Research Article Open Access
Production and Qualitative Analysis of Biosurfactant and Biodegradation of the Organophosphate by Nocardia mediterranie
| Sukirtha TH*, Usharani MV | |
| Department of Environmental Sciences, Bharathiar University, Coimbatore-46, Tamil Nadu, India | |
| Corresponding Author : | Sukirtha TH Department of Environmental Sciences Bharathiar University Coimbatore-46 Tamil Nadu, India E-mail: sukisavier@gmail.com |
| Received January 21, 2013; Accepted July 06, 2013; Published July 08, 2013 | |
| Citation: Sukirtha TH, Usharani MV (2013) Production and Qualitative Analysis of Biosurfactant and Biodegradation of the Organophosphate by Nocardia mediterranie. J Bioremed Biodeg 4:198. doi: 10.4172/2155-6199.1000198 | |
| Copyright: © 2013 Sukirtha TH, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Synthetic Organophosphorus (OP) compounds are used extensively as agricultural and domestic pesticides and could be used as chemical warfare agents. The degradation of organophosphates in soil has gained a lot of importance due to increase the quantities of its use all over the world. The biodegradation of organophosphates were carried out by bacteria, fungi and algae effectively. The biodegradation of organophosphates in flask-quoted conditions was studied by an isolated Nocardia mediterranei from the soil under laboratory condition. The biodegradation process was carried out by the enrichment technique. The biodegradation rate by Nocardia mediterranei enhanced the rate of biodegradation to 20%–30%. Addition of the biosurfactant produced from the Nocardia mediterranei enhanced the rate of biodegradation to 30%–45%. The produced biosurfactant was found to be specific to organophosphates. The TLC technique is used for the identification of glycolipid compound in the biosurfactant. Identification and quantification analysis of biosurfactant were carried out through Standard by HPLC (Column 18). The chemical structure of the biosurfactant was identified as a glycolipid called Trehalolipid by GC-MS.
| Keywords |
| Organophosphorus (OP); Biosurfactant; Glycolipid; TLC; HPLC; GC-MS; Trehalolipid |
| Introduction |
| Pesticides in general are chemicals used to kill or control insects. These pesticides and their degradation products in various compartments of the environment may find their way into the human body through the food chain, causing various health hazards. Organic pesticides have been in use for more than 40 years. Their success have not been without side effects, such as toxicity to non-target species (including humans), and the production of persistent residues in soil and water. |
| Most pesticides used for insect and weed control are applied directly to soil or subsequently reach the soil, where these chemicals are subjected to microbial metabolism. Thus microbes, the invisible scavenger and force of nature, provide a means to eliminate unwanted residues of pesticides from the environment. An understanding of the mechanism of the pesticides, the mode of attack under different conditions, and the pattern of metabolite formed, would be essential to increase the capability of these organisms through genetic engineering, and maximise the potential benefits to be derived from the management of pesticide residue. Thus, using the utility of microorganisms to degrade many natural and manmade chemicals, and utilising the knowledge of genetic manipulation for enhanced biodegradation, it appears that the possibility of providing biological remedies to the management process of pesticide residue in the environment does exist. |
| Currently among the various groups of pesticides all over the world, the organophosphorus group forms a major and most widely used group, accounting for more than 36% of the total world market. They are used for agriculture in the home, in gardens and in veterinary practice. Organophosphorus pesticides were first developed in Germany during the Second World War in the form of Tetra Ethyl Pyro-Phosphate (TEPP), a bi-product of nerve gas development. They work by inhibiting acetyl cholinesterase, an important enzyme in the nervous system. |
| Organophosphorus insecticides are all esters of phosphoric acid and are also called organophosphates. They are mainly used to control a variety of sucking, chewing and boring insects, such as spider mites and aphids, which attack crops like cotton, sugarcane, peanuts, tobacco, as well as vegetables, fruits and ornamental plants. Although organophosphates are biodegradable in nature, their residues are found in the environment. Considering their toxicity, research on biodegradation of organophosphates is being carried out all over the world [1]. Very important members of this group are Parathion, Methyl Parathion, Chloropyrifos, Malathion, Dichlorvos, Monoprotophos and Dimethoalx. Among these, Methyl Parathions, Dichlorvos and Chlorpyrifos are currently cause outbreaks of diseases by poisoning. |
| The objective of this study is the identification of Nocardia mediterranie from soil contaminated with organophosphates (Methylparathion, Dichlorvos and Chlorpyrifos), examination of the biosurfactant production and its role in organophosphate degradation, analysis of the degradation of the organophosphate by spectrophotometer, qualitative analysis of the biosurfactant by TLC and identification and quantitative analysis of biosurfactant by HPLC. |
| Materials and Methods |
| Identification of Nocardia mediterranei |
| The organism (MTCC NO: 14) was identified by the following character and biochemical tests [2]. |
| • Colony morphology |
| • Gram staining |
| • Casein hydrolysis |
| • Citrate test |
| • Urease test |
| • Nitrate reduction test |
| • Carbohydrate fermentation. |
| Degradation of organophosphates (Methyl Parathion, Dichlorovos and Chlorpyrifos) using a microbial strain |
| The colonies formed in the culture media were taken and subcultured in 100 ml Luria Bertani Broth (LB Broth) for further experiments. The bacterial cells from the LB media were taken and again sub-cultured in the mineral media, which was incorporated with 5, 10 and 25 ppm of Methyl Parathion, Dichlorovos and Chlorpyrifosas the solo carbon source. A control was maintained and incubated for two weeks in rotary shaker at 200 rpm for each organophosphate. The growth rate was measured on the third, fifth, seventh, ninth, eleventh, thirteenth and fifteenth day by measuring the optical density using a photo spectrometer at 660 nm [3]. |
| Screening for biosurfactant production |
| Biosurfactant was usually produced as extra-cellular surfaceactive compounds synthesised by microorganisms growing on waterinsoluble substrates. Biosurfactant were produced by many bacterial genera. The composition, structure and production of variety of surfactants produced by bacteria, yeast and fungi have been described [4,5]. Biosurfactant can be classified into glycolipids, lipopeptides, lipopolysacharides, phospholipids, fatty acids and neutral lipids [5,6]. Production of Biosurfactant was often associated with the capacity of microorganisms to utilise hydrocarbons as substrates [7,8]. Surfactants are amphiphatic molecules that can modify the properties of a liquid medium at a surface by reducing surface tension. |
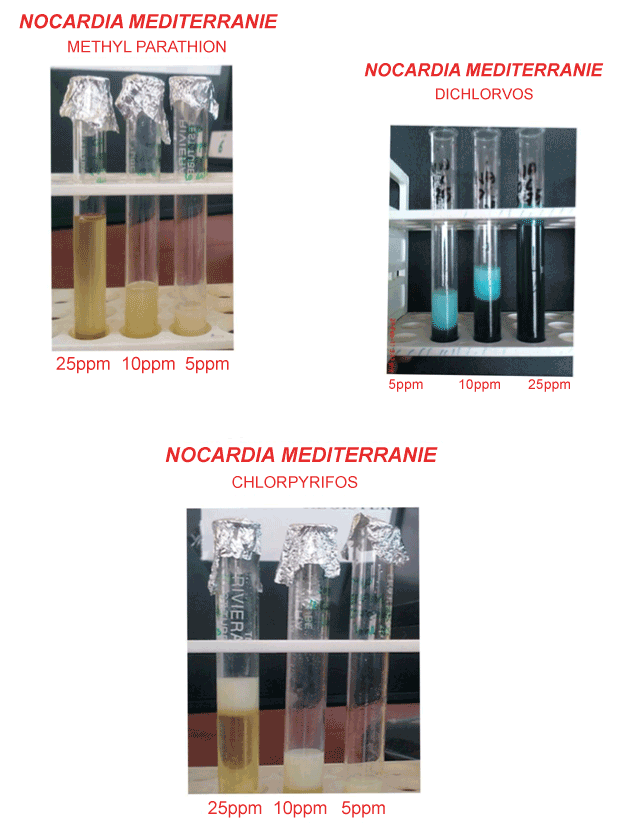
| The culture growth in 100 ml of mineral media incorporated with 5, 10 and 25 ppm Methyl Parathion, Dichlorvos and Chlorpyrifos was taken and centrifuged at 10,000 rpm at 4°C for 15 minutes, and the supernatant was separated. This supernatant was challenged with 5, 10 and 25 ppm Methyl Parathion, Dichlorvos and Chlorpyrifos to check for turbidity, which directly indicated the production of biosurfactant. |
| Test for specificity of biosurfactant (emulsification test) |
| Nine test tubes were taken and 1ml of biosurfactant was added to all test tubes. Then in first three test tubes, 10 μl of 5, 10 and 25 ppm Methyl Parathion was added, in the next three test tubes, 10 μl of 5, 10 and 25 ppm Dichlorvos was added, and in last three test tubes, 10 μl of 5, 10 and 25 ppm Chlorpyrifos was added. After a few seconds, the turbidity was found according to degradation of organophosphate. |
| Role of biosurfactant (Methyl Parathion, Dichlorvos and Chlorpyrifos) degradation |
| The biosurfactant produced by the organism is capable of degrading the pesticide that will enhance the degrading rate [9]. Three conical flasks (100 ml) were taken for each pesticide. First, with mineral medium and 5, 10 and 25 ppm of concerned organophosphate, is kept as control. The second flask contained the mineral medium incorporated with 5, 10 and 25 ppm of concerned organophosphate, 1ml of culture supernatant, which contained the biosurfactant, and inoculated with isolated organism. These flasks were then incubated in the rotary flask at 200 rpm for 2 weeks. The growth rate of each flask was studied on the third, fifth, seventh, ninth, eleventh, thirteenth and fifteenth day by using a UV spectrophotometer at 660 nm. |
| Assay of biosurfactant |
| The assay of the biosurfactant was done in the laboratory conditioned by the following procedure: The culture growth in 100 ml of mineral media incorporated with 5, 10 and 25 ppm of organophosphate (Methyl Parathion, Dichlorvos and Chlorpyrifos), which has an optical density of more than 0.1, was taken and centrifuged at 10,000 rpm at 4°C for 15 minutes. The supernatant was separated and 1 ml of culture supernatant was treated with 10 μl of 5, 10 and 25 ppm organophosphates (Methyl Parathion, Dichlorvos and Chlorpyrifos). It shows turbidity. This indicated the evident production of biosurfactant by the isolated organism to utilise the carbon from organophosphate. The O.D. of the supernatant was checked at 660 nm in UV spectrophotometer with the control. |
| Physical analysis of biosurfactant |
| Dry weight of biosurfactant: First a sterile Petri plate was taken to measure the weight. Then 2 ml of pure biosurfactant was poured on the sterile Petri plate. After that, the biosurfactant-poured petri plate was kept inside the hot-air oven for 30 minutes. Then, the dried plate was checked for weight. The dry weight of the biosurfactant was calculated using the below formula. |
| Formula: Dry weight of biosurfactant=Weight of the plate after drying – Weight of the empty plate |
| Biochemical characterization of biosurfactant |
| Qualitative analysis of biosurfactant by TLC: The biosurfactant was repeatedly washed with acetone and ether-methanol (1:2) or petroleum ether. Wax D was prepared from the purification wax fraction according to the method [10]. |
| Silica gel plate is prepared (1:2), (1 mm thickness), i.e., 30 g in 60 ml distilled water, then allow it to stay for 30 minutes after that. To activate the absorption, silica gel plate is kept in hot-air oven for 1 hour at 100°C. Spot the 20 μl washed biosurfactant on the silica gel plate, the separation of the components was examined by melting point, acidity determination, and thin layer chromatography on silica gel plates with chloroform-methanol (85:15) as developing solvent. Lipids were detected as a brown spot on the plate after spraying with sulphuric acid-dichromate [11], and charring at 150°C [12]. Then Rf value was calculated using the below formula. |
| TLC-Retention Factor (Rf): The retention factor, or Rf, is defined as the distance travelled by the compound divided by the distance travelled by the solvent. |
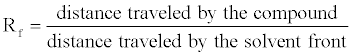 |
| Quantitative analysis of biosurfactant by HPLC |
| To investigate whether the obtained glycolipid biosurfactant included multiple components or not, it was analyzed by LCMS- 2010 EV (SHIMADZU) system, with a standard of Trehalose 6 6’ dimycolate [13,14]. The mobile phase consisted of 70% methanol and 30% methylene chloride, which was injected into a 20 μl in C18 (250 mm×4.6 mm×5 μm) reverse phase column isocratically. The injection flow rate was 0.5 ml min-1 and absorption of the output was detected by detector (SPD-M20A). |
| Structure identification by GC-MS |
| Approximately 4 mg of purified glycolipid mixed with 1 ml chloroform, 0.85 ml of methanol and 0.15 ml of sulfuric acid and heat at 100°C for 140 min. Add 1 ml of distilled water and shake vigorously for 1 min. Leave to stand for phase separation to occur. Remove bottom layer (chloroform layer) containing the fatty acid methyl esters and analyze by GC or GC-MS. GC analyses can be carried out using a DB 35-MS capillary standard non-polar column (30 Mts, ID: 0.25 mm, FILM: 0.25 μm). Analytical conditions that can be used are; injector temperature oven temp 50°C AT 0.5 min rose to 235°C AT 65 C/min hold for 12 min [15]. Electron impact at 70 eV with scan range 50-450 Da and an injection volume of 1 ml. |
| Results and Discussion |
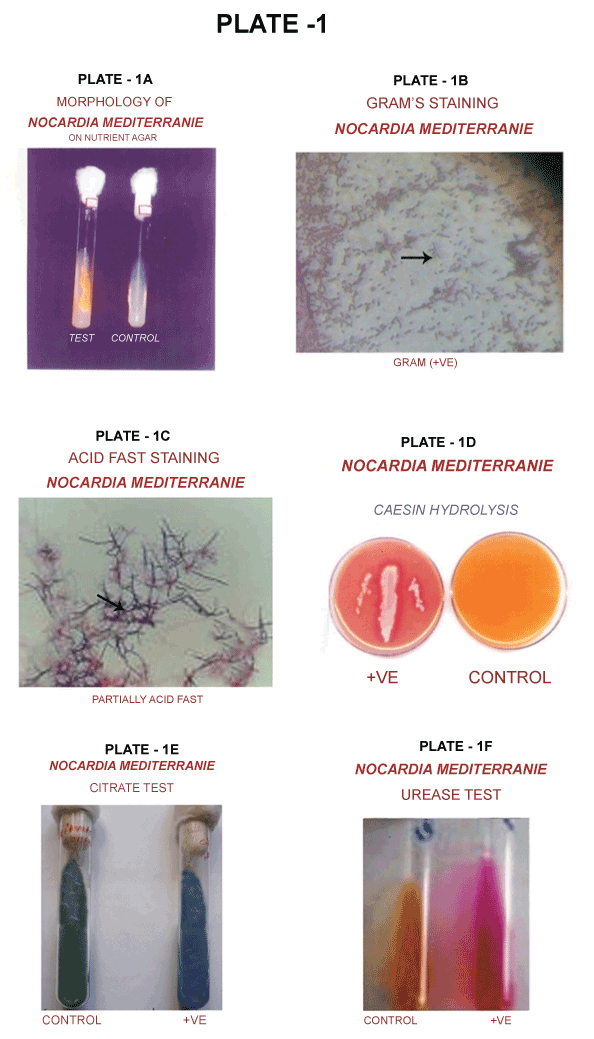
| Identification of Nocardia mediterranie colony morphology in Nutrient Agar |
| Colonies are crugate with a velvety surface due to rudimentary aerial mycelium: Yellowish-brown vegetative mycelium with soluble pigment of orange colour (Plate 1A). |
| Gram’s staining |
| The 24 hours-old bacterial culture was smeared and stained by Gram’s Method. Gram positive rod-shaped bacteria with branched filaments were examined under light microscope (Plate 1B). |
| Acid fast bacilli staining |
| The culture was smeared and stained for acid fast bacilli with carbol fuchsin as the primary stain, 70% of H2SO4 as decolouriser, and malachite green as counter stain. Partially acid fast bacilli were observed under light microscope (Plate 1C). |
| Casein hydrolysis |
| Twenty-four-hours culture was streaked on the casein agar and incubated for 48 hours. A clear zone was formed around the streaked area, which indicated that the Nocardia mediterranei had hydrolyzed casein (Plate 1D). |
| Citrate test |
| The culture was streaked in Simmon citrate medium and incubated for 48 hours. The green colour medium was converted to blue colour, which indicated that the isolated organism had utilized citrate (Plate 1E). |
| Nitrate test |
| Nitrate reduction was performed and it was found that the isolated organism was able to reduce nitrate. |
| Urease test |
| Urea hydrolysis by urease enzymes produced by the organism was examined. It was found that the Nocardia mediterranei had hydrolysed urea (Plate 1F). |
| Carbohydrate fermentation |
| Carbohydrate fermentation test was done by using different sugars, like glucose, inositol, maltose, Sorbitol and lactose, as the carbon source and the produced acid, except sorbitol. |
| Degradation of organophosphate |
| The obtained O.D. of the culture growth is shown in Tables 1-3 and in the optical density of culture is directly proportional to the culture mass. Since actinomycetes groups are slow growers, thus the culture mass was not extremely high. Whereas the culture mass of bacteria and fungi were high when compared to actinomycetes because of their fast replication mode. Thus, the optical density of culture in the present study was low when compared to the optical density of culture reported by Awasthi et al. [9]. |
| Specificity of biosurfactant (emulsification test) |
| The turbidity was found according to degradation of organophosphate. The result was shown in Plate 2. The accelerated degradation of organophosphate in the presence of biosurfactant can either be due to the utilization of microbial cells. The former seems remote, because presence of other carbon sources such as glucose or other different carbohydrate in the culture medium has earlier shown to have no enhancement of Dichlorvos biodegradation despite the increased in microbial mass [9]. This can be due to its enhanced solubilisation or increased affinity towards microbial cells as shown for other hydrocarbons [16,17]. In the earlier study co-cultures [9] were used to degrade organophosphates, and the biosurfactant produced by it was used for the enhanced degradation process. Whereas, in the present study, a single organism was use to degrade Methyl Parathion, Dichlorvos and Chlorpyrifos, and the biosurfactant produced by it was able to enhance the degradation rate as reported in the earlier report. |
| Assay of biosurfactant |
| Absorbance of biosurfactant with organophosphate (Methyl parathion, Dichlorvos and Chlorpyrifos) shown in Table 4. |
| Percentage of Organophosphate Degradation |
| The degradation rate of 5, 10 and 25 ppm organophosphate by Nocardia sp was conducted under laboratory conditions, as evident from the 1.5% of organophosphate in the standard 100%. The percentage of organophosphate in the control sample was 94%, whereas the remaining 6% perhaps disappeared by volatilization. The percentage of Organophosphate in the test sample is 74%, in which the remaining 26% was degraded by the microbial activity; this result indicated the capability of the identified organism (Nocardia sp) to degrade organophosphate (Methyl Parathion to Phosphorous, Dichlorvos to Dimethyl phosphate and Chlorpyrifos to Trichloro-2- pyriclinol). The results obtained are shown in Table 5. |
| In the study of the concentration of Methyl Parathion is high, as in bulk disposal and spill condition (EPA 198°C) for an applied concentration of 10 and 15 ppm Methyl Parathion, losses of only 0.1% were observed after 52 days. In another simulated spill study, 42.7% of a concentration of 48,900 ppm of Methyl Parathion still remained in the soil after 1 year, and 35.8% remained after almost 4 year. |
| Comparative study of Organophosphates (methyl parathion, Dichlorvos and Chlorpyrifos) |
| After 15 days of incubation, Nocardia mediterranei showed 40% of hydrocarbon. Complete mineralization could not be achieved within this period [18]. It has been reported that 10%-30% of the residual hydrocarbon remains when bioremediation treatment is applied, but when the treatment time was prolonged, increase in degradation has been observed [18]. In the present study, the treatment could not be prolonged beyond 20 days to substantiate this observation. The reasons for low ultimate degradation rates include low bioavailability of contaminant, the accumulation of recalcitrant compound, inhibiting metabolites, and the lack of microbial growth factor [19]. Inorganic nutrients from organophosphates limit the extent of hydrocarbon degradation in the environment. |
| 5ml of LB broth that had a good growth of the isolated organism was taken and sub-cultured in mineral media, which contained 5, 10 and 25 ppm of organophosphate (Methyl Parathion, Dichlorvos and Chlorpyrifos) as a carbon source, and was incubated in rotary shaker at 200 rpm, at room temperature, for two weeks. This resulted in the growth of the organism in the mineral medium and its growth rate was measured in UV spectrophotometer at 660 nm. Then, for the comparative study of these organophosphates (Methyl Parathion, Dichlorvos and Chlorpyrifos) done by t-test. From this T-test we can conclude that Chlorpyrifos was finely degraded by Nocardia mediterranei when compared with the Methyl Parathion & Dichlorvos. Since the organism was isolated from the soil contaminated with organophosphate it may also give an effective result in-situ degradation of organophosphates. The obtained O.D. of the culture growth is shown in Table 6. |
| Physical analysis of biosurfactant |
| Dry weight of biosurfactant: The dry weight of biosurfactant was calculated using the below formula. The result is shown in Table 7. |
| Formula: Dry weight of biosurfactant=Weight of the plate after drying-Weight of the empty plate. |
| Biochemical Characterisation of Biosurfactant |
| Qualitative analysis of biosurfactant by TLC: Then Rf value was calculated and the result was shown in Plate 3. |
| Rf value=0.71 |
| Biosynthesis of biosurfactant from a variety of bacteria and yeast has been reported [20-24], most commonly involving rhamnolipids, trehalose and sophorolipids. These contain various hydroxyl fatty acids and carbohydrates, and are characterized by unique surfactant properties [25]. Partial biochemical characterization of the biosurfactant produced by our isolates indicated they were heteropolymers. The analysis of the purified biosurfactant by TLC showed respective single spot of lipid (with the exception of DDV2), protein and carbohydrate that we could not identify further. These surfactants were subsequently classified as peptidoglycolipids and glycoprotein. It must be noted that glycoprotein and peptidoglycolipids are not very common or popular biosurfactant produced by microorganisms [23,26]. Nevertheless, such polymeric bioactive compounds were reported recently [23,27]. |
| Quantitative analysis of biosurfactant by HPLC |
| The result was shown in Table 8. The cellular envelope of Mycobacterium tuberculosis is highly distinctive and harbors a wealth of unique lipids possessing diverse structural and biological properties. However, the ability to conduct global analyses on the full complement of M. tuberculosis lipids has been missing from the repertoire of tools applied to the study of this important pathogen. We have established methods to detect and identify lipids from all major M. tuberculosis lipid classes through liquid-chromatography-mass-spectrometry (LC/MS) lipid profiling. This methodology is based on efficient chromatographic separation and automated ion identification through accurate mass determination and searching of a newly created database (Mtb LipidDB) that contains 2,512 lipid entities. We demonstrate the sensitive detection of molecules representing all known classes of M. tuberculosis lipids from a single crude extract. We also demonstrate the ability of this methodology to identify changes in lipid content in response to cellular growth phases. This work provides a customizable framework and resource to facilitate future studies on mycobacterial lipid biosynthesis and metabolism [28]. |
| Structure analysis by GC-MS |
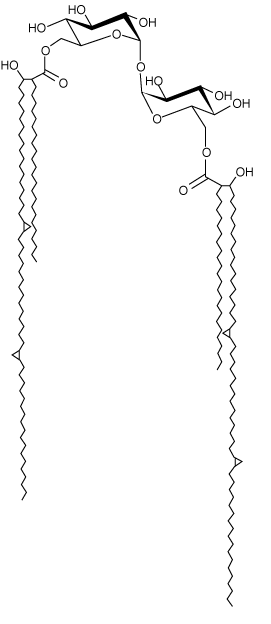
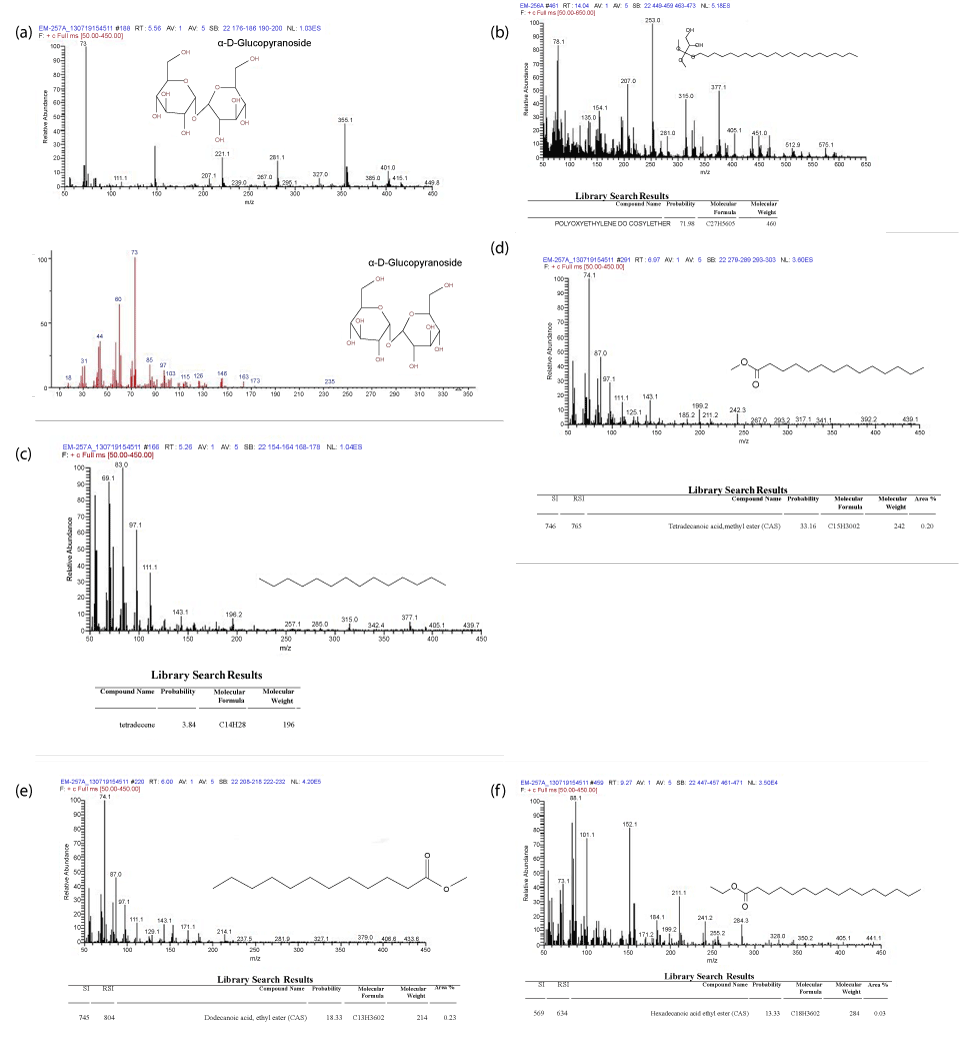
| Structure of Trehalsoe 6 6’ dimycolate Plate 4. |
| The results of GC-Mass spectra result of Biosurfactant sample of Nocardia mediterranei shown in Plate 5a,b,c,d,e and f. Analysis of the intact trehalose lipid is extremely useful for determination of the molecular weight of the glycolipids present. Since each type of trehalose lipid is present as a mixture due to different fatty acid compositions, intact MS can be used to identify all individual structures. The molecular weight along with GC-MS analysis of the fatty acids present is generally enough for total characterization [29]. |
| Different types of trehalose containing glycolipids are known to be produced by several microorganisms belonging to the mycolates group, such as Mycobacterium, Rhodococcus, Arthrobacter, Nocardia and Gordonia. Different structures have been elucidated particularly in Rhodococcus genus. Trehalolipids have gained increased interest for their potential applications in a number of fields due to their ability to lower interfacial tension and increase pseudosolubility of hydrophobic compounds. In comparison to other microbial glycolipids, trehalolipids have generally shown contrasting results and achievements with both cases of inhibition and enhancement of biodegradation rates. One of the important challenges regarding potential use of trehalose lipids in a variety of applications are the optimization of their production and downstream processing. Rhodococcal glycolipids are often bound to cellular envelopes and are usually produced along with other surfaceactive lipids. In this review, we highlight the current knowledge of trehalolipid biosurfactant applications and the latest successful strategies employed to reduce the cost of their production [4,30,31]. |
| Summary and Conclusion |
| On the basis of the study, it was concluded that the identified organism, Nocardia mediterranei, was able to degrade organophosphates (Methyl Parathion, Dichlorvos and Chlorpyrifos) under laboratory conditions. By using statistical analysis (t-Test), I found out that Chlorpyrifos was finely degraded by Nocardia mediterranei when compared with the other two (Methyl Parathion and Dichlorvos). The biosurfactant produced by this organism was found to be specific to organophosphates and it also plays a major role in enhancing the degradation processes of organophosphates. Physical and Biochemical analysis of the biosurfactant were carried out through Standard and TLC technique. Structure Identification and quantification analysis of the biosurfactant were carried out through Standard by HPLC & GC-MS (Column 18).The chemical structure of the biosurfactant was identified as a glycolipid called Trehalolipid (Trehalose 6 6’ dimycolate). |
| Acknowledgements |
| The authors are thankful to the Nehru Arts and Science College, T.M.Palayam, Coimbatore- 105 for providing facilities.The authors are also thankful to Ms.Renuka, for the GC-MS analysis was done at The South India Textile Research Association, Coimbatore. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 | Table 3 | Table 4 |
| Table 5 | Table 6 | Table 7 | Table 8 |
Figures at a glance
 |
 |
 |
 |
 |
| Plate 1 | Plate 2 | Plate 3 | Plate 4 | Plate 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16753
- [From(publication date):
July-2013 - Dec 24, 2025] - Breakdown by view type
- HTML page views : 11636
- PDF downloads : 5117
