Research Article Open Access
Production of Ethanol from High Dry Matter of Pretreated Loblolly Pine by an Evolved Strain of Saccharomyces Cerevisiae
| Gary Matthew Hawkins, Debashis Ghose, Jordan Russel, Joy Doran-Peterson* | |
| Department of Microbiology, University of Georgia, Athens, GA, USA | |
| Corresponding Author : | Joy Doran-Peterson Department of Microbiology University of Georgia, Athens, GA, USA Tel: +1-706-542-3906 E-mail: jpeterso@uga.edu |
| Received June 06, 2013; Accepted July 24, 2013; Published July 26, 2013 | |
| Citation: Hawkins GM, Ghose D, Russel J, Peterson JD (2013) Production of Ethanol from High Dry Matter of Pretreated Loblolly Pine by an Evolved Strain of Saccharomyces Cerevisiae. J Bioremed Biodeg 4:195. doi: 10.4172/2155-6199.1000195 | |
| Copyright: © 2013 Hawkins GM, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Obtaining the highest possible yields from a batch fermentation process is desirable for a cellulosic ethanol facility, as this leads to greater profitability by increasing distillation efficiency and reducing costs. One way to increase yields is by increasing the percentage of solids fermented in a batch process which leads to increased fermentable sugars available and thus increased theoretical maximum yields. One factor limiting the solids loading is that during biomass pretreatment a number of inhibitory compounds are released. These confound the fermentation process in a variety of ways, and as the solids concentration increases so do the concentrations of these inhibitors. There for ea biocatalytic strain that is able to ferment high concentrations of pretreated biomass and withstand the inhibitors present in the fermentation media is desirable. Pine wood has proven to be a particularly difficult biomass type from which to obtain high ethanol titers when fermenting concentrations of solids much greater than 10% dry weight. We previously described a strain of Saccharomyces cerevisiae, AJP50, which is able to ferment high concentrations (17.5% dry wt/v) of sulfur dioxide steam exploded pine wood. Present research details the performance of four isolates of AJP50 and their ability to produce ethanol from pretreated pine. We report ethanol yields of over 50 g/l (91% of maximum theoretical yield) from 22.5% dry wt/v of pine fermented using a simultaneous saccharification and fermentation process with strain GHP4 as the biocatalyst.
| Keywords |
| Ethanol; Softwood; Saccharomyces; Fermentation |
| Introduction |
| Cellulosic ethanol represents a potential replacement liquid transportation fuel to offset the use of petroleum derived gasoline [1,2]. Pine wood represents a potential feedstock that could be used to generate fuel and is widely available, particularly in the northern hemisphere [3,4]. Pine wood has been an important forestry resource for both the lumber and pulp and paper industries; the current value of saw timber is $25/ton while pulpwood is $10/ton [5].The quality of the wood is considerably less important for biofuel production than for lumber production, thus prices for an ethanol production facility would be closer to and potentially lower than pulpwood prices [6,7]. The infrastructure and technology for the growth and harvest of this feedstock is already in place allowing for the rapid implementation of pine feedstocks into biofuel pipelines [8]. Pine wood is not without disadvantages to its use as a bioethanol feedstock, one major hurdle to overcome is the release of inhibitory compounds during the pretreatment of the biomass which are then carried over into the fermentation process. |
| During pretreatment a variety of chemical compounds are released that can inhibit the activity of the biocatalytic organism in many ways [9,10]. The wide variety of inhibitory compounds released is due to the complex structure of biomass [11]; these can be sorted into three general categories: furans, aliphatic acids, and aromatics. The effects of furans and the aliphatic acids are better understood than the effects of aromatics, possibly due to the greater variety of aromatic compounds produced during pretreatment. This variety is due to the complex and variable structure of lignin. These different compounds have been shown to inhibit cellular metabolism [12,13], destabilize membranes [14], cause reactive oxygen species damage [15], and acidify the cytoplasm [16]. The presence of these inhibitors is a major factor confounding ethanol production from high concentrations of pretreated biomass; a barrier to an efficient biomass based ethanol fermentation production process [17]. Fermentation of a high concentration of solids could increase maximum ethanol yields and lower process costs by increasing distillation efficiency. However, a high solids loading also brings with it high concentrations of the aforementioned inhibitory compounds, so the development of a strain that is able to tolerate high concentrations of inhibitory compounds, in addition to the high solids content, is desirable in a cellulosic feedstock based process [9,18]. |
| To overcome these challenges we developed Saccharomyces cerevisiae strain AJP50 [19]. This strain was generated by adaptation and directed evolution from industrial strain XR122N(North American Bioproducts Corportation, Duluth, GA) and is able to catalyze the fermentation of high solids of sulfur dioxide pretreated pine wood [19]. We previously described the ability of AJP50 to withstand the presence of a number of these compounds in defined model fermentation media and its ability to efficiently produce ethanol from 17.5% dry wt/vol of pine. In order to address the question of the maximum solids fermentable by our strain we developed a medium to ensure the retention of the AJP50 phenotype during culturing and isolation; allowing for a large inoculum to be prepared in 24 h. Isolates of AJP50 were then inoculated into various high solids pine fermentations to determine the maximum ethanol titers which could be obtained using a simultaneous saccharification and fermentation process. |
| Methods |
| Cell growth and maintenance |
| Methods used to obtain the AJP50 isolates described in this study have been published previously [19]. YPD media (20 g/l peptone, 10 g/l yeast extract, 20 g/l glucose) supplemented with all 13 inhibitors (YPDI media, Table 1) was used throughout this study. In brief, AJP50 glycerol freezer stocks were first inoculated into YPDI broth at 4.0×105 cells/ml and incubated at 37°C for 24 h before being inoculated onto YPDI agar. This was incubated at 37°C for seven days at which point individual, isolated colonies were inoculated into YPDI broth media. Cell culture samples were frozen at -80°C in 40% w/v glycerol and designated GHP 1, GHP2, GHP 3, and GHP 4. YPDI broth cultures to be used to inoculate pine fermentations and growth curve experiments were inoculated directly from glycerol freezer stocks at 2.0×106 cells/ ml and incubated for 24 h at 37°C with 200 rpm shaking, reaching cell densities of greater than 5.0×107 cells/ml. |
| Pine fermentation experiments |
| Pine wood chips were pretreated in a single step SO2 steam explosion reactor [20] prior to fermentation as described previously [19]. The name of the sample indicates pretreatment conditions; for example pine sample 3-210-10 was pretreated with 3% (w/v) SO2 then held at 210°C in the process reactor for 10 min. All pretreated pine wood samples were stored at 4°C without any washing, pressing, or other method of inhibitor abatement. Three samples were chosen for fermentation in this study. Sample A was treatment 3-210-10, sample B was treatment 2.5-213-5, and sample C 5-217-2. |
| The moisture content of the biomass was determined using an IR- 35 Moisture Analyzer (Denver Instrument, Denver, Colorado) and a mass equivalent to the desired dry weight placed into baffled 125 ml flasks and autoclaved for 20 min at 121°C (which could be considered an additional pretreatment of the biomass performed for all samples). Prior to cell inoculation, cellulolytic enzymes (Novozymes Inc., Franklinton, NC) at 15 FPUcellulase/g dry wt pretreated pine and 60 CBU cellobiase/g dry wt pretreated pine along with Tryptic Soy Broth (TSB) media without dextrose (Difco, Detroit, MI) were added to the flask and brought to a final volume of 50 ml with sterile water. Cellulolytic enzymes were combined, diluted in TSB media then filter sterilized via 2 micron filters. Hemocytometer readings from YPDI grown cell cultures were used to estimate the number of cells/ml. The appropriate volume of culture was removed to sterile centrifuge bottles and centrifuged at 5,000 rpm for 15 min before inoculation into the fermentation media at an initial concentration of 2×107 cells/ml. Fermentations were maintained in baffled flasks at 37°C, pH 5.0, with 200 rpm shaking over the course of the experiment. |
| Fermentations of 22.5% dry wt/v were also performed in 150 ml total volume using small scale bioreactors [21]. Mixing was via magnetic stir bars as opposed to the orbital shaker used for the shake flasks. Other than the increased volume and magnetic stirring all conditions for bioreactor fermentations were identical to those in the shake flask fermentations. |
| Structural analysis of the pretreated pine wood samples has been previously described [19]. Table 2 lists the pretreatment conditions, percent cellulose and hemicellulose, and theoretical maximum yields at 17.5, 20, and 22.5% dry weight solids for all pine samples used in this study. Theoretical maximum yields were calculated as follows: total fermentable carbohydrate (cellulose+hemicellulose)×dry weight of pine×0.53(molecular ratio of ethanol/ polymer carbohydrate)×0.9 for conversion efficiency of 6C sugars. Samples were taken for analysis from each fermentation at the indicated time points, and the ethanol concentration was determined using gas chromatography as previously described [21]. |
| Inhibitor growth assays |
| Growth of the strains in model fermentation media was performed using a Bioscreen C machine (Oy Growth Curves Ab Ltd. Helsinki, Finland).100 well microtiter plates were used in these experiments. Media in each well consisted of TSB media, 13 inhibitors at either the concentration listed in Table 1 (1X) or 1.2 times this concentration (1.2X), 2% w/v glucose, with an initial pH 5.0. Cells were counted after 24 h growth in YPDI media using a hemocytometer and 4.0×105 cells/ ml were inoculated into each well. 20 replicate wells were prepared from each culture. The plates were incubated at 37°C without shaking or agitation; culture optical density was measured hourly at 580 nm. |
| Results and Discussion |
| 12 and 17.5% dry wt/vol fermentations |
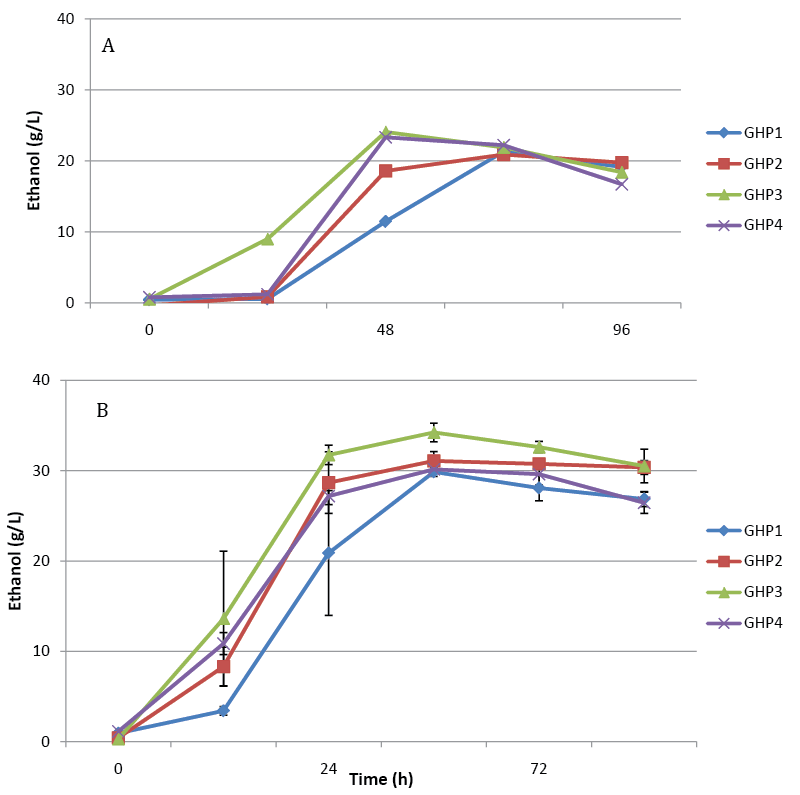
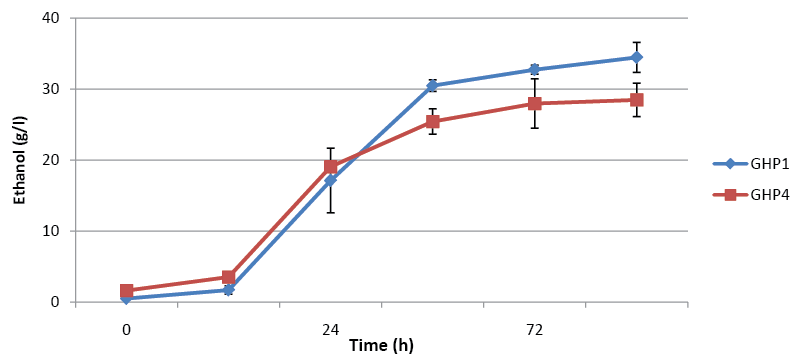
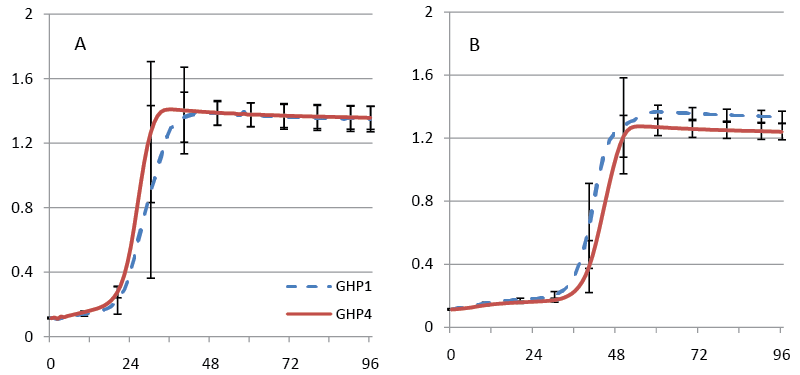
| We previously described two phenotypes of Saccharomyces strain AJP50, the ability to ferment high concentrations of pine solids, and the ability to grow in media that contained biomass-derived fermentation inhibitors. In order to assess the retention of the first phenotype two sets of fermentations were performed with four clonal populations isolated from AJP50 named strain GHP1, GHP2, GHP3, and GHP4. In initial experiments, the isolates were inoculated at a low concentration of cells, 4.0×105 cells/ml, into 12% dry wt/vol of sample A (Figure 1A). All four isolates produced essentially 100% of the theoretical maximum ethanol yield in these experiments. These four isolates were selected for inoculation in higher solids, 17.5% dry wt/vol, fermentations of sample A. These fermentations received a higher inoculum of 2.0×107 cells/ml, which is approximately 2 g dry wt/l, an industrially relevant inoculum level. As in the 12% dry wt/vol fermentations, all four isolates performed well reaching essentially 100% of the theoretical maximum (Figure 1B). that all four isolates retained the ability to ferment high concentrations of pine wood after culturing in YPDI media. In order to obtain higher ethanol titers, pine sample B was selected for fermentation. This sample contains higher concentrations of fermentable carbohydrates than sample A; resulting in higher theoretical maximum ethanol yields (Table 2). These fermentations were performed using only isolates GHP1 and GHP4. Both isolates were able to produce over 30 g/L of ethanol, but neither reached the 100% maximum theoretical yield observed with sample A. GHP1 reached 80% of maximum theoretical yield and GHP4 66% of maximum theoretical yield (Figure 2). Ethanol titers were not significantly increased over what was observed from 17.5% dry wt/vol sample A; to further increase the amount of fermentable sugars 20% and 22.5% dry wt/vol fermentations were performed. If the percentage of the theoretical maximum amount of ethanol obtained remained constant, the increase in percentage dry weight fermented would allow for higher ethanol titers. |
| 20% dry wt/vol fermentations with GHP1 and GHP4 |
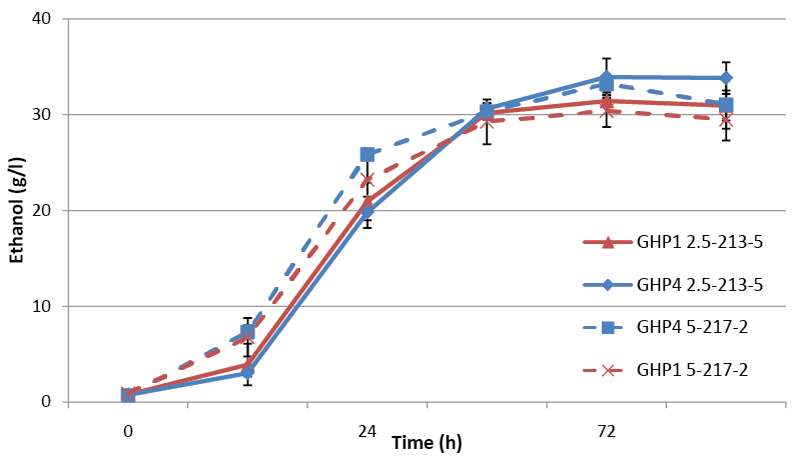
| 20% dry wt/vol fermentations were performed using pine samples B and C. Sample C was selected because it had performed favorably in previous experiments (data not shown) and had 42.3% fermentable sugars. Although ethanol was produced from both pine samples at 20% dry wt/ vol, neither reached greater than 85% of the theoretical maximum ethanol yield (Figure 3). For sample B, GHP1 reached 64% of maximum theoretical yield, a slightly lower ethanol titer than was observed in 17.5% dry wt/vol of the same pine sample. Isolate GHP4 was able to produce 69% of maximum theoretical yield, an increase of 3% from the 17.5% fermentations. For sample C higher percentages of the maximum theoretical were realized but ethanol titers remained similar to those observed in 20% dry wt/vol of sample B, reflecting the lowered amounts of fermentable sugar. GHP1 produced 75% of maximum theoretical yield and GHP4 82%. In order to further increase fermentable sugars in the fermentation media 22.5% fermentations were attempted with GHP4. |
| 22.5% dry wt/vol fermentations |
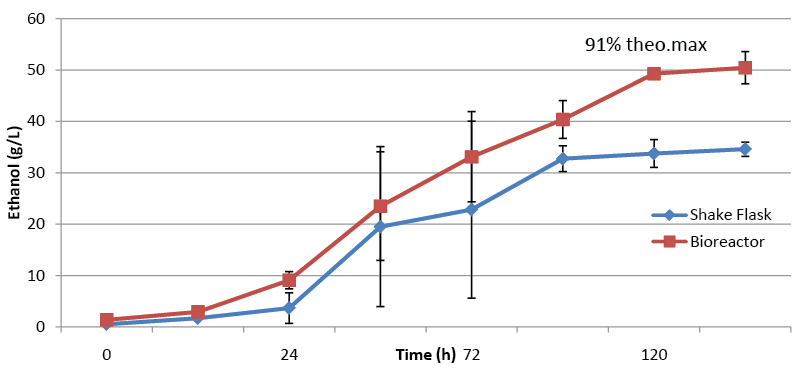
| Sample B was selected for fermentations at 22.5% dry wt/vol as it possessed the highest concentrations of fermentable carbohydrate and therefore the greatest theoretical amounts of ethanol could be produced from this sample. As in the 20% dry wt/vol, ethanol production was observed in shake flask fermentations of 22.5% dry wt/vol Sample C (Figure 4). The ethanol titer was almost identical to that observed in 20% fermentations, resulting in a decrease in the percentage of the maximum theoretical yield from 69% to 62%. The stalling ethanol titers as the dry weight of pine fermented increased caused concerns that the biomass was being insufficiently mixed with the cellulolytic enzymes; leading to incomplete sugar release from the biomass. In order to promote more efficient mixing of the slurry a bioreactor equipped with a magnetic stir bar was used in 150ml fermentations of 22.5% dry wt/vol. These bioreactor fermentations outperformed the shake flasks reaching just over 50g/l (91%of the maximum theoretical yield) of ethanol (Figure 4). These results showed inefficient mixing may be responsible for lower than optimal ethanol yields observed in shake flasks, and strain GHP4 is able to efficiently ferment very high dry weights of pretreated pine with adequate mixing. |
| Growth in model fermentation media |
| To assess the inhibitor tolerances of GHP1 and GHP4 after 24 h of grown in YPDIgrowth curves were constructed using YPDI grown cultures of these strains. When inhibitors of identical concentration (1X model fermentation media) to those listed in Table 1 were added to the media, all three strains showed similar growth (Figure 5). The inhibitor concentrations in 1x model fermentation media were based on 12% dry wt/vol fermentations; in order to better reflect what may be found in higher solids fermentations the concentrations of inhibitors added into the media were increased. In media containing 1.2 times the concentrations in Table 1, GHP1 and GHP4 reached maximal optical density at 50h; later than what was observed at 1X concentration. These data suggests that GHP1 and GHP4 retain the inhibitor resistant growth phenotype of AJP50. |
| Conclusions |
| Four isolates obtained from AJP50 populations on inhibitor supplemented media retain the desirable characteristics of the original strain when cultured for 24 hours in YPDI media. At least two of these strains also retain the ability to efficiently grow in defined media containing selected compounds typically found in pine wood biomass fermentations. Isolate GHP4 is able to produce ethanol from 22.5% dry wt/vol of sulfur dioxide steam exploded pretreated pine wood at over 90% of the theoretical maximum, provided ample agitation of the pine slurry. |
| Acknowledgements |
| The authors would like to thank Amruta Jangid for her work in generating strain AJP50 and C2Biofuels and Georgia Tech for producing the pretreated pine. Partial funding was provided by US DOE-DE-EE-0000410. |
References
|
Tables and Figures at a glance
| Table 1 | Table 2 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14777
- [From(publication date):
July-2013 - Nov 10, 2025] - Breakdown by view type
- HTML page views : 9991
- PDF downloads : 4786
