Research Article Open Access
Simultaneous Chromium Removal and Power Generation Using Algal Biomass in a Dual Chambered Salt Bridge Microbial Fuel Cell
| Singhvi P1 and Chhabra M2* | |
| 1Biological Science and Bio-Engineering, Indian Institute of Technology Bombay, Powai, Mumbai, 400076, India | |
| 2Centre for Excellence in Biologically Inspired System Science and System Science, Indian Institute of Technology-Rajasthan, Jodhpur, Rajasthan 342011, India | |
| Corresponding Author : | Dr. Meenu Chhabra Assistant Professor in Biologically Inspired System Science Indian Institute of Technology-Rajasthan Jodhpur, Rajasthan 342011, India Tel: +91- 0291-2449056 E-mail: meenuchhabra@iitj.ac.in |
| Received February 13, 2013; Accepted June 05, 2013; Published June 07, 2013 | |
| Citation: Singhvi P, Chhabra M (2013) Simultaneous Chromium Removal and Power Generation Using Algal Biomass in a Dual Chambered Salt Bridge Microbial Fuel Cell. J Bioremed Biodeg 4:190. doi: 10.4172/2155-6199.1000190 | |
| Copyright: © 2013 Singhvi P, et al. This is an open-a ccess article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Microbial Fuel Cell (MFC) is a bio-electrochemical device used essentially for power generation and is now emerging as a sustainable waste treatment system. In this paper, we report detoxification of chromium VI contaminated water using algae biomass in a dual chamber salt bridge MFC. The device showed high chromium removal efficiency with 98% removal achieved in 96 h at pH 2. Initial pH of the cathode solution had a significant effect on device performance with acidic pH values favouring high chromium removal, Open Circuit Potential (OCV), power density and anodic COD removal. The power density value of 207 mW/m 2 and open circuit potential of 760 mV was attained at pH 2. Algae biomass driven MFC thus, proved to be an efficient bioremediation device with good power production.
| Keywords |
| Microbial Fuel Cell; Algal biomass; Chromium VI detoxification |
| Introduction |
| The industries namely electroplating, leather tanning, metal finishing and petroleum refining releases tons of hazardous wastes containing chromium annually worldwide [1]. The concentration of chromium in these wastes is far above the discharge limit set by Central Pollution Control Board (CPCB)-India and Environmental Protection Agency (EPA)-US. Chromium (Cr) contamination in water is a serious environmental threat as it is a strong oxidizing agent, a potential carcinogen and extremely toxic metal to the living world [2,3]. US-EPA regulates chromium as one of the priority pollutants and the maximum permissible limit for Cr (VI) in water is 0.05 mg/l. Chromium exists in two oxidation states denoted as Cr (VI) and Cr (III). Cr (III) is less toxic, less mobile and easily precipitates at neutral pH. Cr (VI), on the other hand, is highly toxic and water soluble [1,4]. The primary mechanism of detoxifying chromium containing waters is to reduce the Cr (VI) to Cr (III) state. A number of physico-chemical methods achieve Cr (VI) reduction and detoxification [5-8] however, these methods appear unsuitable owing to excessive sludge production, the use of expensive chemicals, regeneration of the adsorption matrix etc. The bioremediation using chromium resistant microbes is the most suitable method particularly in the natural environments. In this case, however, the rate of Cr (VI) addition to water exceeds the rate of natural attenuation [4]. Therefore, prior treatment of Cr (VI) is essential before discharge in natural water bodies and this paper reports a sustainable technology for the same [9]. |
| In the recent years, Microbial Fuel cells (MFC) have emerged as one of the most pragmatic applications in this direction. Technologies based on MFC represent the novel approach of waste to energy generation. MFC converts the chemical energy of the waste/biomass into electric energy by using microorganisms as catalyst [10-13]. |
| The working principle of MFC is similar to that of an electrochemical cell. Micro-organisms anaerobically oxidize biomass in the anodic chamber thereby reducing anode. Electrons then flow through an external circuit to the cathode constituting the flow of current. The protons flow through a proton exchange membrane or a salt bridge which separates an anode and cathode chamber. The final electron acceptor lies in the cathode. In conventional MFC cathode, oxygen acts as an electron acceptor which finally reduces to water. A wide range of electron acceptors works well in the MFC cathode which includes metal ions like Fe (III), Cr (VI) and Mn (V) [14,15]; inorganic electron acceptors like sulphates [15]; micro-organisms such as denitrifying bacteria and sulphate reducers [16,17]. The cell potential is the difference between the potential of cathode and anode. |
| MFC like the one which uses Cr (VI) as cathode electron acceptor thus serves the dual purpose of producing electrical energy while detoxifying and reducing Cr. Wang et al. [15] reports detoxification of Cr containing wastewater in the MFC using nutrient buffer solution as a source of electrons. Tandukar et al. [18] reports biological detoxification of Cr waters again using nutrient buffer solution in both anode and cathode chamber. Similarly, Hsu et al. [19] achieved Cr concentrations within acceptable limits of discharge by using Shwenella sp. in MFC cathode. |
| In the pursuit of a sustainable process of Cr wastewater treatment, we report in this paper heterogeneous algae biomass as a source of electrons for Cr reduction. Algae biomass easily grows on the surface of the ponds and will be mass cultivated in the near future for bioenergy. Algae represented by the molecular formula C106H263O110N16P offer a good source of oxidizable organic matter [20]. The main objectives of the study are: investigating the potential of algae as a source of electrons for Cr water detoxification, to study the rate of chromium removal and effect of pH on the power density and COD removal. |
| Materials and Methods |
| Sample collection and processing |
| Algae biomass was collected from the surface of stagnant water. The biomass was washed several times using running tap water. The culture was propagated in the laboratory using algae cultivation broth (Himedia laboratories) in glass beakers covered with porous lid (Figure 1A). Incubation was done outdoor with air sparged in using an aquarium air pump to ensure mixing and gas exchange. |
| The wet algae biomass was dried in sun for 72 h and finely grounded in a pestle and mortar. The powdered mass was used as the substrate for MFC. The initial COD of the algae biomass was found to be 2000 mg/g. Algae identification was done using a trinocular Olympus microscope at 40 X magnification. |
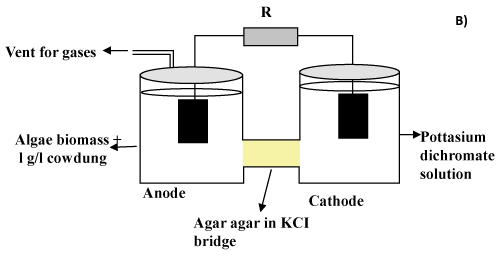
| MFC construction |
| The dual chambered MFC was constructed using two air tight plastic containers (anode and cathode) of 250 ml capacity connected through side openings via tube (length= 6 cm and diameter= 0.7 cm) filled with 2% agar gel in saturated KCl solution. The schematic of the same is shown in Figure 1B. The set up was maintained at room temperature (28°C). Activated charcoal pieces (surface area 10 cm2) were used as the electrodes. The electrical connection between the two electrodes and resisters was done using copper wires. The anode solution (200 ml) consisted of 2% dried algae powder in 50 mM phosphate buffer (pH 7). The chamber was inoculated with 1 g cow dung as the bacterial source. The cathode solution, on the other hand, consisted of potassium dichromate solution (10-200 mg/l) prepared in distilled water. The initial pH of potassium dichromate solution (pH 2,4,6 and 8) at cathode was adjusted using 1 M NaOH and 0.5 M H2SO4 as described previously [15]. |
| Analysis and calculations |
| Chemical Oxygen Demand (COD) of an anodic solution was determined using Merck Spectroquant Kit in the measurement range 25 and 10000 mg O2/L. The method involves oxidation of organic matter in a sample using acidified potassium dichromate (K2Cr2O7) solution in the presence of silver sulfate (Ag2SO4) catalyst. The COD estimation reagents were added to the sample as per manufacturer instructions and the sample digestion was carried out at 148°C for 2 h using a Thermoreactor TR 620 (Merck KgaA, Darmstadt). The green colour generated by Cr3+ ions, as a result of dichromate ion (Cr2O72- ) reduction was measured at 445 nm after cooling the samples using Spectroquant photometer NOVA 60 (APHA 5220D). |
| Chromium VI concentration was determined calorimetrically using Cr VI estimation kit (Merck KgaA, Darmstadt). The method is based on standard methods for investigation of water and wastewater APHA 3500 Cr D. The method involves the reaction of Cr VI with diphenylcarbazide in a weakly phosphoric acid solution to form Cr III and diphenylcarbazone (a red violet complex), measured using Spectroquant photometer NOVA 60 (Merck KgaA, Darmstadt). The samples for Cr VI estimation were supplemented with Cr VI estimation reagents as per manufacturer instructions. The samples were allowed to stand for one minute and absorbance was measured to get Cr VI concentration in mg/l. Chromium removal (%) was estimated as follows: Initial Cr VI concentration–Final Cr VI concentration/ Initial Cr VI concentration X 100. The samples were withdrawn after every 12 h for Cr estimation after a stable open circuit potential was achieved. The polarization curves were recorded by varying the external resistances ranging from 30 to 10000 Ω. The Voltage (V) and current (I) values were read after stabilization using a digital multimeter. Power(P= IV), power density (P/A2, where A is the projected anode surface area) and current density (I/A2) were calculated from the polarization experiments and also using the fixed resister of 200 Ω. |
| Results and Discussion |
| Algae biomass as a substrate for MFC and its bioremediation potential |
| The present study indicated algal biomass as a potential substrate for power generation and bioremediation using MFC´s. Velasquez- Orta et al. [21] reported the potential of both microalgae Chlorella vulgaris and macrophyte Ulva lactuca as a renewable source for electricity production in MFC. Algae biomass presents a rich source of hydrolysable carbohydrates, lipids and proteins. Algae biomass in this study as observed microscopically consisted primarily of green and blue green algae namely Spirogyra sp., Nostoc sp. Oscilatoria sp., Chlamydomonas etc. The heterogeneous algae culture was successfully used for the detoxification of Cr VI water with concomitant power generation. Algae biomass represents a renewable substrate which is cultivable on the mass scale while fixing atmospheric carbon-dioxide [22]. Thus, a bioremediation process based on algae biomass is sustainable keeping in account the cost effectiveness and associated environmental benefits [22]. |
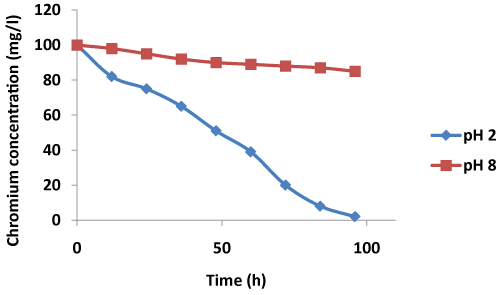
| The effectiveness of algae biomass as an electron source was demonstrated by a 72-95% reduction in the COD of anodic solution after 96 h of incubation. Open circuit potential values of 221 mV to 760 mV indicated anode potential capable of sustaining good power generation from an algae biomass. Moreover, 98% Cr VI removal was achieved at pH 2 indicating high bioremediation potential of algae biomass in the MFC (Table 1). |
| Effect of initial cathode pH on Cr VI removal using algae biomass |
| The reaction at cathode using Cr (VI) as electron acceptor can be written as follows: |
| CrO72- + 14H+ + 6e- → 2Cr3+ + 7H2O (1) |
| Since the reduction of Cr (VI) at cathode requires the participation of H+ ions, the pH of the solution is expected to affect the cathode potential as per the basic Nernst equation. The low pH values or high H+ ion concentration increases the cathode potential which in turn increases the cell potential which is given by the equation 2. |
| Ecell= Ecathode-Eanode (2) |
| As the pH of the cathode solution was increased from 2 to 8, the above said effect was noted on the cell OCV which reduced from 760 mV at pH 2 to 271 mV at pH 8. The rate of Cr VI removal also drastically reduced at high pH values with about 15% removal at 96 h at pH 8 (Figure 2). At pH 2, however, 98% Cr (VI) removal could be achieved (Table 1). Moreover, the Cr (VI) reduction profile was non linear. These observations were in line with observation made by Wang et al. [15] wherein a non linear profile of Cr VI reduction was observed from pH 2-6. The acidic pH values favour the high Cr reduction rate and with an increase in pH the rate declines. At pH 8, the Cr reduction rate was much slower; the reasons could be a low overall cell potential or driving force of the reaction, low concentration of the reactant H+ ions and low anodic COD reduction rate. The rate of anodic substrate oxidation also directly affects the electrochemical reaction rate and as explained by Wang et al. [15] the high substrate concentration at anode and high H+ ion concentration at cathode favours high reaction rates but as the time proceeds, the rate of electron supply from anode reduces due to substrate depletion and H+ concentration at cathode also reduces thereby reducing the overall reaction rates. Algae biomass supported high Cr removal rates with 95% removal achieved in just 96 h at pH 2. At pH 8, nearly 72% reduction in anode COD was observed after 96 h although the Cr removal was much less. The high COD removal could be attributed to bacterial activity other than that of exoelectricigens. |
| Power density values at different chromium concentrations |
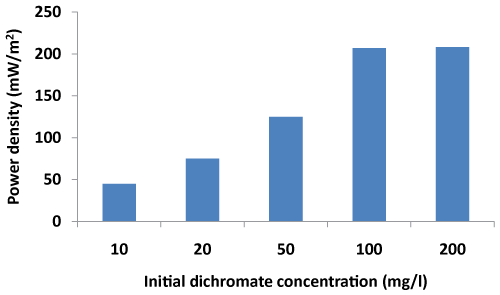
| The power density values were computed for different initial potassium dichromate concentrations at cathode after 96 h of MFC operation at a fixed resistance of 200Ω. It was found that power density increases from 45 mW/m2 to 207 mW/m2 with an increase in initial dichromate concentration from 10 mg/l to 100 mg/l (Figure 3). However, no further increase in power density values was noted as the concentration of dichromate was increased from 100 mg/l to 200 mg/l. These observations are in line with the observations made by You et al. [14] wherein an increase in current density was observed with an increase in permanganate concentration at low external resistance values. Although, the initial concentration of Cr does not have an appreciable effect on the cell potential, but after a long period of operation those cells with high concentrations of acceptor could maintain high cell potential values. Wei et al. [23] also reported that after 72 hours of operation high concentration of potassium ferricyanide could sustain high cell potential values resulting in high power density values. Further increase in power, however, will not be apparent beyond a particular acceptor concentration. The present system showed power density of 206 mW/m2 (pH 2) at an initial dichromate concentration of 100 mg/l. |
| Power density v/s current density at different pH |
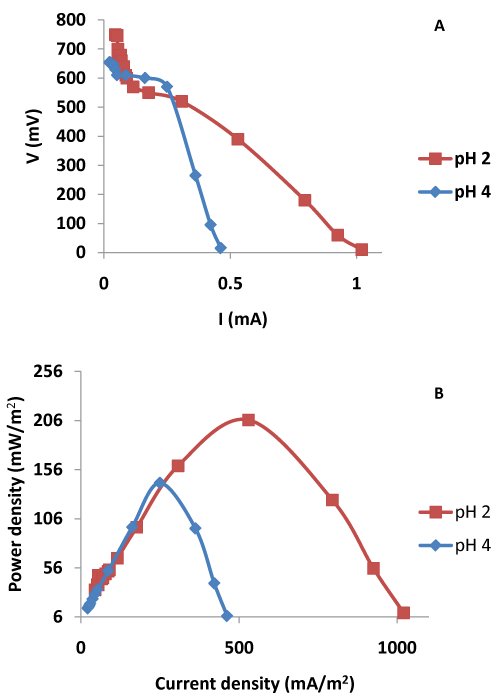
| The power density v/s current density curves were obtained through the polarization experiments at two different pH values. It was found that at an external resistance value of 200 Ω peak power densities were achieved at both the tested pH values (Figure 4). The peak power density values, however, differed with the variations in the pH. At pH 2, the highest power density value of 207 mW/m2 was achieved whereas the highest power density value was restricted to 143 mW/m2 at pH 4. The effect of pH was thus explainable by the theoretical predictions of the Nernst equation wherein the pH affects the cathode potential. Thus, pH 2 was found to be optimal for Cr (VI) bioremediation using algae biomass giving high power output and high current density of 530 mA/ m2 at an external resistance value 200 Ω. Wang et al. [15] reported similar results using nutrient buffer solution for Cr VI reduction. Moreover, the similar effect of cathode pH on manganese reduction and power has been reported by You et al. [14] using potassium permanganate as an electron acceptor. |
| Conclusions |
| The present study shows the effectiveness of algae biomass as a substrate for MFC used for bioremediation purpose. The Cr (VI) removal was successfully achieved with concomitant power generation. This study, therefore, presents an effective and sustainable strategy for Cr VI remediation. |
| Acknowledgements |
| Author MC thanks IIT Jodhpur for financial assistance. Dr. S Harinipriya is gratefully acknowledged for suggesting the MFC electrodes for the study. Author PS thanks Undergraduate Research Initiative program at IIT Jodhpur for infrastructural support and fellowship. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1A | Figure 1B | Figure 2 | Figure 3 | Figure 4 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 16348
- [From(publication date):
June-2013 - Nov 29, 2025] - Breakdown by view type
- HTML page views : 11387
- PDF downloads : 4961
