Research Article Open Access
Stabilizing Role of Nanodiamonds on the Amaranth Transformation in Model Biofilters
| Irina Schneider*, Mihaela Belouhova and Yana Topalova | |
| Department of General and Applied Hydrobiology, Faculty of Biology, Sofia University “St. Kliment Ohridski”, Bulgaria | |
| Corresponding Author : | Irina Schneider Department of General and Applied Hydrobiology Faculty of Biology, Sofia University “St. Kliment Ohridski” 8 Dragan Tzankov Blvd., 1164 Sofia, Bulgaria Tel: +35928167289 E-mail: i.schneider@abv.bg |
| Received April 24, 2012; Accepted May 15, 2012; Published May 17, 2012 | |
| Citation: Schneider I, Belouhova M, Topalova Y (2012) Stabilizing Role of Nanodiamonds on the Amaranth Transformation in Model Biofilters. J Bioremed Biodeg S3:001. doi:10.4172/2155-6199.S3-001 | |
| Copyright: © 2012 Schneider I, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The present article is focused on studying the first phase of a model dye biodegradation, the reduction of the azo bond, and the following amaranth decolorization. This phase is speed-limiting, i.e. it defines the speed of the whole process. The process is with a periodic regime of feeding and is simulated in reactors of the “biofilter’ type in the presence of trivial organic matter (peptone and yeast extract). In the first part of the studies the dynamics of the amaranth and the organic matter (measured as TOC) is followed as well as the decolorization effectiveness and the effectiveness of decreasing concentration of the organic matter. The spatial and temporal distribution of important groups of microorganisms in depth of the inert carrier has been followed, which take part in the biotransformation of the azo dye. The azoreductase activity of the immobilized community is studied. The second part of the article investigates the modulation effect of nanodiamonds on the residual concentration of amaranth and organic matter, as well as on the effectiveness of their biotransformation. The obtained results showed that the medium layer of the inert carrier in the biofilter has the most favorable conditions for carrying out the reduction of the azobond. The key groups of microorganisms in the biofilm which carry out the process are: the anaerobic heterotrophs; the anaerobic azo degraders, the bacteria from genus Pseudomonas and Acinetobacter. Nanodiamonds have a stimulating effect on the technological parameters of the system. It is expressed in maintaining a stable biofilm with a high activity, maintaining an optimal flow of the system and increasing the effectiveness of amaranth decolorization and organic matter removal.
| Keywords | |
| Amaranth decolorization; Biofilm; Microbial and enzymological parameters; Nanodiamonds | |
| Introduction | |
| The textile industry consumes high quantity of clean water (60- 400 lkg-1 dying textile) and chemicals in textile processing [1]. Textile wastewater is a major source of pollutants [2]. It is typically alkaline and has high organic matter concentration, solids, surfactants, oil, heavy metals (such as copper and chromium) and possibly toxic organic matter, including phenols and halogenated organic matter (from processes such as bleaching). The biochemical oxygen demand and chemical oxygen demand are high. As an example, the biochemical oxygen demand varies between 700 and 2000 mgl-1 and the chemical oxygen demand is 2 to 5 times higher than the biochemical oxygen demand level. Dyes often remain undegraded after treatment because they are xenobiotics with a structure resistant to biodegradation. Therefore, dye wastewater is frequently highly colored. Azo dyes make up the majority (60-70%) of the dyes applied in textile processing industries [3]. Wool processing may release bacteria and other pathogens. The textile wastewater requires proper management. The most effective technology for textile wastewater treatment is diphase comprising anoxic and aerobic reactors [4-5]. This technology logically follows biochemical mechanisms of dye biodegradation which includes azobond (-N=N-) reduction under anoxic conditions by azoreductases. The obtained products (aromatic amines) are more toxic and mutagenic than the initial azo dye molecule. Complete aerobic oxidation (mineralization) of aromatic amines is related to cleavage of the aromatic ring by oxygenases under aerobic conditions. | |
| In literature there is a considerable database for textile wastewater treatment under model and real conditions [6-10]. These technologies are based mainly on reactors with activated sludge [6-8]. Reactors with fixed biomass are not widely used for textile wastewater [9,10]. However, the reactors with biofilm have some advantages in comparison to the biobasins with activated sludge, which are: 1) higher metabolic activity because of the concentration of substrates, biomass, enzymes and modulators on the inert material; 2) less hydraulic retention time; 3) smaller volume of the reactor (and less usable land) compensated with more area and active surface of biofilm; 4) less excessive biomass for subsequent treatment; 5) higher resistance of the biofilm to toxic compounds and adverse effects; 6) faster renewal of the biofilm after stress. The disadvantages of this reactor are related to clogging of the biofilter in presence of higher concentration of suspended solids or excessive growth of filamentous microorganisms (because of the impaired ratio between carbon and nitrogen; availability of higher concentration of toxic compounds, etc.). These disadvantages could be eliminated by screening, flow equalization, and then settled to remove suspended solids before biological treatment. | |
| In full-scale wastewater treatment plants it is often found that local facilities are inefficient and scientists are looking for different approaches to improve treatment [6,9,11]. Different opportunities for acceleration and improvement of the effectiveness of wastewater treatment process as acclimation [12], immobilization [13,14], addition of alochtonic microorganisms [15-18], addition of enzymes [19] were reported in the scientific literature. Carbon adsorption is sometimes used to enhance removal [2]. In recent years, the application of nanotechnologies in different scientific areas increases. Nanodiamonds have potential application in medicine and biology, and have proven biocompatibility. These nanoparticles are unecotoxic [20,21]. The modulation effect of nanodiamonds has not been studied on wastewater treatment processes yet. The investigation of amaranth decolorization effectiveness with biofilm; the spatial and temporal distribution of key functional and taxonomic groups of microorganisms in carrier depth and the effect of nanodiamonds on some key technological parameters was the aim of this study. | |
| Materials and Methods | |
| In the present study an azo detoxification process with a model xenobiotic is simulated. The azo dye amaranth is used, its structure being similar to the structure of many dyes used in industry. In the first part of the studies the dynamics of key technological, microbial and enzymological parameters of the process is investigated. The initial process of the azo dye biotransformation, the azobond reduction, is studied. This process is speed-limiting and is the “unlocking” mechanism for the full mineralization of the azo dyes. The dynamics of important physiological and taxonomic groups of microorganisms is followed, as well as the one of the azoreductase activity of the biofilm in the course of the process and of the inert carrier in depth (surface, medium and bottom layer). The second part of the studies follows the dynamics of the technological parameters in two biofilters with quartz sand. In the critical phase of destabilization of the biofilm and decrease of the azoreductase activity (269th hour) nanodiamonds were added to one of the biofilters with the aim to stimulate the decolorization effectiveness. Nanodiamond powder is provided by Prof. Stavri Stavrev from the International Centre for Nanomaterials, Nanotechnologies and Nanomedicine, Smolyan Town, Bulgaria. Nanodiamond powder is added in concentration 3.12 mg.g-1 quartz sand. | |
| Bioprocess and bioreactors | |
| The first phase of dye biodegradation (azobond reduction) was simulated in sequencing batch reactors with fixed biomass. The simulated process was carried out in 0.5 L plastic reactors (Figure 1). The used carrier was quartz sand with size between 0.08 and 0.16 cm. The quartz sand was kindly provided by the Bistritza Drinking Water Treatment Plant (Sofia City, Bulgaria). The thickness of the carrier layer was 3 cm and samples for microbiological and enzymological analyses were taken from the surface layer (1 cm); the middle layer (2 cm) and the bottom layer (3 cm). The biofilters were connected to peristaltic pumps for providing flow system. An electric relay was connected to every peristaltic pump for precise regulation of the operating regime. The sequencing batch reactor was with feeding cycle of 24 hours. The duration of the simulated process was 455 hours. The amaranth concentration, COD and TOC values were monitored as a function of time during decolorization process. The reactors were incubated at 25 ± 2°C in thermostat. | |
| Biological system | |
| Biofilters inocula were prepared from specially treated real Activated Sludge (AS). The AS was taken from biobasins of the Sofia Wastewater Treatment Plant (processing municipal and industrial wastewater). It was treated with UD-20 automatic (3×10 sec. with frequency 22 kHz and vibration amplitude 8 μm) for obtaining homogenic suspension and for accelerating the biomass immobilization on inert material and initial biofilm formation. Moreover during the first 24 hours of the process the biofilter was fed with model wastewater (see the ingredients of the synthetic wastewater), but without included azo dye. The aim was to stimulate the development of the biofilm in conditions without stress factors and to enrich the biomass of the immobilized community. During the process the biofilm was acclimated to model wastewater step by step with a gradual increase of the azo dye concentration from 10 to 47 mg l-1. That adaptation was based on Holdane curve [22]. When the substrate is toxic, its increase leads to the increase the growth rate only of one concentration, specific for every xenobiotic, named critical. The critical concentration of amaranth for investigated biological system and technological parameters was 47 mg l-1. Therefore, that concentration was accepted as the final highest concentration. | |
| Synthetic textile wastewater | |
| The synthetic textile wastewater contained mineral media, 3% nutrient solution and increasing concentration of amaranth from 10 to 47 mg l-1. The mineral composition of synthetic wastewater contained (in gl-1): K2HPO4.3H2O - 5.6, KH2PO4 - 3.4, (NH4)2SO4 - 2.0, MgCl2.6H2O - 0.34, MnCl2.4H2O - 0.001, FeSO4.7H2O - 0.0006, CaCl2.2H2O - 0.026, Na2MoO4.2H2O - 0.002. The nutrient solution was additional source of carbon and hydrogen for co-metabolic biodegradation of amaranth and contained (in gl-1): peptone 10.0, yeast extract 5.0 and NaCl 5.0. | |
| Analytical methods | |
| Color measurements of synthetic wastewater were performed in UV-VIS spectrophotometer (Ultrospec 3000, PHARMACIA BIOTECH LTD., USA) against sample from dye-free model wastewater. The absorbance values of supernatants for amaranth (FLUKA, Germany) were determined at wavelength of 520 nm. The Chemical Oxygen Demand (COD) in water was analyzed according to a standard procedure [23]. Total organic carbon was measured with a TOC/ TN analyzer (TOC-V, SHIMADZU CORP., Japan). The microbial diversity of the biofilm was studied by plate count technique [24]. Immobilized biomass for microbiological analyses was detached from carrier by ultra sonic disintegration (3x5 sec. with frequency 22 kHz and vibration amplitude 8 μm) with UD-20 automatic (TECHPAN, Warsaw, Poland) in 0.9% solution of NaCl. The microbial groups related to organic matter transformation: aerobic (AH) and anaerobic (AnH) heterotrophs were determined by cultivation on Nutrient Agar (SCHARLAU CHEMIE, Barcelona, Spain). The anaerobic azodegraders (AzoD) were cultivated on Nutrient Agar with 50 mgl-1 amaranth. The colonies with media decolorization were determined as azodegraders. The key indicators, microorganisms participating in the transformation of xenobiotic compounds–bacteria from genus Acinetobacter were cultivated according to Kuznetzov and Dubinina [24] with the addition of 200 μM N,N’-dicyclohexylcarbodiimide (DCCD), an inhibitor of membrane-bonded ATPase; bacteria from genus Pseudomonas were cultivated on Glutamate Starch Pseudomonas Agar (SCHARLAU CHEMIE, Barcelona, Spain). The anaerobic microorganisms (AnH and AzoD) were incubated for 7 days in anaerobic jars (MERCK & CO. INC., Darmstadt, Germany) with Anaerocult A (MERCK & CO. INC., Darmstadt, Germany) for creating anaerobic conditions. The petri dishes were incubated at 28 ± 2°C. The anaerobic azo reductase activity was measured according to Grekova-Vasileva et al. [16]. The microbial biomass for enzyme analyses was harvested according to Feist and Hegeman [25]. The quantity of biomass for determination of the specific enzyme activity was measured as concentration of cell protein (Pr) by microbiuret method [26]. | |
| The effectiveness of removal (Eff) of the organic matter (measured as COD and TOC) and amaranth were determined. The following formula for its calculation was used: | |
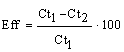 (1) (1) |
|
| Where Ct1 is the concentration of pollutant in influent; Ct2 is the concentration of pollutant in effluent. | |
| Results and Discussion | |
| Technological parameters of a textile wastewater treatment process | |
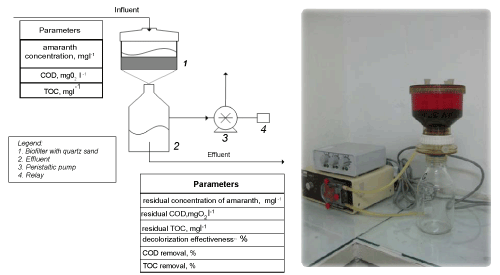
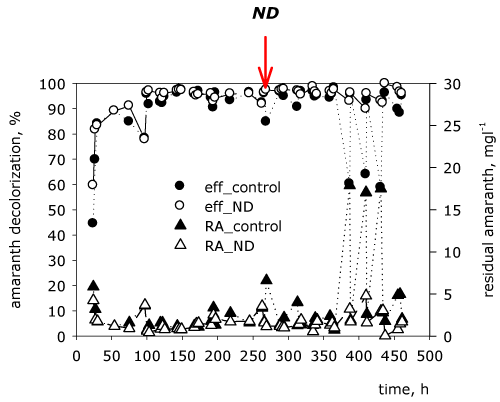
| From the dynamics of the residual concentration of amaranth in the effluent it is found that the process is biphase (Figure 2a). The early phase is read from the beginning to the 365th hour and is related to a high percentage of decolorization of the dye, which is caused by the applied acclimatization algorithm (raising the concentration of amaranth step by step). During the first 75 hours the biofilter is fed with wastewater with dye concentration around 10 mgl-1 with the aim of adapting the biological system to a low concentration of the amaranth, since the first hours are critical regarding the formation of an active and stable biofilm. Initially the residual concentration varies between 3 and 6 mgl-1, but at the 75th hour it decreases to 1.6 mgl-1. The following increase of the concentration of up to 31.6 mgl-1 in the influent doesn’t have a significant effect on the decolorization effectiveness, which reaches up to 98%. Then there is an increase of the incoming concentration of the amaranth of up to 43 mgl-1 at the 269th hour, which results in a decrease of the decolorization to about 84% and an increase of the residual concentration to 6.6 mgl-1. From these data it is found that the concentration of 43 mgl-1 is close to the critical for the biofilm according to the Holdane curve [22]. In order to avoid the destabilization of the immobilized community in the next 96 hours of the experiment the concentration of the dye in the influent is reduced and it varies between 39 and 42 mgl-1. The late phase of the process was from the 365th hour to the end of the experiment and is characterized by lowered activity of decolorization. This is again related to an increase of the concentration of amaranth in the influent, this time up to 47 mgl-1. The residual concentration in the effluent raises upto 17 mgl-1 and the effectiveness of decolorization reduces to 64%. From the obtained results it is found that if the concentration of the dye increases above 50 mgl-1, this could lead to the accumulation of xenobiotic in the biomass of the biofilm, to its thinning as a result of dropping of cells and to low biodegradation activity. | |
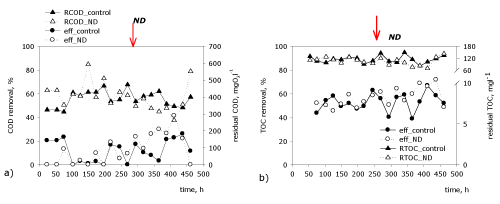
| The organic matter in influent and effluent was measured by the total organic carbon indicator (Figure 2b). The input concentration of the organic matter varies between 230 and 252 mgl-1 depending on the increasing concentrations of amaranth in the influent. It is found that during the whole process the effectiveness of decreasing the organic matter varies between 43 and 67%. These percentages are related to the degradation of the trivial organic matter in the wastewater, added in the form of peptone and yeast extract. The incomplete removal of the organic matter is related mainly to the accumulation of aromatic amines, an intermediate product from the transformation of the azo dyes. | |
| Functional parameters of biofilm | |
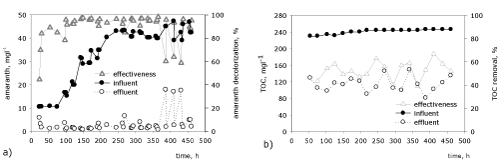
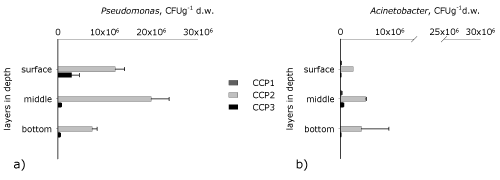
| The spatial distribution in depth of key groups of microorganisms, which take part in the reduction of the azobond is studied in purposefully chosen critical control points - CCP (Figure 3,4). The first CCP (0 hour) studies the biodiversity at the initial biofilm formation, CCP2 (191st hour) follows the changes during the stable functioning of the biofilm and by means of CCP3 (445th hour) the immobilized community is studied at the end of the process. From the data for the 0 hour of the process it is found that the activated sludge (used for inoculation of the biofliters) has the highest quantity of aerobic heterotrophs and bacteria from genus Acinetobacter, but as a whole all studied groups of microorganisms are present. This shows that the used activated sludge has a high biodiversity and metabolic pathways for degrading trivial and toxic pollutants. This is due to the fact that the Sofia Wastewater Treatment Plants treats both municipal and industrial wastewaters, including pollutants from the dyeing industry. During the process it is found that in the course of the adaptation process until the 191st hour (CCP2) the biofilm develops and the quantity of all studied groups increases. | |
| This shows that during the early phase of the process the biofilm has a high biodiversity and a prolific representation of the groups that play a key role in the purification and the biodetoxication process, while functioning with a high effectiveness of decolorization (Figure 2a). At the end of the process (CCP3) as a whole there is a decrease of the quantity of the immobilized microorganisms. This is related to the almost reached critical concentration of the amaranth at the end of the process and with the destabilization and the thinning of the biofilm as a result of this. | |
| As a whole in a spatial aspect the highest number of microorganisms is in the surface and the medium layer of the inert carrier, and in depth it is the lowest. This tendency is probably related to the fact that in depth there is a compression among the separate particles of the quartz sand and the mass transfer is more difficult. Moreover, the concentration of organic matter decreases towards the bottom, which influences the quantity of the microorganisms in the lowest layers of the inert carrier. Despite the general tendency the distribution of the different groups of microorganisms in depth has some specifics, which are defined by the characteristics of each group. | |
| In the biofilter the aerobic heterotrophs have the highest number (Figure 3a). In a vertical aspect the aerobic heterotrophs decrease from the surface to the bottom of the carrier. The reason is that in depth in the inert carrier the redox potential decreases. The surface layer of the biofilm is under conditions of a high redox potential since it is periodically washed with wastewater and physical aerating is carried out. In the medium and bottom layer of the quartz sand anoxic and anaerobic conditions are created as a result of the partial or full depletion of oxygen as a result of the biodegradation processes. Moreover the free spaces between the sand particles are very small and prevent oxygen from entering in depth. The obtained results about the anaerobic heterotrophs at the 191st hour (CCP2) confirm this hypothesis (Figure 3b). It is found that the highest quantity of anaerobes is in the medium layers of the inert carrier, and in the surface layer it is the lowest. In the bottom layer of the inert carrier the occurring conditions are not favorable for the development of the microorganisms. This tendency is present for all studied groups and most probably it is related to the reduced concentration of nutritious substrates. The spatial distribution of the anaerobic heterotrophs at the 455th hour of the process (CCP3) follows the tendency of the aerobic ones. This shows that one part of the aerobes are facultative and can exist and be active under aerobic as well as under anaerobic conditions, or that the aerobes quickly deplete the oxygen and create anaerobic conditions. As a whole the high concentration of the heterotrophs is related to the presence of higher concentrations of organic matter (COD of the influent is around 500 mg テ青?2l-1). These concentrations, however, favor the development of the aerobes [22], whereas the obligate anaerobic microorganisms require at least 3 times higher concentrations of organic matter. | |
| The anaerobic azodegraders are the key functional group of microorganisms, since they are directly related to the biotransformation of amaranth. The quantity of the anaerobic azodegraders at the 0 hour is about 7.103 CFUg-1 d.w., which shows that the used inoculum has a biodegradation potential to degrade such type of pollutant (Figure 3c). At the 191st hour of the process their quantity increases and reaches up to about 3.106 CFUg-1 d.w., the highest number being in the medium layer of the inert carrier. This tendency shows once again that in this layer the conditions are with a lower redox potential and favor the reduction of the azobond. The same trend in a vertical distribution is also found at the 455th hour of the process, but the quantity of the microorganisms decrease. | |
| In the course of the process two taxonomic groups of microorganisms were studied as well, related to the biodegradation of toxic pollutants [22]. It is found that the quantity of the bacteria from genus Pseudomonas is higher than the one of Acinetobacter (Figure 4). In both groups a peak was found at the 191st hour (CCP2), and at the end of the process (CCP3) they decrease as a result of the increase of the initial concentration of the amaranth. At the 191st hour of the process the bacteria from genera Pseudomonas and Acinetobacter have the highest quantity in the medium layer of the inert carrier and follow the tendency of the anaerobic heterotrophs and the anaerobic azodegraders. These two taxonomic groups are related to the decolorization of the amaranth because of their ability to use alternative energy sources and to switch their metabolism depending on the conditions in the reactor. At the end of the process (CCP3) their quantity decreases in depth and follows the tendencies found in the aerobic and anaerobic heterotrophs. | |
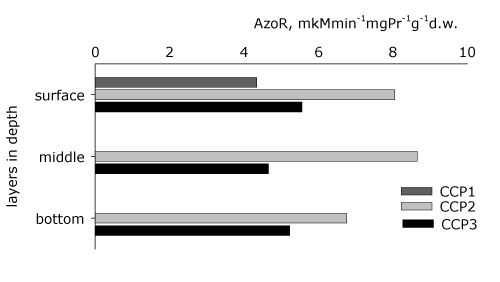
| The anaerobic azoreductase activity of the biofilm was studied spatially and temporally (Figure 5). It is found that at the beginning of the process (CCP1) the azoreductase activity is expressed only in the surface layer of the inert carrier, which is related to the continuous process of forming the biofilm. It is confirmed that the biodegradation potential is highest during the 191st hour of the process (CCP2), as the middle layer of the inert carrier favors azoreduction because of the lower redox potential. The anaerobic heterotrophs and the anaerobic azodegraders (Figure 3b, 3c) have an important role in carrying out these processes, as well as the bacteria from genera Pseudomonas and Acinetobacter (Figure 4a, 4b). At the end of the process (CCP3) a decrease of the decolorization activity of the biofilm is found, which is in parallel with its decreased effectiveness (Figure 2a). | |
| Effect of nanodiamonds on textile wastewater treatment process | |
| This part of the article follows the modulatory effect of nanodiamonds, added at the 269th hour of the process on the transformation of the amaranth. Until the 269th hour the two biofilters with quartz sand function in parallel under the same conditions. It is found that there are no abrupt fluctuations in the functioning of the two reactors before adding a modulator. A decrease of the effectiveness in the control biofilter is registered immediately at the 269th hour, whereas in the biofilter with nanodiamonds the effectiveness remains high (over 97%). The residual concentration of the amaranth in the effluent of the control sample reaches up to 6.6 mgl-1, whereas in the biofilter with nanodiamonds–up to 1.15 mgl-1. A decreased biotransformation activity (up to 58%) is registered for the control variant during the later phase of the process (after the 365th hour). In the biofilter with the nanodiamonds the decolorizing activity of the biofilm remains high (over 95%). The obtained results lead to the conclusion that the added nanodiamonds at the 269th hour of the process are a positive modulator and increase the decolorization effectiveness with 37% at the most critical hours (387th, 410th and 431st hour). They preserve the stability of the model system and a stable high effectiveness of decolorization. Most probably the reason is their high surface activity, which leads to the increase of the contact surface between the target enzymes and the substrate for their functioning–the azo dye (Figure 6). | |
| Another indicator of special significance in water treatment and the evaluation of the water quality is the chemical oxygen demand (COD) as an indicator for the organic load. The obtained values of COD in the effluent don’t differ significantly for the two model biofilters until the addition of nanodiamonds at the 269th hour (Figure 7). The fluctuations in the concentration of the organic matter vary between 312.36 and 593.31 mgテ青?2l-1. Such high values after treatment under anaerobic conditions are found by other researchers as well [27]. After adding nanodiamonds the values for COD are lower in comparison to the control biofilter. The registered decrease in comparison to the control biofilter varies between 15.64 mgテ青?2l-1 and 119.95 mgテ青?2l-1 in the later phase of the process. The obtained high values for COD of the effluent are caused by the accumulation of intermediate metabolites from the reduction of the azobond under anoxic conditions. The aromatic amines obtained in the biotransformation of the amaranth (4-aminonaphthalene-1- sulphonate and 1-amino-2-hydroxynaphthalene-3,6-disulphonate) cannot be degraded without the presence of oxygen, which is necessary for activating the oxygenases and cleavage the aromatic ring. As a whole the low activity (up to 40%) of removing the organic matter is related to degrading the easily used substrates in the model wastewater–the peptone and the yeast extract. They are used by the biological systems as donors of protons for accomplishing a non-specific azoreductase under anoxic conditions. | |
| The obtained data about the organic matter (measured as TOC) are similar. It is found that after adding nanodiamonds the biodegradation effectiveness (70%) rises in comparison with the control biofilter. The residual concentration of the total organic carbon in the effluent after adding the modulator decreases to 69.12 mgl-1 at the most, while it reaches 81.36 mgl-1 for the control biofilter. | |
| In order to study the parallel functioning of the two biofilters before adding the stimulator and to register the effect of nanodiamonds on the studied technological parameters the data are compared in Table 1. | |
| The average value of the flow of wastewater for the two model systems is similar and varies between 22 and 23 mlh-1 before adding the nanodiamonds (Table 1). Similar results are found for almost all studied indicators. After adding the nanoparticles it is found that the biofilter preserves its flow (the flow is 23 mlh-1), whereas the control biofilter increases it. Most probably this is related to the destabilization of the biofilm and it’s thinning as a result of reaching the critical concentration of the amaranth, as it was found in the first part of the studies. The added nanodiamonds have a stabilizing effect on the effectiveness of amaranth decolorization. It is found that the effectiveness of decolorization for the two model systems varies between 90 and 92% before adding a stimulator, and the residual concentration of the dye is between 1.61 and 1.90 mgl-1. After adding the nanodiamonds the average effectiveness of decolorization reaches up to 96%, while the residual concentration remains 1.65 mgl-1. For the control biofilter the average decolorization effectiveness is preserved, but the residual concentration increases up to 4.44 mgl-1. Nanodiamonds have a stabilizing effect also on the biodegradation of organic matter (measured as total organic carbon). After adding them the effectiveness decreases of TOC increases (61%) in comparison with the control biofilter (54%). Moreover, it is found that chemical oxygen demand in the effluent decreases to 366.5 mgテ青?2l-1 in the presence of the modulator, whereas in the control biofilter it is up to 393.7 mgテ青?2l-1. | |
| Conclusions | |
| The obtained results lead to the following more important conclusions: | |
| • The gradual increase of the xenobiotic concentration in the influent has an important role for the adaptation of the microbial community to the model azo dye. The approximate critical concentration of the amaranth for a biofilm without adding nanodiamonds as a stimulator is around 47 mgl-1, whereas after adding a modulator it shifts to higher concentrations. | |
| • The medium layer of the inert carrier in the biofilter has the most favorable conditions for carrying out the first phase of the biotransformation of the amaranth–the reduction of the azobond. The key groups of microorganisms in the biofilm carrying out the process are: the anaerobic heterotrophs; the anaerobic azodegraders, the bacteria from genera Pseudomonas and Acinetobacter. | |
| • The obtained data about the values of COD in the effluent are high because of the accumulation of intermediate products from the azo reduction-aromatic amines. An additional aerobic step is needed for the further decrease of the organic load and the full mineralization of the aromatic amines. | |
| • The nanodiamonds, added at the 269th hour of the process have a stimulating and stabilizing effect on the technological parameters of the system. It is expressed in maintaining a stable biofilm with a high activity; maintaining an optimal flow of the system and increasing the effectiveness of amaranth decolorization and the organic matter removal. | |
| The obtained results from the laboratory studies reveal new opportunities for the water purification technologies. However, they are still at a beginning stage and that’s why there are studies yet to be carried out about the type and the applied concentrations of nanodiamonds, the reduction of losses in washing and dropping of parts of the biofilm, scaling and verification of the process. | |
| Acknowledgements | |
| This work was supported financially by the National Scientific Fund at the Bulgarian Ministry of Education, Youth and Science (Project DO02-294: “Ecological modeling of water purification processes in critical control points of upper sub catchment of Iskar River” and Project DMU03-56: “Innovative ecological approaches for dairy wastewater treatment”). The authors thank Prof. S. Stavrev for nanodiamonds supplying and E. Daskalova for laboratory assistance. | |
References
|
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14045
- [From(publication date):
specialissue-2013 - Dec 21, 2025] - Breakdown by view type
- HTML page views : 9465
- PDF downloads : 4580
