Editorial Open Access
Renaissance of Marine Natural Product Drug Discovery and Development
Yonghong Liu*Guangdong Key Laboratory of Marine Materia Medica, South China Sea Institute of Oceanology, Chinese Academy of Sciences, Guangzhou 510301, People’s Republic of China
- *Corresponding Author:
- Yonghong Liu
Guangdong Key Laboratory of Marine Materia Medica
South China Sea Institute of Oceanology
Chinese Academy of Sciences
Guangzhou 510301, People’s Republic of China
E-mail: yonghongliu@scsio.ac.cn
Received date April 17, 2012; Accepted date April 18, 2012; Published date April 21, 2012
Citation: Liu Y (2012) Renaissance of Marine Natural Product Drug Discovery and Development. J Marine Sci Res Development 2:e106. doi:10.4172/2155-9910.1000e106
Copyright: © 2012 Liu Y. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Marine Science: Research & Development
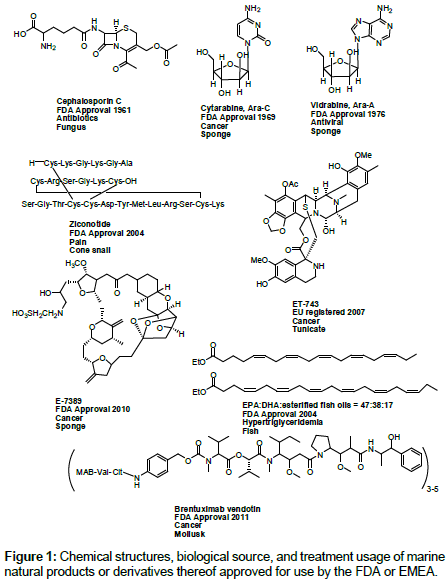
The marine environment has been shown to be the source of chemical diversity structures with promising biological activities. Besides the chemical novelty associated with those compounds, some of them possess novel mechanisms of action as well. To date, eight marine drugs have been approved by the FDA or EMEA registered, they are Cephalosporin C, Cytarabine (Ara-C), Vidrabine (Ara-A), Ziconotide (Prialt), omega-3-acid ethyl esters (Lovaza), ET-743 (Yondelis), E7389 (Halaven), Brentuximab vendotin (SGN-35) [1,2] showed in Figure 1.
These success stories have had to overcome difficulties inherent to marine natural products-derived drugs, such as sustainable source and issues related to structural complexity. Nevertheless, five marinederived agents are now approved, most as “first-in-class” drugs. Additionally, there is abundant pipeline of clinical and preclinical marine compounds to suggest their continued application in human medicine.
The solidness of the marine pharmaceuticals pipeline is evident, clinical trials with Soblidotin (TZT 1027), Tasidotin, Synthadotin (ILX-651), Bryostatin 1, Hemiasterilin (E7974), and Pseudopterosin have been completed, a compound (Plitidepsin) in Phase III trials, six compounds (DMXBA(GTS-21), Plinabulin (NPI 2358), PM00104, Elisidepsin, PM01183, CDX-011) in Phase II trials, and four compounds (Marizomib (Salinosporamide A; NPI-0052), PM060184, SGN-75, ASG-5ME) in Phase I trials have been processed with multitudinous marine natural products being investigated preclinical as clinical candidates.
The global marine preclinical pharmaceutical pipeline remains very active. During the period 1998-2008, the global marine pharmaceutical preclinical pipeline included 592 marine natural products that showed antitumor and cytotoxic activity, and 666 additional chemicals which demonstrated other pharmacological activities (i.e. antibacterial, anticoagulant, anti-inflammatory, antifungal, anthelmintic, antiplatelet, antiprotozoal, and antiviral activities;) with actions on the cardiovascular, endocrine, immune, and nervous systems; and other miscellaneous mechanisms of action [3].
The unexplored marine organisms harbors the most biological and chemical diversity, it is the vastest resource to discover novel structures (to date, more than 21855 compounds were discovered [4]) with novel modes of action. In order to conduct preclinical and clinical trials and further develop a promising lead into a marketed drug, sustainable supply is necessary. The continuous supply problem is the major challenge for marine natural product drug discovery and development. However, modern approaches are available to overcome the obstacles. Proceedings in technologies such as sampling strategies, nanoscale NMR for structure determination, total chemical synthesis, fermentation, and biotechnology are all crucial to the success of marine natural products as drug leads. The high degree of innovation in the field of marine natural products, will lead to a new wave resurgence of new drugs in the coming future [5].
The potential of marine natural products to become new drugs is still on the horizon. With the prominent development of more marine natural products from the current pipeline, the contribution of marine natural products to the future pharmaceuticals seems to be prospecting. New technologies and efficient collaborations between multidisciplinary scientists will be essential to ensure the future success of marine natural products as new therapeutic chemical entities that can make a significant contribution to the cure of human disease.
References
- Mayer AMS, Glaser KB, Cuevas C, Jacobs RS, Kem W, et al. (2010) The odyssey of marine pharmaceuticals: a current pipeline perspective. Trends Pharmacol Sci 31: 255-265.
- Gerwick WH, Moore BS (2012) Lessons from the Past and Charting the Future of Marine Natural Products Drug Discovery and Chemical Biology. Chemistry & Biology 19: 85-98.
- Mayer AMS, Rodriguez AD, Berlinck RGS, Fusetani N (2011) Marine pharmacology in 2007–8: Marine compounds with antibacterial, anticoagulant, antifungal, anti-inflammatory, antimalarial, antiprotozoal, antituberculosis, and antiviral activities; affecting the immune and nervous system, and other miscellaneous mechanisms of action. Comp Biochem Physiol C-Toxicol Pharmacol 153: 191-222.
- Blunt JW, Copp BR, Keyzers RA, Munro MH, Prinsep MR (2012) Marine natural products. Nat Prod Rep 29: 144-222.
- Montaser R, Luesch H (2011) Marine natural products: a new wave of drugs? Future Med Chem 3: 1475-1489.
Relevant Topics
- Algal Blooms
- Blue Carbon Sequestration
- Brackish Water
- Catfish
- Coral Bleaching
- Coral Reefs
- Deep Sea Fish
- Deep Sea Mining
- Ichthyoplankton
- Mangrove Ecosystem
- Marine Engineering
- Marine Fisheries
- Marine Mammal Research
- Marine Microbiome Analysis
- Marine Pollution
- Marine Reptiles
- Marine Science
- Ocean Currents
- Photoendosymbiosis
- Reef Biology
- Sea Food
- Sea Grass
- Sea Transportation
- Seaweed
Recommended Journals
Article Tools
Article Usage
- Total views: 17327
- [From(publication date):
August-2012 - Oct 15, 2025] - Breakdown by view type
- HTML page views : 12504
- PDF downloads : 4823