Research Article Open Access
Seasonal Variation in Arbuscular Mycorrhizal Fungi Root Colonization of Cheatgrass (Bromus tectorum), an Invasive Winter Annual
Ryan R. Busby1,2*, Mark W. Paschke1, Mary E. Stromberger1 and Dick L. Gebhart21Graduate Degree Program in Ecology, Colorado State University, Fort Collins, CO 80523, USA
2United States Army Engineer Research and Development Center, 2902 Newmark Drive, Champaign, IL 61822, USA
- *Corresponding Author:
- Ryan R. Busby
Graduate Degree Program in Ecology
Colorado State University, Fort Collins
CO 80523, USA
Tel: (217) 373-7296
Fax:(217) 373-7266
E-mail: Ryan.R.Busby@usace.army.mil
Received date: February 07, 2012; Accepted date: February 10, 2012; Published date: February 13, 2012
Citation: Busby RR, Paschke MW, Stromberger ME, Gebhart DL (2012) Seasonal Variation in Arbuscular Mycorrhizal Fungi Root Colonization of Cheatgrass (Bromus tectorum), an Invasive Winter Annual. J Ecosys Ecograph S8:001. doi:10.4172/2157-7625.S8-001
Copyright: © 2012 Busby RR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Ecosystem & Ecography
Abstract
Cheatgrass is a highly invasive winter annual grass that is most aggressive in the semi-arid steppe region of western North America. In this region, cheatgrass invasion becomes so severe that virtual monocultures can result. Due to its strategy for growth from autumn to spring, cheatgrass remains active during winter months when most native vegetation is dormant. This shift in host activity could be important for beneficial soil microbes, particularly the arbuscular mycorrhizal fungi (AMF), as they are adapted for coincidental growth with host plants. Many native plant species that are utilized for restoring areas invaded by cheatgrass associate with AMF, so any reduction in these symbiotic fungi could reduce the successful establishment of desirable plant species. Although cheatgrass is recognized as a facultative associate of AMF, its associations with AMF across seasons and throughout its lifespan are not known. We measured AMF colonization of cheatgrass roots from soon after germination through senescence. We found that cheatgrass remains colonized throughout its life. Colonization drops dramatically once soil temperatures approach freezing, but was highest late in the growth cycle of cheatgrass during flowering and seed set. Colonization by AMF never attained levels comparable to highly mycorrhizal plant species. This indicates that cheatgrass is a poor host for AMF throughout its life, and long-term dominance by cheatgrass could alter AMF in soils. Restoring highly invaded sites quickly following invasion might reduce the negative effects of cheatgrass on this important soil microbial community.
Keywords
Arbuscular mycorrhizal fungi; Bromus tectorum; Cheatgrass; Colonization; Seasonal change
Introduction
Cheatgrass (Bromus tectorum L.) is a highly invasive, introduced winter annual grass in western North America [1]. Cheatgrass is very successful at colonizing new sites due to its ability to germinate in autumn when most native vegetation is senescing, remain active throughout the winter when conditions allow, utilize soil moisture before most native vegetation resumes growth in the spring, and reproduce and die when soil moisture becomes limiting or is exhausted [2,3]. Cheatgrass is described as a facultative mycotroph [4], and has been associated with reduced AMF density in invaded soils [5]. Native grasses exhibited lower diversity of AMF species colonizing their roots when grown with cheatgrass than without [6]. Mycorrhizal colonization has reduced cheatgrass biomass [7] or had no effect [8].
Mycorrhizal colonization of roots is highly seasonal, with colonization lowest at cool temperatures, and highest at warm temperatures [9-12]. Generally, this seasonality coincides with host activity, as most plant species are senescent or dormant at cool temperatures, and activity is highest during periods of warm temperatures. This correlation with host activity was further shown in colonization differences between perennial cool- and warm-season grasses at the same site, where cool-season grass colonization peaked in late spring and early autumn and warm-season grass colonization peaked in mid-summer [10].
The only annual grasses investigated for winter AMF colonization are the winter cereal crops wheat (Triticum aestivum L.), barley (Hordeum vulgare L.), and rye (Secale cereale L.) [13]. Several studies indicate that winter wheat is not colonized until spring [14,15], but others have shown winter colonization [16,17]. Hetrick and Bloom [18] found almost no colonization of winter wheat at 10°C, less than 1% colonization at 15°C, with peak colonization occurring between 20° and 25°C.
Because cheatgrass is a winter annual and uses this strategy to gain an advantage over native vegetation, it is important to know how AMF are involved with cheatgrass throughout its life. The goal of this study was to determine how AMF colonization changes over time in cheatgrass roots and test the hypothesis that cheatgrass roots are colonized by AMF throughout its lifespan in invaded soils.
Materials and Methods
Patches were identified (through standing dead shoots) containing dense cheatgrass populations the prior year during the summer of 2007 in an invaded short grass steppe plant community at the Pine Ridge Natural Area (Fort Collins, CO, USA). Two random patches (north site and south site) were selected approximately 150 m apart and monitored until cheatgrass seedlings emerged in autumn. The north site (40.5489°, -105.1435°) is located on an east-facing slope, on a soil classified as a hilly Haplustoll [19]. The south site (40.5473°, -105.1428°) is located on a rocky, southeast-facing hillside, on a Satanta loam (Fine-loamy, mixed, superactive, mesic Aridic Argiustolls) [19]. Six weeks after germination was estimated to have occurred (based on rainfall events and site visits), five cheatgrass individuals from each patch were excavated and their roots were washed in tap water, cut into 3 cm segments, and stored in 70% ethanol. Approximately every 3 weeks after this initial harvest, an additional five cheatgrass individuals were collected in the same manner from each patch. A total of 11 sampling dates occurred between October 2007 and June 2008, giving a total of 110 individual samples (55 per site). Collection was repeated until cheatgrass individuals began to senesce in the spring. Soil temperature data at a depth of 15 cm was obtained from the Colorado State University Fort Collins Weather Station (approximately 5.5 km from study sites) at their website:
http://climate.colostate.edu/~autowx/fclwx_about.php (Accessed 7-11-2008).
Root samples were washed in tap water, cleared to remove pigments in 2.5% KOH for 30 min. at 90°C, re-rinsed with tap water, and acidified in 1% HCl for 4 h. Roots were then stained in acid glycerol containing 0.05% trypan blue for 30 minutes at 90°C and destined in acid glycerol for 30 minutes at 90°C [20]. Root samples were divided into three subsamples each (n = 110, N = 330). Each subsample was observed under 400 X magnification and the presence of hyphae, vesicles, and arbuscules were determined using 100 root intersections per subsample with a crosshair reticle [21].
Colonization count data were arcsine square root transformed, and normality was confirmed through residuals plots, box plots and Wilks-Shapiro normality tests. Colonization data were analyzed using the proc mixed random effects model in SAS version 9.1 (The SAS Institute, Cary, NC, USA), with site, date, their interaction, subsamples nested within samples, and samples nested within sites as effects. Nesting of samples and subsamples was incorporated to minimize pseudo replication, with treatments applied at the site level and samples (individual plants) treated as independent replicates within each site.
Results and Discussion
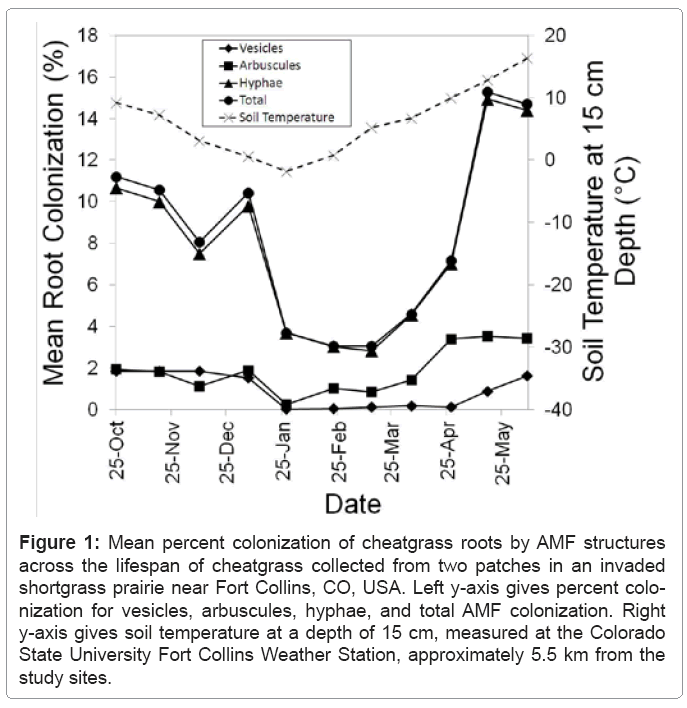
Cheatgrass roots were colonized during every sampling date from soon after germination through senescence, although mean colonization was never greater than 20% (Figure 1). Subsample vesicle colonization ranged from 0% to 7%, arbuscule colonization ranged from 0% to 10%, hyphal colonization ranged from 0% to 33%, and total colonization ranged from 0% to 34%. Means (and standard errors) for all samples and subsamples across all sampling dates and both sites were: vesicle % colonization: 0.93 (± 0.08), arbuscule % colonization: 1.89 (± 0.12), hyphae % colonization: 8.04 (± 0.34), and total % colonization: 8.35 (± 0.35). Vesicle, arbuscule, and hyphae percent colonization do not sum to the total percent colonization because one colonized intersection was often colonized by multiple structures, but the intersection only contributed 1% to the total percent colonization.
Figure 1: Mean percent colonization of cheatgrass roots by AMF structures across the lifespan of cheatgrass collected from two patches in an invaded shortgrass prairie near Fort Collins, CO, USA. Left y-axis gives percent colonization for vesicles, arbuscules, hyphae, and total AMF colonization. Right y-axis gives soil temperature at a depth of 15 cm, measured at the Colorado State University Fort Collins Weather Station, approximately 5.5 km from the study sites.
Colonization decreased significantly during the month of January once soil temperatures approached freezing, and did not begin to increase until soil temperature began to warm in March, with a peak colonization of 15.3% in May when florets appeared (Figure 1). Peak colonization occurred during the May 17 sampling interval, when florets were produced. However, colonization decreased in the subsequent June 8 sampling interval, when senescence of cheatgrass was occurring (Figure 1). However, vesicle colonization was still increasing during this final sampling interval. Population variance was not affected by variance of samples within patches or subsamples within samples (Table 1). Population variance in AMF colonization was due entirely to site and date effects, and their interaction (Table 1). Sampling date was significant for all colonization structures, but site was only significant for arbuscule colonization (Table 1). South site mean arbuscule colonization was 1.37% ( 0.14), while the north site mean arbuscule colonization was 2.41% (± 0.18). The interaction between site and date had a significant effect on population variance for all colonization structures (Table 1).
| Colonization Structure | ||||||||
|---|---|---|---|---|---|---|---|---|
| Vesicles | Arbuscules | |||||||
| Effect | Variance Component | Error df | F | p | Variance Component | Error df | F | p |
| Date | 0.002 | 10 | 6.88 | 0.003 | 0.002 | 10 | 2.98 | 0.050 |
| Site | 0.000 | 9 | 0.96 | 0.354 | 0.001 | 10 | 7.99 | 0.019 |
| Sample(Site) | < 0.001 | 290 | 1.98 | 0.097 | < 0.001 | 290 | 1.32 | 0.263 |
| Subsample(Sample) | 0.000 | 290 | 0.94 | 0.501 | 0.000 | 290 | 0.50 | 0.889 |
| Date x Site | < 0.001 | 290 | 3.08 | 0.001 | 0.001 | 290 | 4.86 | < 0.001 |
| Residual | 0.003 | 0.005 | ||||||
| Hyphae | Total | |||||||
| Effect | Variance Component | Error df | F | p | Variance Component | Error df | F | p |
| Date | 0.006 | 10 | 4.88 | 0.010 | 0.006 | 10 | 4.99 | 0.009 |
| Site | < 0.001 | 10 | 2.83 | 0.122 | < 0.001 | 11 | 2.62 | 0.134 |
| Sample(Site) | < 0.001 | 290 | 1.54 | 0.191 | < 0.001 | 290 | 1.99 | 0.096 |
| Subsample(Sample) | 0.000 | 290 | 0.62 | 0.797 | 0.000 | 290 | 0.46 | 0.916 |
| Date x Site | 0.003 | 290 | 7.48 | < 0.001 | 0.003 | 290 | 7.11 | < 0.001 |
| Residual | 0.006 | 0.006 | ||||||
Table 1: Analysis of variance for effects of date, site, sample, subsample, and date x site interaction on percent root colonization of cheatgrass by AMF structures.
The significant site x date interaction was most likely due to the difference between sites at the end of the sampling effort, as the different sites were situated on different soils and slope aspects. These differences could greatly affect the growth of plants, even in close proximities to one another. While site was significant for arbuscule colonization, the ecological importance of a 1% difference in arbuscule colonization is probably small. The north site population senesced earlier than the south site population, and during the final sampling the south site individuals were much greener than the north site individuals, as cheatgrass turns purple following seed set. This resulted in a much greater reduction in arbuscular, hyphal, and total colonization during the final sampling interval at the north site, and much greater vesicle colonization at the north site, relative to the south site. Although this is the first study to observe AMF colonization changes in an invasive winter annual grass throughout its life span, cross-seasonal comparisons at a broader landscape level would likely be more variable, and if compared to coexisting native vegetation would be much more informative.
As vesicles are C storage structures for the AMF, an increase in vesicles during host senescence indicates the AMF were storing increasing levels of C as the C supplied by the host plant began to diminish, most likely to increase spore production once the C source began to diminish. Bentivenga and Hetrick [10] observed high colonization of perennial grass roots long after senescence of the host plant, and speculated that at the later stages of host growth, AMF may turn parasitic in order to maximize C availability for sporulation. Arbuscules are believed to be exchange sites, where AMF provide P and other soil resources to the plant in exchange for C. As the host senesces or otherwise reduces the flow of C to its fungal symbionts, it would be expected that a decrease in arbuscule presence would be observed.
AMF colonization of host roots has been previously observed to decrease significantly at 15°C, and stop almost completely at 10°C [22]. We observed a similar trend for colonization of cheatgrass roots, and increases in colonization occurred only after the soil temperature rose above 10°C at the 15 cm depth in spring (Figure 1). The reduction in colonization at low temperatures is thought to result from reduced C supplied by the plant host [23]. Gavito et al. [23] found that C supply was reduced at low temperatures while P transfer from AMF to the plant host remained constant at temperatures between 10 and 25°C. However, other studies have shown that AMF colonization reduces plant growth at low temperatures [22] due to the AMF consuming host C at low temperatures but not providing P to the host [18]. Because of the variation in interactions between AMF species and plant hosts [24], it is possible that certain AMF species are more parasitic than others on cheatgrass at low temperatures.
The native vegetation that is replaced by cheatgrass includes many perennial species that are active throughout the summer and dormant through the winter, including shrubs, forbs and C4 grasses. Large differences in AMF species sporulation and abundance have been observed in single host plant species during the growing season, and between C3 and C4 grasses both within and between sampling dates [10]. Many of the AMF species adapted to growth during warm soil temperatures with hosts active during this period would not have access to a suitable host in soils where cheatgrass is the only host, as cheatgrass would be senescing while they are becoming active. Thus, the loss of seasonal host activity due to cheatgrass dominance most likely favors AMF species adapted to early season growth.
Cheatgrass invasion often completely overwhelms native vegetation, creating virtual monocultures that exclude other plant species [1]. Colonization of cheatgrass by AMF never attained colonization levels comparable to highly mycorrhizal plant species.
This indicates that cheatgrass is a poor host for AMF throughout its life, and long-term dominance by cheatgrass could alter AMF in soils. Cheatgrass-invaded soils have been observed to contain lower AMF density than soils dominated by native vegetation [5]. This alteration could hinder restoration of invaded sites, but restoring highly invaded sites quickly following invasion might reduce the negative effects of cheatgrass on this important soil microbial community.
Acknowledgements
The authors would like to thank Jennifer Shanahan and the Fort Collins Natural Resources Department for permitting the collection of cheatgrass samples from Pine Ridge Natural Area.
References
- Knapp PA (1996) Cheatgrass (Bromus tectorum L) dominance in the Great Basin Desert. Global Environmental Change 6: 37-52.
- Stewart G, Hull AC (1949) Cheatgrass (Bromus tectorum L.) - An ecologic intruder in southern Idaho. Ecology 30: 58-74
- Klemmedson JO, Smith JG (1964) Cheatgrass (Bromus tectorum L.) Bot Rev 30: 226-262.
- Goodwin J (1992) The role of mycorrhizal fungi in competitive interactions among native bunchgrasses and alien weeds: A review and synthesis. Northwest Sci 66: 251-260.
- Al-Qawari AA (2002) Relationships Among Nitrogen Availability, Vesicular-Arbuscular Mycorrhizae, and Bromus tectorum in Disturbed Rangeland Sites in Colorado. Ph.D Dissertation, Colorado State University, Fort Collins.
- Hawkes CV, Belnap J, D'Antonio C, Firestone MK (2006) Arbuscular mycorrhizal assemblages in native plant roots change in the presence of invasive exotic grasses. Plant and Soil 281: 369-380.
- Rowe HI, Brown CS, and Claassen VP (2007) Comparisons of mycorrhizal responsiveness with field soil and commercial inoculum for six native montane species and Bromus tectorum. Restoration Ecology 15: 44-52.
- Allen EB, Allen MF (1982) The role of vesicular-arbuscular mycorrhizae in some colonizing annuals. Bulletin of the Ecological Society of America 63: 180.
- Rabatin SC (1979) Seasonal and edaphic variation in vesicular-arbuscular mycorrhizal infection of grasses by Glomus tenuis. New Phyto 83: 95-102.
- Bentivenga SP, Hetrick BAD (1992) Seasonal and temperature effects on mycorrhizal activity and dependence of cool- and warm-season tallgrass prairie grasses. Can J Bot 70: 1596-1602.
- Sanders IR, Fitter AH (1992) The ecology and functioning of vesicular-arbuscular mycorrhizas in co-existing grassland species. I. Seasonal patterns of mycorrhizal occurrence and morphology. New Phyto 120: 525-533.
- Lutgen ER, Muir-Clairmont D, Graham J, Rillig MC (2003) Seasonality of arbuscular mycorrhizal hyphae and glomalin in a western Montana grassland. Plant and Soil 257: 71-83.
- Jakobsen I, Nielsen NE (1983) Vesicular-arbuscular mycorrhiza in field-grown crops. I. Mycorrhizal infection in cereals and peas at various times and soil depths. New Phyto 93: 401-413.
- Hetrick BAD, Bockus WW, Bloom J (1984) The role of vesicular-arbuscular mycorrhizal fungi in the growth of Kansas winter wheat. Can J Bot 62: 735-740.
- Mohammad MJ, Pan WL, Kennedy AC (1998) Seasonal mycorrhizal colonization of winter wheat and its effect on wheat growth under dryland field conditions. Mycorrhiza 8: 139-144.
- Buwalda JG, Stribley DP, Tinker PB (1985) VA-mycorrhiza of winter and spring cereals. J Agri Sci 105: 649-657.
- Dodd JC, Jeffries P (1986) Early development of VAM in autumn-sown cereals. Soil Biol Biochem 18: 149-154.
- Hetrick BAD, Bloom J (1984) The influence of temperature on colonization of winter wheat by vesicular-arbuscular mycorrhizal fungi. Mycologia 76: 953-956.
- United States Department of Agriculture (1980) Soil survey of Larimer County Area, Colorado. Washington, DC.
- Koske RE, Gemma JN (1989) A modified procedure for staining roots to detect VA mycorrhizas. Mycological Research 92: 486-488.
- McGonigle TP, Miller MH, Evans DG, Fairchild GL, Swan JA (1990) A new method which gives an objective measure of colonization of roots by vesicular-arbuscular mycorrhizal fungi. New Phyto 115: 495-501.
- Liu A, Wang B, Hamel C (2004) Arbuscular mycorrhiza colonization and development at suboptimal root zone temperature. Mycorrhiza 14: 93-101.
- Gavito ME, Olsson PA, Rouhier H, Medina-Penafiel A, Jakobsen I, et al. (2005) Temperature constraints on the growth and functioning of root organ cultures with arbuscular mycorrhizal fungi. New Phyto 168: 179-188.
- Gustafson DJ, Casper BB (2006) Differential host plant performance as a function of soil arbuscular mycorrhizal fungal communities: experimentally manipulating co-occurring Glomus species. Plant Ecology 183: 257-263.
Relevant Topics
- Aquatic Ecosystems
- Biodiversity
- Conservation Biology
- Coral Reef Ecology
- Distribution Aggregation
- Ecology and Migration of Animal
- Ecosystem Service
- Ecosystem-Level Measuring
- Endangered Species
- Environmental Tourism
- Forest Biome
- Lake Circulation
- Leaf Morphology
- Marine Conservation
- Marine Ecosystems
- Phytoplankton Abundance
- Population Dyanamics
- Semiarid Ecosystem Soil Properties
- Spatial Distribution
- Species Composition
- Species Rarity
- Sustainability Dynamics
- Sustainable Forest Management
- Tropical Aquaculture
- Tropical Ecosystems
Recommended Journals
Article Tools
Article Usage
- Total views: 15427
- [From(publication date):
June-2013 - Dec 22, 2025] - Breakdown by view type
- HTML page views : 10642
- PDF downloads : 4785