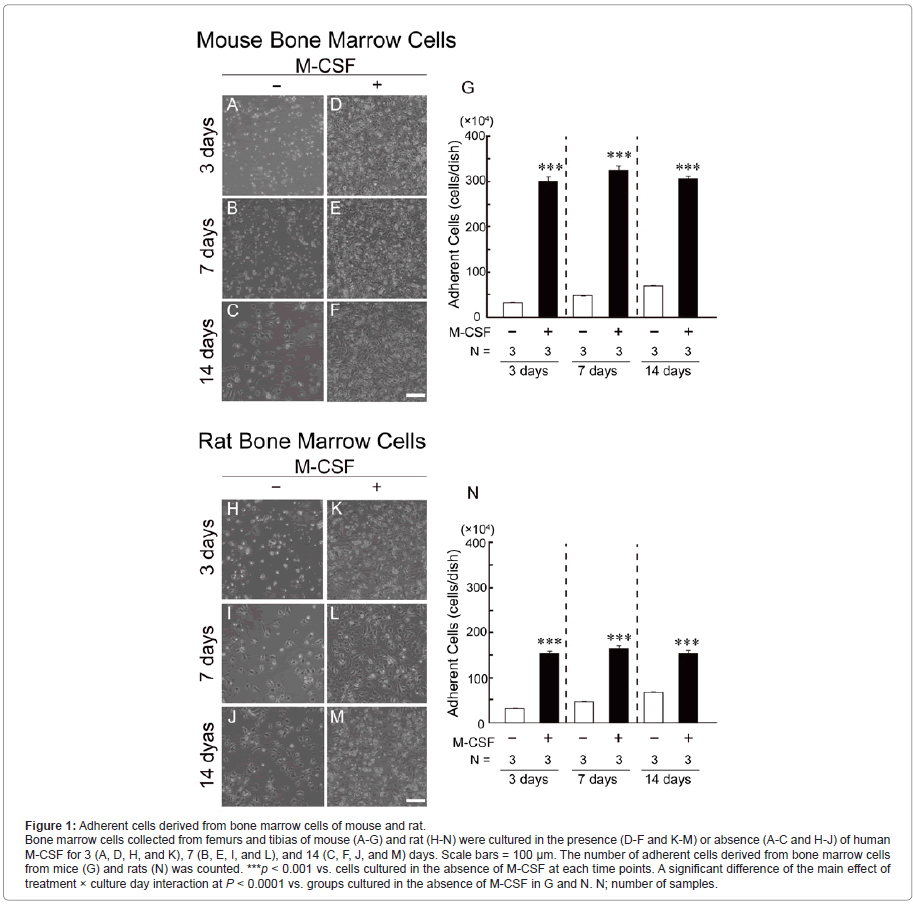
Number of adherent cells derived from mouse and rat bone marrow cells
During the cultivation of bone marrow cells collected from mouse (Figure 1, A-G) and rat (Figure 1, H-N), adherent cells on the culture dish were gradually increased even in the absence of human M-CSF (Figure 1, A-C and H-J and white columns in Figure 1, G and N). In the presence of human M-CSF (2.0 x 104 units/mL), although the number of adherent cells originated from mice (Figure 1, D-F) and rats (Figure 1, K-M) was markedly increased even at 3 days after the cultivation, it was no more changed after 3 days (black columns in Figure 1, G and N). In addition, the number of adherent cells derived from mouse bone marrow cells was more abundant than those derived from rat bone marrow cells.
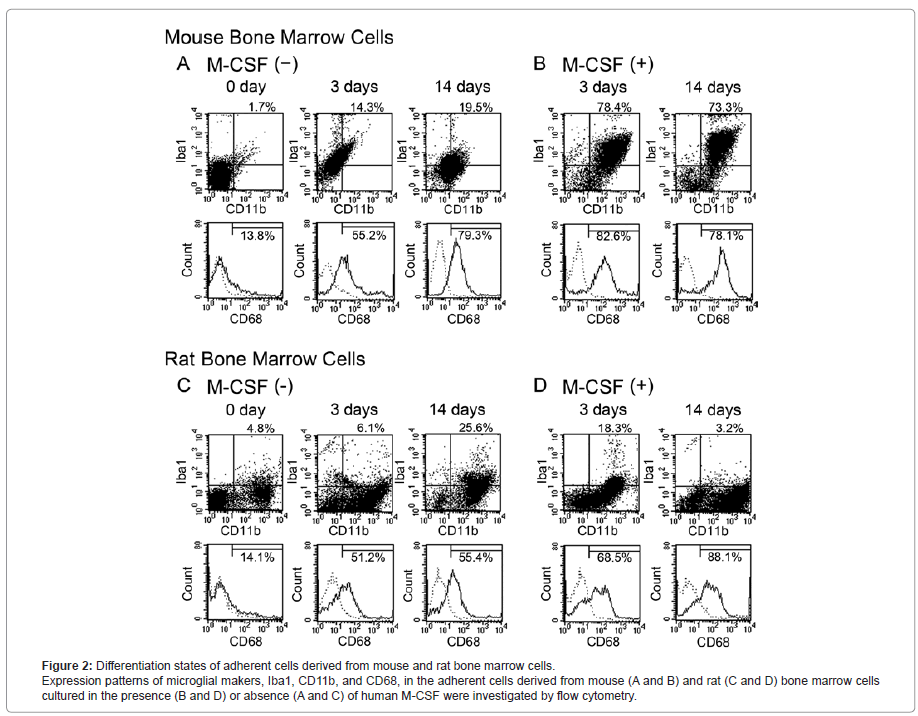
Differentiation state of mouse and rat bone marrow-derived cells
Differentiation states of mouse (Figure 2, A and B) and rat (Figure 2, C and D) bone marrow-derived cells cultured in the presence (Figure 2, B and D) and absence (Figure 2, A and C) of human M-CSF were investigated by flow cytometry. In the study just after the cell collection (0 day in Figure 2, A and C), bone marrow cells from mice and rats scarcely expressed microglial antigens such as Iba1 and CD68. Although mouse bone marrow cells also did not express CD11b at 0 day, approximately a half of the cells originated from rat were CD11b-immunopositive (CD11b+).
In the absence of human M-CSF, mouse bone marrow-derived cells gradually expressed microglial markers in a culture day- dependent manner, and 19.5% of the cells were CD11b and Iba1 double-immunopositive (CD11b+/Iba1+) and 79.3% of the cells were CD68-immunopositive (CD68+) at 14 days after the cultivation (Figure 2A). At the same time point, 25.6% and 55.4% of rat bone marrow- derived cells were CD11b+/Iba1+ and CD68+, respectively (Figure 2C).
In the presence of human M-CSF, the number of CD11b+/Iba1+ cells and CD68+cells derived from mouse bone marrow cells was markedly increased and reached at the maximum (78.4% and 82.6%, respectively) at 3 days after the cultivation (Figure 2B). On the other hand, the number of CD11b+/Iba1+ cells derived from rat bone marrow cells reached at the maximum (18.3%) at 3 days after the cultivation, and that of CD68+ cells reached at maximum (88.1%) at 14 days after the cultivation (Figure 2D). Thus, the mouse bone marrow cells seem to be more sensitive to human M-CSF than the rat bone marrow cells and effectively expressed several microglial markers.
Although we further investigated the expressions of the makers of astrocytes (GFAP), oligodendrocytes (ODC), neurons (βIII-T) and glial progenitor (NG2), these makers were not detected in the mouse and rat bone marrow-derived cells under all culture conditions examined in this study (data not shown).
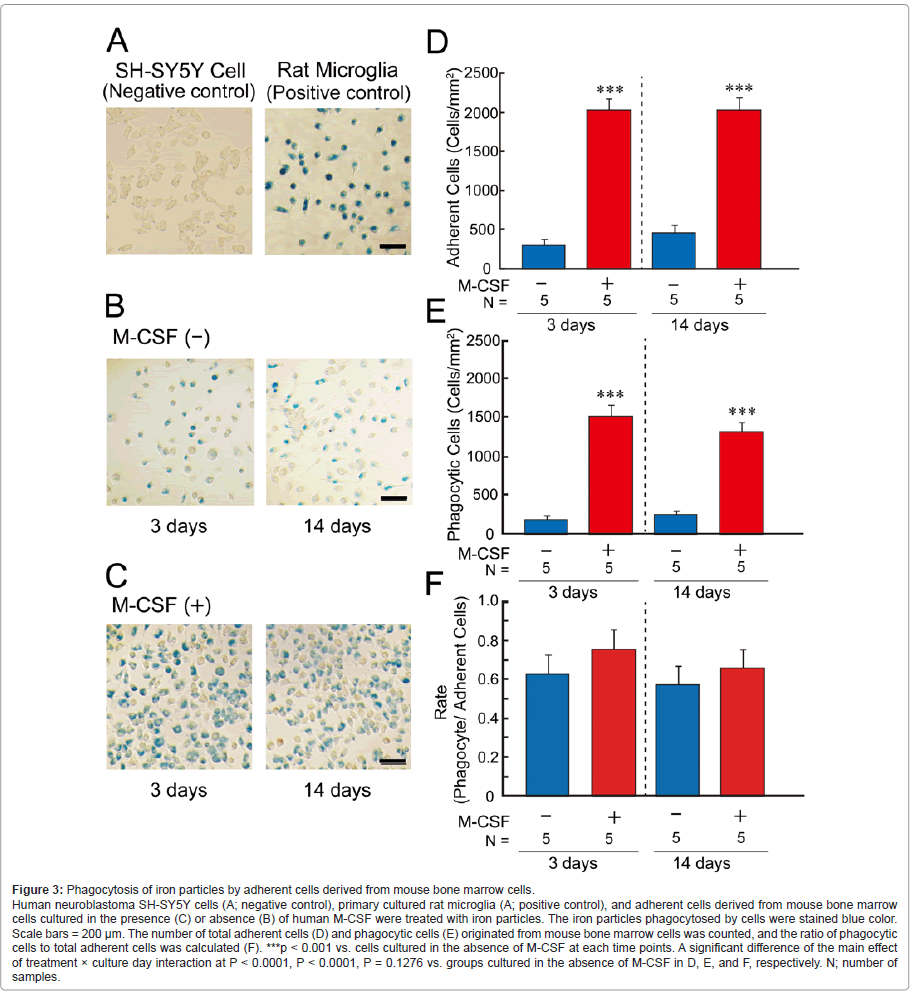
Phagocytosis of iron particles by mouse bone marrow- derived cells
We next examined phagocytic ability using iron particles (Figure 3). Adherent cells originated from mouse bone marrow cells, but not originated from rats, were used in this assay because the mouse bone marrow cells were more sensitive to human M-CSF than the rat bone marrow cells and more effectively differentiated to microglia- like cells (Figure 2). Iron particles (blue color in Figure 3, A-C) were markedly phagocytosed by primary-cultured rat microglia, but not human neuroblastoma SH-SY5Y cells (Figure 3A) [19,23]. In the presence (Figure 3C) and absence (Figure 3B) of human M-CSF, a part of adherent cells were stained blue color. Although the number of adherent cells (Figure 3D) and blue-colored cells (phagocytic cells; Figure 3E) was markedly increased by the treatment with human M-CSF (red columns in Figure 3, D and E), the ratio of phagocytic cells to total adherent cells were unchanged by the treatment (Figure 3F).
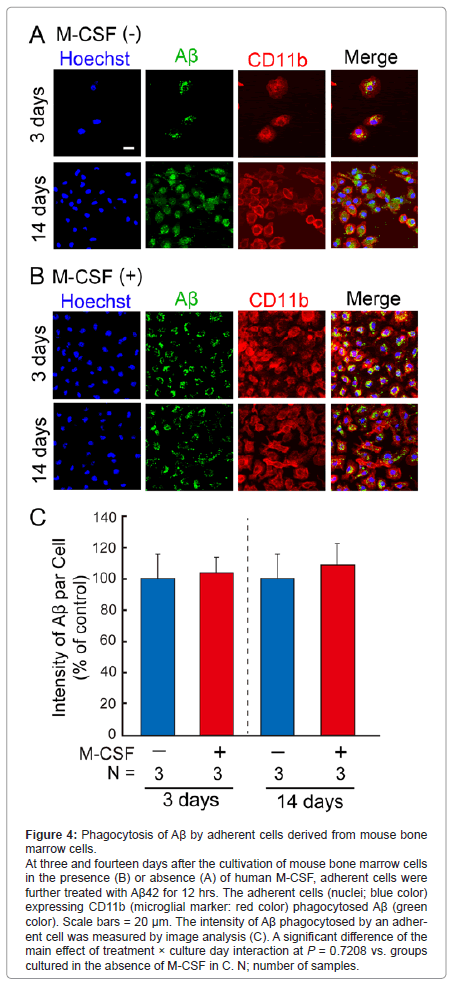
Phagocytosis of Aβ by mouse bone marrow-derived cells
We next examined phagocytic ability of adherent cells derived from mouse bone marrow cells using Aβ42 peptides (Figure 4). In the absence of human M-CSF (Figure 4A), the number of adherent cells was gradually increased (blue color in Figure 4A), and the adherent cells expressing CD11b (red color in Figure 4A) phagocytosed Aβ (green color in Figure 4A). In the presence of human M-CSF (Figure 4B), the number of adherent cells reached at confluent at 3 days after the seeding (blue color in Figure 4B), and the adherent cells expressing CD11b (red color in Figure 4B) markedly phagocytosed Aβ (green color in Figure 4B). We further measured intensity of Aβ phagocytosed by the adherent cells (Figure 4C). The intensity in a phagocytic cell was unchanged by the treatment with human M-CSF.
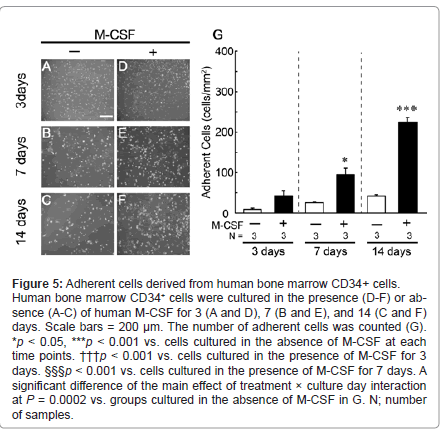
Number of adherent cells derived from human bone marrow CD34+ cells
During the cultivation of human bone marrow CD34+ cells (Figure 5), adherent cells on the culture dish were slightly increased in the absence of human M-CSF (Figure 5, A-C and white columns in G). In the presence of human M-CSF, the number of adherent cells was markedly increased in a culture day-dependent manner (Figure 5, D-F and black columns in G).
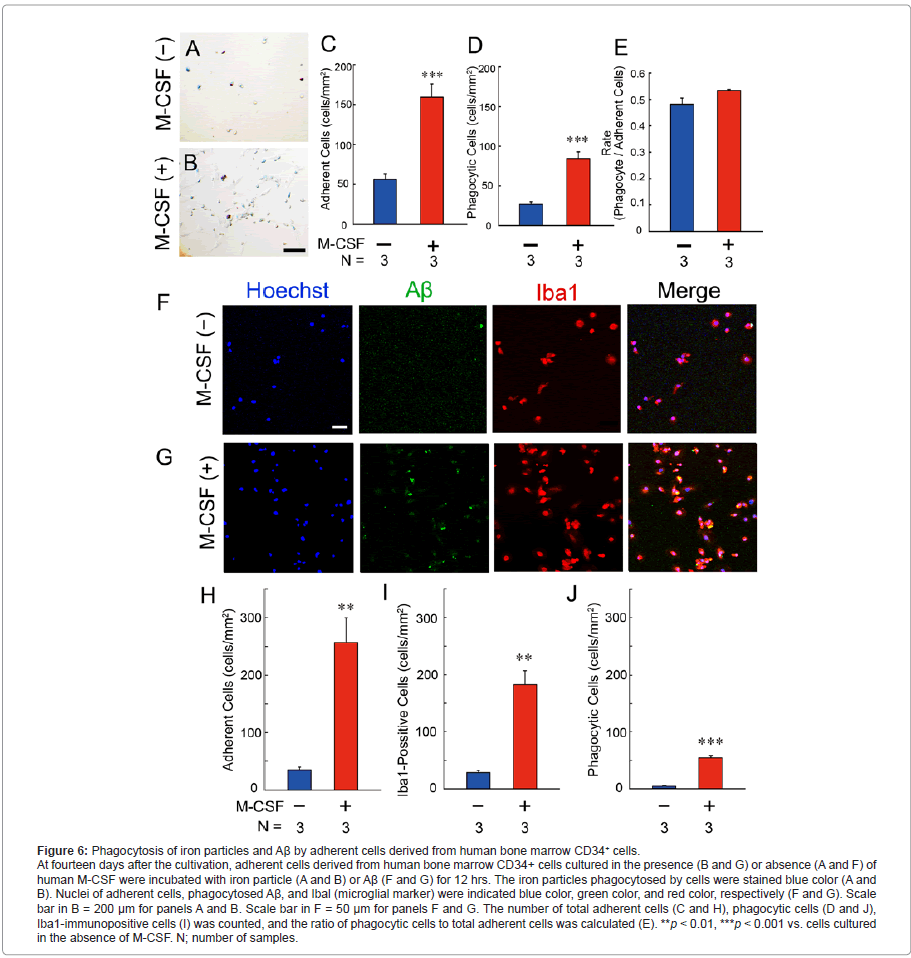
Phagocytic ability of adherent cells derived from human bone marrow CD34+ cells
At fourteen days after the cultivation, we examined phagocytic ability of the adherent cells derived from human bone marrow CD34+ cells using iron particles (Figure 6, A-E). In contrast to the negative control of human SH-SY5Y cells (Figure 3A), a part of adherent cells was stained blue color in the presence (Figure 6B) and absence (Figure 6A) of human M-CSF. Although the number of total adherent cells (Figure 6C) and blue-colored cells (phagocytic cells; Figure 6D) was markedly increased by the treatment with human M-CSF (red columns in Figure 6, C and D), the ratio of phagocytic cells to total adherent cells were unchanged by the treatment (Figure 6E).
We further examined Aβ phagocytosis using Aβ42 peptides (Figure 6, F-J). In the absence of human M-CSF, although total adherent cells (blue color in Figure 6F and blue column in Figure 6H) mostly expressed Iba1 (red color in Figure 6F and blue column in Figure 6I), a few cells phagocytosed Aβ (green color in Figure 6F and blue column in Figure 6J). In the presence of human M-CSF, the total number of adherent cells (blue color in Figure 6G and red column in Figure 6H), Iba1-immunopositive cells (red color in Figure 6G and red column in Figure 6I), and Aβ-phagocytic cells (green color in Figure 6G and red column in Figure 6J) was markedly increased.
Discussion
Previous study demonstrated that exogenously administered microglia effect to the clearance of Aβ in the experimental ex vivo study using unfixed brain sections from both AD cases and transgenic mice [17]. Therefore, we previously prepared rat primary cultured microglia from brains of new bone Wistar rats and directly transplanted it into the lateral ventricle of the Aβ-injected rats [19]. The trace of transplanted microglia was examined using the magnetic resonance imaging, and we found that exogenous microglia accumulated on Aβ deposit and increased Aβ clearance in the brain of Aβ-injected rats. In the study, we transplanted with microglia into the lateral ventricle, whereas several groups reported that intra-arterially injected microglia can migrate through an intact and/or injured blood-brain barrier (BBB) to the brain parenchyma [24,25]. Therefore, we speculate that the transplantation with freshly prepared exogenous human microglia from systemic circulation may be effective for the Aβ clearance in human brain, and it positively suggests the cell therapeutic strategies for AD.
In the clinical application of this cell therapeutic strategy for human AD, preparation of exogenous human microglia is an important step. Although it is possible to prepare human microglia from fetal and adult human brain [26,27], ethical and technical difficulties may preclude the clinical application. Previous in vivo studies suggested that mouse bone marrow-derived cells can migrate across the BBB, differentiate into parenchymal microglia [28,29], and furthermore accumulate on the Aβ plaques in mouse models of AD [30,31]. In vitro studies also showed that microglia-like cells can be prepared from the mouse bone marrow cells by the treatment with M-CSF [20] or granulocyte-M-CSF (GM-CSF) [32]. In this study, we treated human bone marrow cells as well as those from mice and rats with human M-CSF and investigated their abilities of proliferation and phagocytosis.
We first examined bone marrow cells from mice and rats. The mouse bone marrow cells were more sensitive to human M-CSF than the cells from rats and effectively increased the number of cells that expressed microglial protein markers (Figure 2,B and D). To address the reason for the different sensitivity between the cells from mice and rats, we performed an alignment analysis of M-CSF in human and mouse or human and rat and found that the homology of mouse M-CSF (70.3%) is higher than that of rat M-CSF (61.8%) (Supplementary Figure S1 and Supplementary Text).This result may explain the reason, at least in part, why the mouse bone marrow cells were more sensitive to human M-CSF than the cells from rats. We further examined phagocytic analysis using mouse bone marrow- derived cells and found that the cells can phagocyte Aβ (Figure 4). Although the amount of Aβ phagocytosed by single phagocytic cell was at the same level, the number of Aβ phagocytic cell was markedly increased by the treatment of human M-CSF.
We subsequently demonstrated that human bone marrow-derived cells were able to differentiate to the microglia-like Aβ phagocytic cells (Figure 6, F and G) and the treatment with M-CSF markedly increased the number of phagocytic cells (Figure 6J). Although the ratio of phagocytic cells to total adherent cells were unchanged by the treatment with human M-CSF (Figure 6E), the number of Aβ phagocytic cells was markedly increased by the treatment (Figure 6, D and J). The stable preparation and enough supply of the cells for the transplantation are important issue on the clinical application. In addition, it have been reported that the treatment with M-CSF acidifies the lysosomes of mouse microglia and increases the degradation of Aβ [33,34]. Therefore, the preparation of microglia- like Aβ phagocytic cells by the treatment with human M-CSF may have beneficial advantages in the cell therapeutic strategies for AD.
As far as we know, no study clearly demonstrates the Aβ phagocytosis in bone marrow-derived cells, especially human origin. Thus, the novel findings in the present study are summarized as following; (i) bone marrow-derived microglia-like cells are able to phagocytose Aβ, and (ii) proliferation of the Aβ phagocytic cells can be significantly increased by the treatment of M-CSF. Taken together, we positively suggest that the transplantation of microglia-like Aβ phagocytic cells derived from human bone marrow cells, furthermore embryonic stem (ES) cells and/or induced pluripotent stem (iPS) cells could be a powerful tool for the cell therapeutic approach in AD. Further studies in the biology and regulation of microglia-like cells derived from stem cells may provide a novel insight into the cell therapeutic strategies for AD.
Acknowledgements
We thank Dr. Toshio Kaneda (Faculty of Pharmaceutical Sciences, Hoshi University) and Dr. Osamu Honmou (Sapporo Medical University, School of Medicine) for helpful discussions. This study was supported by Frontier Research Programs from the Ministry of Education, Culture, Sports, Science and Technology of Japan; grants-in-aid from Japan Society for the Promotion of Science; and Kyoto Pharmaceutical University Fund for the Promotion of Scientific Research.
References
- Selkoe DJ (1999) Translating cell biology into therapeutic advances in Alzheimer's disease. Nature 399: A23-A31
- Lyketsos CG, Carrillo MC, Ryan JM, Khachaturian AS, Trzepacz P, et al. (2011) Neuropsychiatric symptoms in Alzheimer's disease. Alzheimers Dement 7: 532-539.
- Neuroinflammation working group (2000) Inflammation and Alzheimer’s disease. Neurobiol Aging 21: 383-421.
- Giulian D, Haverkamp LJ, Yu JH, Karshin W, Tom D, et al. (1996) Specific domains of β-amyloid from Alzheimer plaque elicit neuron killing in human microglia. J Neurosci 16: 6021-6037.
- Walker DG, Lue LF (2005) Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer’s disease and other neurodegenerative diseases. J Neurosci Res 81: 412-425.
- Kitamura Y, Yanagisawa D, Takata K, Taniguchi T (2009) Neuroprotective function in brain microglia. Current Anaesthesia & Critical Care 20: 142-147.
- Turrin NP, Rivest S (2006) Tumor necrosis factor a but not interleukin 1β mediates neuroprotection in response to acute nitric oxide excitotoxicity. J Neurosci 26: 143-151.
- Wiltfang J, Esselmann H, Bibl M, Smirnov A, Otto M, et al. (2002) Highly conserved and disease-specific patterns of carboxyterminally truncated Aβ peptides 1-37/38/39 in addition to 1-40/42 in Alzheimer's disease and in patients with chronic neuroinflammation. J Neurochem 81: 481-496.
- Games D, Adams D, Alessandrini R, Barbour R, Borthelette P, et al. (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F β-amyloid precursor protein. Nature 373: 523-527.
- Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, et al. (1996) Correlative memory deficits, Aβ elevation, and amyloid plaques in transgenic mice. Science 274: 99-102.
- Holcomb L, Gordon MN, McGowan E, Yu X, Benkovic S, et al. (1998) Accelerated Alzheimer-type phenotype in transgenic mice carrying both mutant amyloid precursor protein and presenilin 1 transgenes. Nat Med 4: 97-100.
- Hardy J, Selkoe DJ (2002) The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science 297: 353-356.
- Dodart JC, Bales KR, Ganno KS, Greene SJ, DeMattos RB, et al. (2002) Immunization reverses memory deficits without reducing brain Aβ burden in Alzheimer's disease model. Nat Neurosci 5: 452-457.
- Janus C, Pearson J, McLaurin J, Mathews PM, Jiang Y, et al. (2000) Aβ peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer's disease. Nature 408: 979-982.
- Morgan D, Diamond DM, Gottschall PE, Ugen KE, Dickey C, et al. (2000) Aβ peptide vaccination prevents memory loss in an animal model of Alzheimer's disease. Nature 408: 982-985.
- Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, et al. (1999) Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature 400: 173-177.
- Bard F, Cannon C, Barbour R, Burke RL, Games D, et al. (2000) Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat Med 6: 916-919.
- Nicoll JA, Wilkinson D, Holmes C, Steart P, Markham H, et al. (2003) Neuropathology of human Alzheimer disease after immunization with amyloid-β peptide: a case report. Nat Med 9: 448-452.
- Takata K, Kitamura Y, Yanagisawa D, Morikawa S, Morita M, et al. (2007) Microglial transplantation increases amyloid-β clearance in Alzheimer model rats. FEBS Lett 581: 475-478.
- Noto D, Takahashi K, Miyake S, Yamada M (2010) In vitro differentiation of lineage-negative bone marrow cells into microglia-like cells. Eur J Neurosci 31: 1155-1163.
- Kaneko I, Tutumi S (1997) Matters arising: conformations of β-amyloid in solution. J Neurochem 68: 438-439.
- Takata K, Kitamura Y, Kakimura J, Shibagaki K, Tsuchiya D, et al. (2003) Role of high mobility group protein-1 (HMG1) in amyloid-β homeostasis. Biochem Biophys Res Commun 301: 699-703.
- Kitamura Y, Shibagaki K, Takata K, Tsuchiya D, Taniguchi T, et al. (2003) Involvement of Wiskott-Aldrich syndrome protein family verprolin-homologous protein (WAVE) and Rac1 in the phagocytosis of amyloid-β (1-42) in rat microglia J Pharmacol Sci 92: 115-123.
- Hayashi Y, Tomimatsu Y, Suzuki H, Yamada J, Wu Z, et al. (2006) The intra-arterial injection of microglia protects hippocampal CA1 neurons against global ischemia-induced functional deficits in rats. Neuroscience 142: 87-96.
- Imai F, Suzuki H, Oda J, Ninomiya T, Ono K, et al. (2007) Neuroprotective effect of exogenous microglia in global brain ischemia. J Cereb Blood Flow Metab 27: 488-500.
- Lee SC, Liu W, Roth P, Dickson DW, Berman JW, et al. (1993) Macrophage colony-stimulating factor in human fetal astrocytes and microglia. Differential regulation by cytokines and lipopolysaccharide, and modulation of class II MHC on microglia. J Immunol 150: 594-604.
- Lue LF, Walker DG, Rogers J (2001) Modeling microglial activation in Alzheimer's disease with human postmortem microglial cultures. Neurobiol Aging 22: 945-956.
- Hess DC, Abe T, Hill WD, Studdard AM, Carothers J, et al. (2004) Hematopoietic origin of microglial and perivascular cells in brain. Exp Neurol 186: 134-144.
- Simard AR, Rivest S (2004) Bone marrow stem cells have the ability to populate the entire central nervous system into fully differentiated parenchymal microglia. FASEB J 18: 998-1000.
- Malm TM, Koistinaho M, Pärepalo M, Vatanen T, Ooka A, et al. (2005) Bone-marrow-derived cells contribute to the recruitment of microglial cells in response to β-amyloid deposition in APP/PS1 double transgenic Alzheimer mice. Neurobiol Dis 18: 134-142.
- Simard AR, Soulet D, Gowing G, Julien JP, Rivest S (2006) Bone marrow-derived microglia play a critical role in restricting senile plaque formation in Alzheimer's disease. Neuron 49: 489-502.
- Hinze A, Stolzing A (2011) Differentiation of mouse bone marrow derived stem cells toward microglia-like cells. BMC Cell Biol 12: 35.
- Majumdar A, Cruz D, Asamoah N, Buxbaum A, Sohar I, et al. (2007) Activation of microglia acidifies lysosomes and leads to degradation of Alzheimer amyloid fibrils. Mol Biol Cell 18: 1490-1496.
- Majumdar A, Capetillo-Zarate E, Cruz D, Gouras GK, Maxfield FR (2011) Degradation of Alzheimer's amyloid fibrils by microglia requires delivery of ClC-7 to lysosomes. Mol Biol Cell 22: 1664-1676.
Relevant Topics
- Addiction Recovery
- Alcohol Addiction Treatment
- Alcohol Rehabilitation
- Amphetamine Addiction
- Amphetamine-Related Disorders
- Cocaine Addiction
- Cocaine-Related Disorders
- Computer Addiction Research
- Drug Addiction Treatment
- Drug Rehabilitation
- Facts About Alcoholism
- Food Addiction Research
- Heroin Addiction Treatment
- Holistic Addiction Treatment
- Hospital-Addiction Syndrome
- Morphine Addiction
- Munchausen Syndrome
- Neonatal Abstinence Syndrome
- Nutritional Suitability
- Opioid-Related Disorders
- Relapse prevention
- Substance-Related Disorders
Recommended Journals
Article Tools
Article Usage
- Total views: 15668
- [From(publication date):
specialissue-2013 - Dec 15, 2025] - Breakdown by view type
- HTML page views : 10876
- PDF downloads : 4792