Reduced Phosphorylation of Histone Variant H2Ax in the Organ of Corti is Associated with Otoprotection from Noise Injury
Received: 27-Nov-2012 / Accepted Date: 10-Jan-2013 / Published Date: 16-Jan-2013 DOI: 10.4172/2161-119X.1000131
Abstract
Research on the molecular bases of noise induced hearing loss has revealed that noise exposure produces multiple independent and complementary biochemical cascades that could damage DNA. The phosphorylation of Ser139 of histone variant H2Ax (γ-H2Ax) occurs within one minute following DNA damage and spans two million DNA bases on either side of the damage. In the current study we investigated whether noise exposure could induce γ-H2Ax within the organ of Corti. Cumulative signal strength was employed to quantify the absolute level of γ-H2Ax in mathematical energy units. The results indicated that noise exposure could increase the level of γ-H2Ax in the organ of Corti. Furthermore, treatment with a DNA repair enhancing chemotype (carboxy alkyl ester) reduced the noise induced increase of γ-H2Ax which was associated with an accelerated rate of functional recovery from the noise exposure. The combined results implicate molecular mechanisms of DNA damage and repair in the pathophysiology of noise induced hearing loss.
Keywords: DNA damage and repair, Noise induced hearing loss,Carboxy alkyl esters
252897Introduction
Research on the molecular bases of Noise Induced Hearing Loss (NIHL) has revealed that noise exposure produces multiple independent and complementary biochemical cascades that may damage DNA [1-5]. For instance, chinchillas exposed to impulse noise revealed DNA strand breaks within five minutes following the exposure as detected by the TUNEL assay [2]. Furthermore, electrochemical high-performance liquid-chromatography revealed the presence of the 8-hydroxy-2’-deoxyguanosine DNA base adduct in the rat cochlea within eight hours post-noise exposure [6]. These DNA damage products are particularly toxic since they inhibit gene express by stalling the procession of the RNA polymerase-II holocomplex during transcription [7,8]. Furthermore, the inhibition of gene expression or the manufacturing of mutated gene products would affect normal cell and tissue functions. One line of research has demonstrated that [Ca]2+ rapidly accumulates in the hair cells and supporting cells following over-stimulation through an ATP regulated pathway that involves the release of [Ca]2+ reservoirs from mitochondria into the cytosol [9,10]. This is important because high cytosolic levels of [Ca]2+ may stimulate mitochondria to release enzymes that induce DNA strand breaks [11]. Indeed, previous demonstrations have shown that noise exposure stimulate mitochondria to release endonuclease-G and the apoptosis inducing factor [12,5]. Each of these enzymes can translocate to the nucleus and independently induce DNA strand breaks. Furthermore, noise stimulate both the intrinsic and extrinsic-caspase mediated cell death pathways and each pathway deploys the DNA fragmentation factor enzyme to precipitate DNA strand breaks [13,4].
Among the various types of DNA damage products, DNA doublestrand breaks (DSB) are considered the most lethal because they threaten the stability of whole chromosomes [14]. Therefore, their appearance in a cell triggers a global response that involves the activation of at least three kinases, Ataxia Telangiectasia Mutated (ATM), DNA-dependent protein kinase (DNA-PK) and ataxia telangiectasia/RAD3 related (ATR), that phosphorylate Ser-139 of histone variant H2Ax (γ-H2Ax) at the site of the DSB [15]. Hundreds of γ-H2Ax may precipitate around the DSB within one minute [16]. For instance, in human cells γ-H2Ax can be detected as far as ~2 megabases on either side of the break [17]. Such amplification of a single DSB makes it possible to detect individual cells suffering episodes of DSBs. The specificity of γ-H2Ax for DSBs is an established phenomenon in DNA damage research and immunolabeling of γ-H2Ax in tissue sections is a standard approach for indentifying cells or tissues that are experiencing DSB [18,19]. Immunolabeling for γ-H2Ax is also used in the clinic as a biodosimeter of DSBs following exposure to radiotherapy, X-ray examinations or computed tomography (CT) scans [20]. Furthermore, it has been demonstrated that γ-H2Ax immunolabeling is the most sensitive method for detecting a DSB [21]. Since DSBs and other types of DNA damage products threaten the stability of the genome, a considerable amount of research has focused on identifying biomedical approaches to increase the repair of such lesions.
Small molecular weight compounds called Carboxy Alkyl Esters (CAEs) have been shown to maintain genomic stability under toxic conditions and have being standardized to increase DNA repair capacity when administered to humans and rodents [22-31]. In a randomized age and gender matched human study, two groups of subjects were treated with CAE (250 and 350 mg) for 8 consecutive weeks [30]. A third group of subjects served as control by not receiving CAEs. Blood cells were removed from the subjects in each group and exposed to hydrogen peroxide to induce free radical DNA damage. There was a statistically significant increase in enzymatic DNA repair activity as evidenced by higher levels of repaired DNA single strand breaks in the groups that received CAE treatment compared to the control group. Additionally, CAE had no detectable toxicity in humans. Further research on humans have confirmed these results, by demonstrating that CAE augments DNA repair capacity which then improves health outcomes such as immune function, anti-inflammation, osteoarthritis relief and positive psychotropic effects [32,26,29]. Animal experiments have also confirmed that CAE treatment augments DNA repair capacity. For instance, female W/Fu rats treated with CAE (up to 160 mg/kg) for 28 days by gastric gavage then exposed to 12 Gy whole body irradiation to induce wide-spread genomic damage, showed significantly (p < 0.05) improved DNA repair capacity relative to control rats who were not treated with CAE [29]. Several other studies in rats and mice have confirmed these results by showing that CAE augments DNA repair capacity which then increases whole-body defenses such as leukocyte recovery from DNA damage, improved mitogenic responses, improved proliferation of lymphocytes, improved life-span of cells and enhanced immune function [29,22]. A recent study has revealed that CAE treatment augments the recovery of auditory sensorineural functions following noise injury which suggest the possibility of reduced DNA damage in the cochlea with CAE treatment [33].
In the current study, we investigated whether or not noise exposure could increase immunolabeling of γ-H2Ax in the organ of Corti and whether CAE treatment would counteract the effect of the noise. We were particularly interested in an early time point (1 day) after the noise exposure because permanent cochlear impairment might be predicated on early genomic stress [2,6].Therefore, we monitored cochlear function out to 4 weeks post-noise exposure to determine whether there was an association between events observed at 1 day post-exposure and the severity of permanent cochlear impairment.
Materials and Methods
Animals and experimental design
Experiments were conducted on male Long-Evans rats (250-300g) that were acquired from Harlan Laboratories, Inc. (Livermore, CA. USA). All experimental protocols were approved by the Institutional Animal Care and Use Committee at the Loma Linda Veteran’s Affairs Hospital. A total of 48 animals were used in the experiments. After arriving at the vivarium, the animals were allowed to acclimate for one week. Baseline Distortion Product Otoacoustic Emissions (DPOAE) was then collected on each animal to verify cochlear function. The animals were assigned to one of four groups based on their DPOAE recordings in order to counterbalance cochlear function between the groups. The four groups included a CAE group (n = 10), a noise+CAE group (n = 11), a control group (n =14) and a noise exposure group (n = 13). A formulation of CAE that has been standardized to increase DNA repair activity was prepared by Optigenex Inc (Hoboken, NJ.USA) as reported previously [31,33]. The animals in the CAE and CAE+noise groups were administered 160 mg/kg of CAE by gastric gavage for 28 consecutive days [28,33]. The control group was also treated via gastric gavage with distilled water (dissolving agent for CAE) for 28 consecutive days. The noise group did not receive any treatment beyond being exposed to the nose dose (see below). Noise exposure of the noise and CAE+noise groups occurred on the 29th day (one day after the 28 days of water or CAE treatment). Then postexposure DPOAE measurements and tissue harvesting were conducted from each group on the 30th day (1-day after the noise exposure). Tissues were harvested to evaluate whether or not γ-H2Ax expression increased in the organ of Corti after noise exposure and whether or not CAE treatment could reduce the expression of γ-H2Ax in the organ of Corti at an early time point (1-day post trauma) after the exposure. To evaluate whether a putative effect on γ-H2Ax expression is associated with long-term recovery of cochlear function, some of the animals were allowed to survive for 4 weeks post-noise exposure and cochlear function was evaluated again with DPOAE. Table 1 describes the different animal groups, their treatment regimen and the experimental design.
| Baseline DPOAE | CAE treatment | Noise | DPOAE+Sacrifice | DPOAE recovery | |
|---|---|---|---|---|---|
| Groups | Day 0 | 28 days of treatment | Day 29 | Day 29 | 1-week and 4-week post-noise |
| Control | (N=14) | water | + | (N=9) | |
| Noise | (N=13) | noise | + | (N=8) | |
| CAE | (N=10) | CAE | + | ||
| Noise+CAE | (N=11) | CAE | noise | + | (N=6) |
Abbreviations: CAE, Carboxy alkyl esters; DPOAE, distortion product otoacoustic emissions
Table 1: Experimental design.
Distortion Product Otoacoustic Emissions (DPOAE)
The cubic 2f1 - f2 DPOAE is particularly sensitive to cochlear noise damage. Therefore DPOAEs (2f1 - f2 level as a function of increasing stimulus frequency, commonly referred to as a DP-gram) were recorded as described previously [34]. Briefly, each animal was anesthetized with ketamine/xylazine (44/7 mg/kg, im.) while normal body temperature was maintained using a direct current (dc) heating unit built into the surgical table.
The cubic 2f1 - f2 DPOAE was recorded with two primaries, f2 and f1 ; where f2 is higher than f1 at an f2/f1 ratio of 1.25. The primaries (L) were presented in 0.1-octave increments from 3.2 to 63 kHz. The levels of the primaries were set to L1 - 10 = L2 or as indicated in the figures. The frequency and level ratios of the primaries were selected to maximize the 2f1 - f2 SPL recorded from the ear canal [35-37]. A customized signal presentation, acquisition and analysis program written in LabVIEW version 7.1 (National Instruments, Austin, TX. USA) was used to drive a PCI-4461 computer-based DSP board (National Instruments, Austin, TX., USA) for generation of the primaries and Fourier analysis of the response.
Noise exposure
Both the noise-only group and the CAE+noise group were exposed in the same noise exposure chamber at the same time. The noise exposure paradigm has been described previously [33]. Briefly, the animals were exposed to an octave band of noise (OBN) centered at 8 kHz. The intensity of the noise was 105 dB SPL and the duration was 4 hours. The animals were awake during the exposure and were free to move around in a wire-cloth enclosure within a 40 L noise chamber. The noise was generated by a Function Generator (Sanford Research System, Menlo Park, CA. USA) coupled to a Frequency Device (Frequency Device Inc., Haverhill, MA. USA). Vifa D25AG-05 speakers (Vifa International A/S, Videbaek, Denmark) located approximately 5 cm above the animals’ wire-cloth enclosure was used to present the noise. The frequency spectrum of the noise was verified in the noise chamber containing the rats with a sound level meter (Quest Electronics, Oconomowoc, WI. USA) close to the animals’ pinnae.
Immunolabeling
Animal and tissue preparation: Immunolabeling of γ-H2Ax within cells in tissue sections is a standard method of detecting DSB [18-20]. Therefore, we employed γ-H2Ax immunolabeling and quantification to determine differences between the groups. Twenty anesthetized animals (control group = 5, noise group = 5, CAE group = 5 and CAE+noise group = 5) were sacrificed by transcardial perfusion with phosphate-buffered saline (PBS; 10mM, pH7.4) followed by periodate-lysine-paraformaldehyde fixative [38].The heads were then removed, skinned and post-fixed in 4% paraformaldehyde overnight at 22°C. Formic acid (10%) was used for chemical decalcification of the heads as described previously [34]. Then the heads were paraffin embedded and sectioned at 5μm in the midmodiolar plane. The sections were incubated in a heated water bath and mounted on subbed slides for immunolabeling. Kidney tissues were simultaneously harvested, postfixed, paraffin embedded, sectioned and mounted on subbed slides.
Immunoperoxidase procedure
Tissue sections were de-paraffinized in zylene, hydrated in graded ethyl alcohol and water then incubated in 0.9 % H2O2 for 10 minutes. They were then heated for 20 minutes at 90-98°C in a low pH (0.80- 3.06) sodium citrate-citric acid buffer (antigen retrieval) and then rinsed thoroughly with PBS. Afterwards, the sections were pre-treated with a blocking solution of normal goat serum, 10% Triton X-100 and 2% bovine serum albumin (BSA; Sigma, St. Louis, MO, USA) in PBS for 1 hour. The primary antibody was diluted in the blocking solution at a 1:100 concentration. The primary antibody (anti- γ-H2Ax, Ser139) is commercially available (Santa Cruz Biotechnology, Inc., Santa Cruz, CA. USA). The specificity of the antibody has been confirmed previously [39-41]. Nevertheless, negative control experiments were conducted such that sections were incubated with blocking serum without the primary antibody [42]. Such negative control sections were processed at the same time as the experimental sections and received simultaneous and identical treatments. Furthermore, positive control experiments were conducted by immunolabeling tissue that is known to exhibit constitutive expression of γ-H2Ax [43] (Figure 1). After incubating the sections with the primary antibody (or blocking serum for negative control sections) for 48 hours at 4°C, the sections were rinsed with PBS. They were then treated with a biotinylated anti-rabbit secondary antibody (Vector Laboratories, Temecula, CA. USA) diluted 1:100 in PBS + 2% BSA for 24 hours at 4°C. The sections were then rinsed in PBS, incubated with preformed avidinbiotin- peroxidase enzyme complexes (Vectastain ABC reagent; Vector Laboratories, Inc., Burlingame, CA. USA) for one hour, rinsed again with PBS and then treated with a solution of Trizma pre-set crystals (1.58 g; Sigma-Aldrich, St. Louis, MO. USA) to stabilize peroxidase enzyme reactions. The peroxidase enzyme complexes were then used to oxidize 3.3’-diaminobenzidine tetrahydrochloride to produce a brown chromogen.
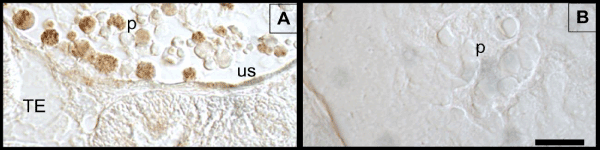
Figure 1: γ-H2Ax labeling in kidney. Panel A shows γ-H2Ax immunolabeling in the Long-Evans rat kidney (positive control). A few podocytes (P) that line the urinary space (US) are labeled while other podocytes are not labeled. The tubular epithelia (TE) in panel A shows little or no staining. Panel B reveals that omitting the antibody from the immunolabeling procedure resulted in no staining within podocytes (negative control). The scale bar (20 μm) in panel B applies to panel A.
Quantitative morphometry
Equipment: A Leica DM2500 upright microscope (Leica Microsystems Inc., Bannockburn, IL. USA) was used for brightfield microscopy. A ProgRes® CFscan digital camera (JENOPTIK Laser, Jena, Germany) mounted on the Leica DM2500 was used for digital image capturing. Image-Pro® plus version 6.3 (Media Cybernetics Inc., Bethesda, MD, USA) for Windows™ was used to control image capturing, pixel thresholding and bitmap analyses. A Dell Optiplex GX620 with an Intel Core2 processor was used for software operations.
Photomicroscopy: The microscope current drain, light source and temperature were standardized to ensure accurate and consistent reading of each tissue section. The intensity of the light to a 1.4 megapixel charged-coupled-device color sensor was monitored over an 8 hour period by capturing a blank field (cover slip, mounting media and glass slide). The light intensity fluctuated by only 0.1% (a modest difference in mean gray value of 0.2748) within the first hour of being turned on then stabilized when the lamp housing reached a temperature of 62 ± 1°C. All photomicrographs were taken when the microscope light intensity stabilized. To further ensure consistent spatial and temporal illumination for each tissue section during microscopy, the mean gray value (g) for blank fields adjacent to each tissue section was maintained at ≥ 254g. In addition to providing consistent illumination, this high level illumination had the added advantage of masking background staining which was significantly lighter (light brown stain) than the antibody staining which exhibited a heavy brown stain and thus unmasked by the illumination. Photomicrographs of the organ of Corti were taken with an N-PLAN 40x/0.65 objective lense. These photomicrographs (680 x 512 pixels) were saved in uncompressed tagged-image file format for later retrieval. They were then converted from 48 bits/pixel to 24 bits for subsequent analyses. To further remove background staining from each photomicrograph a predetermined threshold criterion was applied via Image-Pro’s threshold algorithm. This threshold criterion was empirically determined from pilot experiments. For instance, the acellular tectorial membrane of Corti’s organ is sometimes stained after the immunolabeling procedure. This is due to the trapping of the secondary antibody within the microfibrillar matrix of the tectorial membrane and the failure of wash steps to completely dislodge the secondary antibody. The thresholding criteria employed in this study was trained on masking background levels of staining such as the level of background found in the tectorial membrane. Therefore, applying the threshold criteria increased the signal-to-noise ratio. After thresholding, an area of interest (AOI) field was selected within each organ of Corti. Bitmap readings were then taken within the AOI. The bitmaps record the positional matrix (n1 x n1) and brightness (b) of each primary colored (red, green and blue or RGB) pixel (n1 x n1 x 3). These bitmap readings were then used in the determination of absolute chromogen levels by the cumulative signal strength technique.
Cumulative signal strength: Cumulative signal strength is an objective technique that quantifies the absolute amount of immunolabeling (staining intensity) in photomicrographs [43,44]. This is achieved by measuring the mathematical energy (E) within each n1 x n1 x 3 pixel of an N1 x N1 x 3 photomicrograph. The computation is as follows [46].
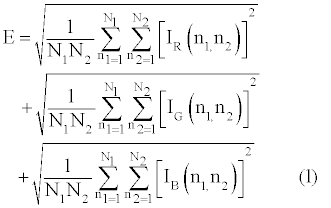
where Ii(n1,n2) is the energy from a particular hue (i = R, G or B) at position n1 x n2 in the AOI frame. In photomicrographs from bright field microscopy Ii(n1,n2) = 255 - bi(n1,n2), where b is the brightness value or gray value. The E that reflects specific staining of the γ-H2Ax antibody (E γ-H2Ax) is the absolute value of the difference between antibody induced immunolabeling (Eantibody[+]) and that induced by the absence of the antibody (Eantibody[-]) also known as the negative control;
E γ-H2Ax = (Eantibody[+]) - (Eantibody[-]) (2)
Statistical analysis
Statistical analyses were conducted with Prism 5, version 5.03 (GraphPad Software, Inc., La Jolla, CA. USA). The DPOAE data was analyzed for group effects such that analysis of variance (ANOVA) testing was conducted on 2f1 - f2 DPOAE levels to determine significant differences between the groups. Post-hoc testing employed Dunnett’s paired comparison analyses. The intensity of γ-H2Ax immunolabeling was recorded from midmodiolar cochlear sections. For each animal, duplicate readings were recorded and 10 cochlear sections were used for each group (a total of 40 sections). The apical, middle and basal cochlear coils were evident in the sections therefore, 120 (40 x 3) cochlear coils were studied. Statistical differences between groups were determined with ANOVA and Bonferroni’s multiple comparison testing.
Results
γ-H2Ax labeling
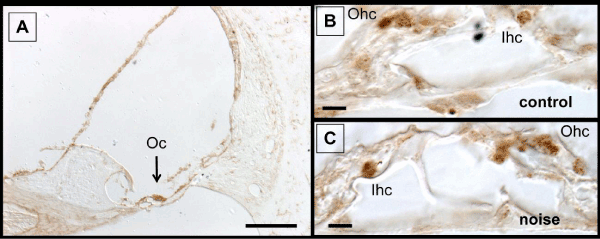
It is known that the kidney exhibit consistent immunolabeling of γ-H2Ax which is believed to represent DSB that form in noncoding regions of the genome called gene deserts [43,47]. Therefore, kidney sections (Figure 1) served as positive and negative controls for the immunolabeling experiments. Figure 1A reveals γ-H2Ax labeling in podocytes of the renal corpulse (positive control). This labeling was abolished when the antibody is omitted from the immunolabeling procedure (Figure 1B, negative control). Immunolabeling for γ-H2Ax was also detected within the organ of Corti. The labeling was present under normal (control) conditions and after noise or CAE treatment. Figure 2A is a representative example of the staining. Several cochlear structures are labeled but labeling in the organ of Corti is particularly prominent. Panels B and C from Figure 2 demonstrates that γ-H2Ax labeling could be detected within hair cells and supporting cells with and without noise exposure. This persistent expression of γ-H2Ax is consistent with previous research that has shown persistent expression of DNA repair proteins in the organ of Corti [42,48,49]
Figure 2: γ-H2Ax labeling in scala media. Panel A shows that several structures are labeled but the most prominent is the organ of Corti. γ-H2Ax labeling can be detected in the organ of Corti with (panel C) and without (panel B) noise exposure. The labeling is predominantly localized in hair cells and supporting cells. Abbreviations: Oc, organ of Corti; Ohc, outer hair cell; Ihc, inner hair cell. The scale bar in panel A is 100 μm and the scale bars in panels B and C are 10 μm.
γ-H2Ax levels
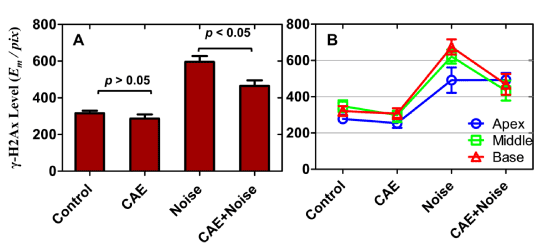
The prominence of γ-H2Ax labeling in the organ of Corti made it difficult to make subjective judgments on the level of expression between the groups. Therefore, to determine differences in staining intensity, the level of γ-H2Ax in the organ of Corti was quantified by determining the absolute amount of chromogen per pixel by employing the cumulative signal strength technique [44-46]. This technique measures the signal energy as a function of pixel, where energy is defined in the mathematical sense. Therefore, the values are unit less and thus reported as mathematical energy units per pixel (EM /pix). Figure 3A reveals that γ-H2Ax level in the control group was similar to that in the CAE group. However, noise exposure induced an increase in γ-H2Ax. This increase was also observed after co-treatment with CAE and noise. Note that γ-H2Ax level was lower in the CAE+noise treated group relative to that in the noise-only group. Figure 3B reveals γ-H2Ax levels for each cochlear turn (apical, middle and basal coils) as a function of the treatment groups. In all cochlear turns, γ-H2Ax levels were similar between the control and CAE groups. However, γ-H2Ax levels were highest among the noise and CAE+noise groups. In the middle and basal turn γ-H2Ax levels were higher in the noise group compared to the CAE+noise group. Therefore, inspection of individual cochlear turns further supported the notion that CAE treatment reduced noise induced levels of γ-H2Ax.
Figure 3: γ-H2Ax levels within the organ of Corti. γ-H2Ax levels were quantified in mathematical energy units per pixel (Em /pix) and displayed in panels A-B as a function of the treatment conditions. Note that there were no significant differences in γ-H2Ax level between the control and CAE conditions. However, there was a marked increase after noise or co-treatment with CAE and noise. The level of γ-H2Ax is significantly lower in the CAE+noise condition relative to the noise-only condition. This effect is further supported in Panel B where γ-H2Ax levels for individual cochlear turns were quantified. Errors bars are standard errors of the means.
Statistical analyses were conducted on γ-H2Ax level measurements. A one-way ANOVA where treatment condition served as a between subjects factor revealed that there was a statistically significant difference in γ-H2Ax levels between the four groups (F[3,36] = 30.33, p < 0.01). Bonferroni pair wise contrast revealed no statistically significant differences between the control and CAE groups (p > 0.05). But these groups exhibited significantly lower γ-H2Ax levels compared to the noise exposed group (p < 0.01). Furthermore, the noise exposed group exhibited significantly higher γ-H2Ax levels compared to the CAE+noise group (p < 0.05). These statistical calculations suggest that CAE treatment reduced noise induced γ-H2Ax within the organ of Corti. This conclusion was further supported by statistical calculations on γ-H2Ax level measurements from individual cochlear turns. For instance, the control and CAE group showed no significant difference (p > 0.05) in γ-H2Ax levels regardless of cochlear turn (apical, middle or basal). However, the noise group and the CAE+noise group were significantly (p < 0.05) different at the basal and middle turns. In these cochlear turns, γ-H2Ax was significantly (p < 0.05) lower within the CAE+noise group. The pooled results indicate that CAE treatment reduced noise induced γ-H2Ax levels in the basal and middle cochlear turns. This is significant because basalward turns are known to be more vulnerable to cell death and the loss of cochlear function after exposure to the same noise dose used in the current study [33].
Protection from noise injury
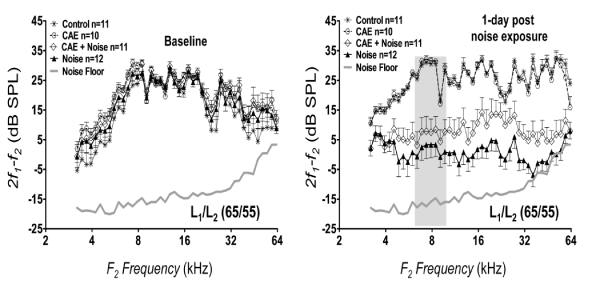
To evaluate protection from noise injury, DP-grams of 2f1 - f2 were recorded. These recordings were obtained at baseline and 1 day following noise exposure. Figure 4 shows the response of 2f1 - f2 DPOAE as a function of f2 frequency driven with primary levels (L) at 65/55 dB SPL. Baseline recordings revealed that all groups had large 2f1 - f2 levels that exceeded the noise floor by at least 6 dB. At 1 day post-noise exposure, the control groups (vehicle-control and CAE treated groups) showed equivalent 2f1 - f2 levels (two-way ANOVA: F[1,43] = 2.09, p > 0.05). However there was a significant difference (two-way ANOVA: F[1,43] = 56.37, p < 0.01) between the noise treated groups where the CAE+noise group exhibited higher (better) levels than the noise-only group. Furthermore, the highest frequency components (≥ 32 kHz) of the noise-only group was suppressed into the noise floor (average = 1.27 dB above noise floor) while that of the CAE+noise group remained above the noise floor (average = 7.31 dB above noise floor). These findings suggest that 2f1 - f2 DPOAE levels from the CAE+noise group was better preserved than that of the noise group as early as 1 day postnoise exposure.
Figure 4: Protection from noise injury. The levels of 2f1 - f2 as a function of f2 frequency (DP-gram; L1/L2 = 65/55) are shown for each treatment group at baseline and 1 day post-noise exposure. The vertical gray bar in this and all figures represent the frequency range of the damaging noise. Both the noise-only group and the CAE+noise group were exposed in the same noise exposure chamber at the same time. Note that the CAE+noise group exhibited better (higher) levels than the noise only group at 1 day post-noise exposure. Errors bars are standard errors of the means.
Recovery from noise injury
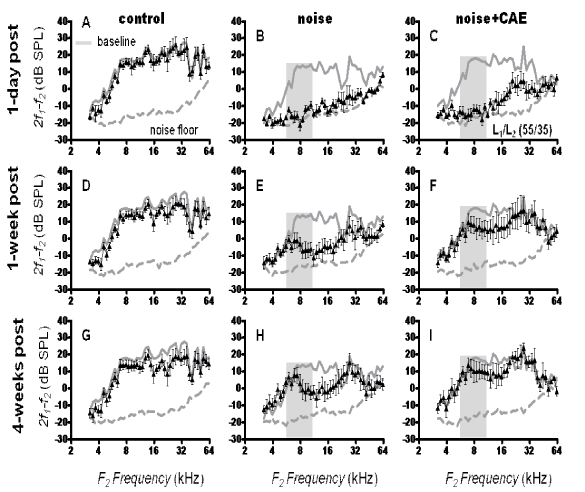
Recordings of the 2f1 - f2 DPOAE were conducted out to 4 weeks post-noise exposure to evaluate functional recovery from the noise exposure. The stimulus primary levels were set at 55/35 (L1/L2) which increases the sensitivity of the 2f1 - f2recordings [50]. Figure 5 illustrates the change in 2f1 - f2 levels for three time points (1-day, 1-week and 4-weeks post noise exposure). In the control group, 2f1 - f2 levels were robust and exhibited modest variations between time points. However, in the noise exposed group there was wide-spread loss of 2f1 - f2 levels at 1 day post exposure. For instance, the 2f1 - f2 DPOAE levels were reduced to approximate the noise floor over the entire f2 frequency range. At 1 week post-noise exposure there was some recovery in 2f1 - f2 levels but the frequency range between ~8-24 kHz showed prominent loss. At 4 weeks post-noise exposure there was still a prominent loss in the ~8-24 kHz range. These results indicate that the noise exposure resulted in permanent loss 1½ -octave above the center frequency (8 kHz) of the OBN. Indeed, permanent cochlear loss in Long-Evans rats exposed to an 8 kHz OBN at 105 dB SPL for 4 hours typically occurs at 4 weeks post-exposure [51,52].
Figure 5: Recovery from noise injury. DP-grams (L1/L2 = 55/35) obtained at 1 day, 1 week and 4 weeks post noise exposure are illustrated for the control, noise and CAE+noise groups. Both the noise-only group and the CAE+noise group were exposed in the same noise exposure chamber at the same time. Note that the CAE treated group showed faster recovery than the noise exposure group. The continuous gray lines in each panel represent baseline recordings and the broken gray lines are the noise floor. Errors bars are standard errors of the means.
The capacity of the CAE+noise group to recover from noise injury is shown in Figure 5. At 1 day post-noise exposure the CAE+noise group showed loss of 2f1 - f2 levels. For instance, frequencies below ~16 kHz were suppressed in to the noise floor. However, high frequencies between ~16-32 kHz were not reduced into the noise floor which is in contrast with the noise-only treatment where the entire frequency spectrum was suppressed into the noise floor. These data support that of Figure 4 in suggesting that CAE may provide early protection against noise injury. Indeed, at only 1 week post-noise exposure the CAE+noise group showed recovery while the noise-only group still exhibited a prominent loss. One week following noise exposure is considered an early time point because functional recovery is still occurring. Interestingly, the CAE+noise group showed almost complete recovery of 2f1 - f2 levels at 4 weeks post-noise exposure. This indicates that the CAE treated group was able to recover from the noise exposure. Statistical analyses were conducted on the 2f1 - f2 levels obtained at 4 weeks post noise exposure. A one-way ANOVA revealed significant (F[2,129] = 11.17, p < 0.01) main effects for the groups. Dunnett’s multiple comparison testing demonstrated that there was a statistically significant (p < 0.01) difference between the control and noise groups but there was no significant (p > 0.05) difference between the control and CAE+noise groups.
Discussion
In the current study, we found that noise exposure could increase immunolabeling of γ-H2Ax in the organ of Corti. This is consistent with previous studies that have shown that noise exposure increase the level of DNA strand breaks and the 8-hydroxy-2’-deoxyguanosine DNA base adduct in the cochlea [2,6]. Furthermore, a previous study used immunoblots to demonstrate an increase in γ-H2Ax expression in lysates from the ear following whole-body X-irradiation (20 Gy) of C57BL mice [53]. The current data also showed that, CAE treatment may reduce noise induced elevation of γ-H2Ax. This observation is consistent with the DNA repair literature where CAE treatment has been shown to increase the repair of DNA damage products [22-31]. An interesting observation from the current study is that immunolabeling for γ-H2Ax could be detected under normal conditions as well as after noise exposure. This indicates that the organ of Corti may be experiencing basal levels of DSBs under normal conditions. Indeed, previous research has shown a persistent DNA damage response among hair cells and supporting cell under normal conditions [38,49].
It is known that the normal metabolism of specific cell-types may perpetuate a persistent DNA damage response due to the precipitation of DSB as detected with γ-H2Ax immunolabeling [43,54,55]. This basal (normal) level of DSB is believed to localize in gene deserts (nonprotein coding genes) and do not affect the normal functions of the cell [47]. However, a consequence of this particular cellular phenotype is hyper-sensitivity to cell death because such cells are challenged with meeting the demands of endogenous genomic stress in addition to increased stress from exogenous stressors [55]. “This phenomenon has been described in a process called basal demand interference and might help to explain the selective vulnerability of hair cells to a large variety of stressors [42,49,56]. Several endogenous mechanisms including, NADPH oxidase (NOX)-3 activity, oxidative redox activity and the functions of nitric oxide synthase-I, II and II may account for a persistent DNA damage response in the organ of Corti [49]. Ultimately, a persistent DNA damage response under normal conditions is known to preclude DNA repair during episodes of increased stress [42].”This is particularly important because failure to repair damage DNA can result in mutated gene fragments (miscoding) and/or altered DNAprotein interactions (epigenetic dys-regulation) that alter cellular functions and/or induce various forms of cell death [58,59].
It is known that noise exposure alters the expression of a large variety of genes and induce multiple forms of cell death [60-62]. Furthermore, noise exposure is associated with human neoplastic neuromas (e.g., acoustic neuromas and sonocarcinogensis) which support the notion that noise stress can be mutagenic [63-65]. Therefore, efforts to increase the capacity of cells to repair damage DNA might be otoprotective against noise injury. For instance, it is known that cells with efficient DNA repair capacity can better resist mutagenesis, cell death and loss of cellular functions compared with cell that are less efficient [66,67]. Given that noise exposure damages DNA as early as 5 minutes post-exposure, it is tempting to speculate that this early genomic damage may underlie subsequent cascades that lead to miss-regulation of gene expression, cochlear dysfunction and ultimately cellular patterns of death and survival. If this is the case, then efforts to optimize DNA repair capacity in the initial stages of injury might provide a novel means of preserving cochlear function. For instance, the reduction in γ-H2Ax that we observed at 1 day post noise exposure was associated with preserved cochlear function at 4 weeks after the noise exposure. Therefore, the current study provides a basis for further research focused on biomedical strategies of augmenting DNA repair capacity in the cochlea.
Acknowledgements
This work was supported by a CDA-2 (C7600-W) Award from the Rehabilitation Research and Development Service of the Office of Research and Development United States Department of Veterans Affairs. The Loma Linda Veterans Affairs Medical Center provided facilities for conducting the experiments. The authors would also like to thank the Department of Otolaryngology and Head & Neck Surgery, School of Medicine, Loma Linda University Medical Center for research development support.
References
- Henderson D, Bielefeld EC, Harris KC, Hu BH (2006) The role of oxidative stress in noise-induced hearing loss. Ear Hear 27: 1-19.
- Hu BH, Henderson D, Nicotera TM (2006) Extremely rapid induction of outer hair cell apoptosis in the chinchilla cochlea following exposure to impulse noise. Hear Res 211: 16-25.
- Le Prell CG, Yamashita D, Minami SB, Yamasoba T, Miller JM (2007) Mechanisms of noise-induced hearing loss indicate multiple methods of prevention. Hear Res 226: 22-43.
- Murai N, Kirkegaard M, Järlebark L, Risling M, Suneson A, et al. (2008) Activation of JNK in the inner ear following impulse noise exposure. J Neurotrauma 25: 72-77.
- Yamashita D, Miller JM, Jiang HY, Minami SB, Schacht J (2004) AIF and EndoG in noise-induced hearing loss. Neuroreport 15: 2719-2722.
- Van Campen LE, Murphy WJ, Franks JR, Mathias PI, Toraason MA (2002) Oxidative DNA damage is associated with intense noise exposure in the rat. Hear Res 164: 29-38.
- Kathe SD, Shen GP, Wallace SS (2004) Single-stranded breaks in DNA but not oxidative DNA base damages block transcriptional elongation by RNA polymerase II in HeLa cell nuclear extracts. J Biol Chem 279: 18511-18520.
- Satou K, Hori M, Kawai K, Kasai H, Harashima H, et al. (2009) Involvement of specialized DNA polymerases in mutagenesis by 8-hydroxy-dGTP in human cells. DNA Repair (Amst) 8: 637-642.
- Gale JE, Piazza V, Ciubotaru CD, Mammano F (2004) A mechanism for sensing noise damage in the inner ear. Curr Biol 14: 526-529.
- Mann ZF, Duchen MR, Gale JE (2009) Mitochondria modulate the spatio-temporal properties of intra- and intercellular Ca2+ signals in cochlear supporting cells. Cell Calcium 46: 136-146.
- Crowe SL, Movsesyan VA, Jorgensen TJ, Kondratyev A (2006) Rapid phosphorylation of histone H2A.X following ionotropic glutamate receptor activation. Eur J Neurosci 23: 2351-1361
- Han W, Shi X, Nuttall AL (2006) AIF and endoG translocation in noise exposure induced hair cell death. Hear Res 211: 85-95.
- Wang J, Ruel J, Ladrech S, Bonny C, van de Water TR, et al. (2007) Inhibition of the c-Jun N-terminal kinase-mediated mitochondrial cell death pathway restores auditory function in sound-exposed animals. Mol Pharmacol 71: 654-666.
- Khanna KK, Jackson SP (2001) DNA double-strand breaks: signaling, repair and the cancer connection. Nat Genet 27: 247-254.
- Stiff T, O'Driscoll M, Rief N, Iwabuchi K, Löbrich M, et al. (2004) ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiation. Cancer Res 64: 2390-2396.
- Rogakou EP, Boon C, Redon C, Bonner WM (1999) Megabase chromatin domains involved in DNA double-strand breaks in vivo. J Cell Biol 146: 905-916.
- Rogakou EP, Pilch DR, Orr AH, Ivanova VS, Bonner WM (1998) DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J Biol Chem 273: 5858-5868.
- Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, et al. (2009) DNA damage response and cellular senescence in tissues of aging mice. Aging Cell 8: 311-323.
- Redon CE, Weyemi U, Parekh PR, Huang D, Burrell AS, et al. (2012) γ-H2AX and other histone post-translational modifications in the clinic. Biochim Biophys Acta 1819: 743-756.
- Redon CE, Nakamura AJ, Martin OA, Parekh PR, Weyemi US, et al. (2011) Recent developments in the use of γ-H2AX as a quantitative DNA double-strand break biomarker. Aging (Albany NY) 3: 168-174.
- Rothkamm K, Löbrich M (2003) Evidence for a lack of DNA double-strand break repair in human cells exposed to very low x-ray doses. Proc Natl Acad Sci U S A 100: 5057-5062.
- Akesson C, Lindgren H, Pero RW, Leanderson T, Ivars F (2003) An extract of Uncaria tomentosa inhibiting cell division and NF-kappa B activity without inducing cell death. Int Immunopharmacol 3: 1889-1900.
- Akesson Ch, Pero RW, Ivars F (2003) C-Med 100, a hot water extract of Uncaria tomentosa, prolongs lymphocyte survival in vivo. Phytomedicine 10: 23-33.
- Mammone T, Akesson C, Gan D, Giampapa V, Pero RW (2006) A water soluble extract from Uncaria tomentosa (Cat's Claw) is a potent enhancer of DNA repair in primary organ cultures of human skin. Phytother Res 20: 178-183.
- Pero RW, Giampapa V, Vojdani A (2002) Comparison of a broad spectrum anti-aging nutritional supplement with and without the action of a DNA repair enhancing cat's claw extract. J Anti Aging Med. 5: 345-355.
- Pero RW, Amiri A, Sheng Y, Welther M, Rich M (2005) Formulation and in vitro/in vivo evaluation of combining DNA repair and immune enhancing nutritional supplements. Phytomedicine 12: 255-263.
- Pero RW, Lund H, Leanderson T (2009) Antioxidant metabolism induced by quinic acid. Increased urinary excretion of tryptophan and nicotinamide. Phytother Res 23: 335-346.
- Sheng Y, Bryngelsson C, Pero RW (2000) Enhanced DNA repair, immune function and reduced toxicity of C-MED-100, a novel aqueous extract from Uncaria tomentosa. J Ethnopharmacol 69: 115-126.
- Sheng Y, Pero RW, Wagner H (2000) Treatment of chemotherapy-induced leukopenia in a rat model with aqueous extract from Uncaria tomentosa. Phytomedicine 7: 137-143.
- Sheng Y, Li L, Holmgren K, Pero RW (2001) DNA repair enhancement of aqueous extracts of Uncaria tomentosa in a human volunteer study. Phytomedicine 8: 275-282.
- Sheng Y, Akesson C, Holmgren K, Bryngelsson C, Giamapa V, et al. (2005) An active ingredient of Cat's Claw water extracts identification and efficacy of quinic acid. J Ethnopharmacol 96: 577-584.
- Lamm S, Sheng Y, Pero RW (2001) Persistent response to pneumococcal vaccine in individuals supplemented with a novel water soluble extract of Uncaria tomentosa, C-Med-100. Phytomedicine 8: 267-274.
- Guthrie OW, Gearhart CA, Fulton S, Fechter LD (2011) Carboxy alkyl esters of Uncaria tomentosa augment recovery of sensorineural functions following noise injury. Brain Res 1407: 97-106.
- Guthrie OW, Xu H (2012) Noise exposure potentiates the subcellular distribution of nucleotide excision repair proteins within spiral ganglion neurons. Hear Res 294: 21-30.
- Whitehead ML, Stagner BB, Lonsbury-Martin BL, Martin GK (1995) Effects of ear-canal standing waves on measurements of distortion-product otoacoustic emissions. J Acoust Soc Am 98: 3200-3214.
- Whitehead ML, Stagner BB, McCoy MJ, Lonsbury-Martin BL, Martin GK (1995) Dependence of distortion-product otoacoustic emissions on primary levels in normal and impaired ears. II. Asymmetry in L1,L2 space. J Acoust Soc Am 97: 2359-2377.
- Whitehead ML, McCoy MJ, Lonsbury-Martin BL, Martin GK (1995) Dependence of distortion-product otoacoustic emissions on primary levels in normal and impaired ears. I. Effects of decreasing L2 below L1. J Acoust Soc Am 97: 2346-2358.
- Guthrie OW (2008) Dys-synchronous regulation of XPC and XPA in trigeminal ganglion neurons following cisplatin treatment cycles. Anticancer Res 28: 2637-2640.
- Verma R, Rigatti MJ, Belinsky GS, Godman CA, Giardina C (2010) DNA damage response to the Mdm2 inhibitor nutlin-3. Biochem Pharmacol 79: 565-574.
- Li C, Fan S, Owonikoko TK, Khuri FR, Sun SY, et al. (2011) Oncogenic role of EAPII in lung cancer development and its activation of the MAPK-ERK pathway. Oncogene 30: 3802-3812.
- Gupta K, Chakrabarti A, Rana S, Ramdeo R, Roth BL, et al. (2011) Securinine, a myeloid differentiation agent with therapeutic potential for AML. PLoS One 6: e21203.
- Guthrie OW (2008) Preincision complex-I from the excision nuclease reaction among cochlear spiral limbus and outer hair cells. J Mol Histol 39: 617-625.
- Dmitrieva NI, Cai Q, Burg MB (2004) Cells adapted to high NaCl have many DNA breaks and impaired DNA repair both in cell culture and in vivo. Proc Natl Acad Sci U S A 101: 2317-2322.
- Matkowskyj KA, Schonfeld D, Benya RV (2000) Quantitative immunohistochemistry by measuring cumulative signal strength using commercially available software photoshop and matlab. J Histochem Cytochem 48: 303-312.
- Matkowskyj KA, Cox R, Jensen RT, Benya RV (2003) Quantitative immunohistochemistry by measuring cumulative signal strength accurately measures receptor number. J Histochem Cytochem 51: 205-214.
- Hu JJ, Ambrus A, Fossum TW, Miller MW, Humphrey JD, et al. (2008) Time courses of growth and remodeling of porcine aortic media during hypertension: a quantitative immunohistochemical examination. J Histochem Cytochem 56: 359-370.
- Dmitrieva NI, Cui K, Kitchaev DA, Zhao K, Burg MB (2011) DNA double-strand breaks induced by high NaCl occur predominantly in gene deserts. Proc Natl Acad Sci U S A 108: 20796-20801.
- Guthrie OW (2009) DNA repair proteins and telomerase reverse transcriptase in the cochlear lateral wall of cisplatin-treated rats. J Chemother 21: 74-79.
- Guthrie OW, Carrero-MartÃÂnez FA (2010) Real-time quantification of Xeroderma pigmentosum mRNA from the mammalian cochlea. Ear Hear 31: 714-721.
- Avan P, Bonfils P, Gilain L, Mom T (2003) Physiopathological significance of distortion-product otoacoustic emissions at 2f1-f2 produced by high- versus low-level stimuli. J Acoust Soc Am 113: 430-441.
- Chen GD, Fechter LD (2003) The relationship between noise-induced hearing loss and hair cell loss in rats. Hear Res 177: 81-90.
- Lorito G, Giordano P, Prosser S, Martini A, Hatzopoulos S (2006) Noise-induced hearing loss: a study on the pharmacological protection in the Sprague Dawley rat with N-acetyl-cysteine. Acta Otorhinolaryngol Ital 26: 133-139.
- Koike M, Mashino M, Sugasawa J, Koike A (2008) Histone H2AX phosphorylation independent of ATM after X-irradiation in mouse liver and kidney in situ. J Radiat Res 49: 445-449.
- Firsanov D, Vasilishina A, Kropotov A, Mikhailov V (2012) Dynamics of γH2AX formation and elimination in mammalian cells after X-irradiation. Biochimie 94: 2416-2422.
- Wang X, Michaelis EK (2010) Selective neuronal vulnerability to oxidative stress in the brain. Front Aging Neurosci 2: 12.
- Guthrie OW (2008) Aminoglycoside induced ototoxicity. Toxicology 249: 91-96.
- Guthrie OW, Li-Korotky HS, Durrant JD, Balaban C (2008) Cisplatin induces cytoplasmic to nuclear translocation of nucleotide excision repair factors among spiral ganglion neurons. Hear Res 239: 79-91.
- Hatahet Z, Purmal AA, Wallace SS (1994) Oxidative DNA lesions as blocks to in vitro transcription by phage T7 RNA polymerase. Ann N Y Acad Sci 726: 346-348.
- Huang H, Das RS, Basu AK, Stone MP (2011) Structure of (5'S)-8,5'-cyclo-2'-deoxyguanosine in DNA. J Am Chem Soc 133: 20357-20368.
- Taggart RT, McFadden SL, Ding DL, Henderson D, Jin X, et al. (2001) Gene Expression Changes in Chinchilla Cochlea from Noise-Induced Temporary Threshold Shift. Noise Health 3: 1-18.
- Lee SC, Bohne BA, Harding GW (2008) Cochlear base-apex differences in cell death pathways following exposure to low-frequency noise. Otorhinolaryngol J 2: 29-43.
- Fryatt AG, Mulheran M, Egerton J, Gunthorpe MJ, Grubb BD (2011) Ototrauma induces sodium channel plasticity in auditory afferent neurons. Mol Cell Neurosci 48: 51-61.
- Preston-Martin S, Thomas DC, Wright WE, Henderson BE (1989) Noise trauma in the aetiology of acoustic neuromas in men in Los Angeles County, 1978-1985. Br J Cancer 59: 783-786.
- Frenzilli G, Lenzi P, Scarcelli V, Fornai F, Pellegrini A, et al. (2004) Effects of loud noise exposure on DNA integrity in rat adrenal gland. Environ Health Perspect 112: 1671-1672.
- Ghabili K, Shoja MM, Tubbs RS, Rahimi-Ardabili B, Ansarin K (2007) Sonocarcinogenesis: loud noise may cause malignant transformation of cells. Med Hypotheses 69: 1156.
- Kenyon J, Gerson SL (2007) The role of DNA damage repair in aging of adult stem cells. Nucleic Acids Res 35: 7557-7565.
- Lomonaco SL, Xu XS, Wang G (2009) The role of Bcl-x(L) protein in nucleotide excision repair-facilitated cell protection against cisplatin-induced apoptosis. DNA Cell Biol 28: 285-294.
Citation: Guthrie OW, Xu H (2013) Reduced Phosphorylation of Histone Variant H2Ax in the Organ Of Corti Is Associated With Otoprotection from Noise Injury. Otolaryngology 3:131. DOI: 10.4172/2161-119X.1000131
Copyright: © 2013 Guthrie OW, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Select your language of interest to view the total content in your interested language
Share This Article
Recommended Journals
Open Access Journals
Article Tools
Article Usage
- Total views: 15862
- [From(publication date): 5-2013 - Nov 17, 2025]
- Breakdown by view type
- HTML page views: 10972
- PDF downloads: 4890