Special Issue Article Open Access
Synthesis of Poly-3-Hydroxybutyrate Reduces Maintenance Demand In Bacteria Growing Slowly on Methyl Tert-Butyl Ether
| Thore Rohwerder*, Hauke Harms and Roland H. Müller | |
| Helmholtz Centre for Environmental Research - UFZ, Department of Environmental Microbiology, Permoserstr. 15, 04318 Leipzig, Germany | |
| Corresponding Author : | Thore Rohwerder Helmholtz Centre for Environmental Research - UFZ Department of Environmental Microbiology, Permoserstr. 15 04318 Leipzig, Germany, Tel: +493412351317 Fax: +493412351351 E-mail: thore.rohwerder@ufz.de |
| Received: November 10, 2011; Accepted: December 14, 2011; Published: December 16, 2011 | |
| Citation:Rohwerder T, Harms H, Müller RH (2011) Synthesis of Poly-3- Hydroxybutyrate Reduces Maintenance Demand In Bacteria Growing Slowly on Methyl Tert-Butyl Ether. J Bioremed Biodegrad S1:003. doi:10.4172/2155-6199.S1-003 | |
| Copyright: © 2011 Rohwerder T, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Related article at Pubmed Pubmed  Scholar Google Scholar Google |
|
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
Availability of usable energy for biosynthesis and maintenance is a primary condition of life. In the case of microbial degradation of so-called recalcitrant compounds, growth is often prevented due to low energy production rates not exceeding maintenance requirements. However, any process that reduces biomass maintenance under these threshold conditions will increase the chance that productive degradation can occur. We have developed a growth model to explain the surprising observation that the growth of Aquincola tertiaricarbonis L108 and other bacterial strains on the groundwater pollutant methyl tert-butyl ether (MTBE) and its degradation product tert-butyl alcohol is accompanied by the accumulation of poly-3-hydroxybutyrate (PHB). The growth-coupled production of this storage polymer is remarkable since slow growth of all MTBE degraders indicates the difficulty to utilize this substrate. The modified model distinguished an active biomass fraction for which maintenance energy is required and a passive PHB fraction without maintenance requirements. Consequently, the presence of a PHB fraction saved specific maintenance costs and due to PHB accumulation, calculated overall growth yields were increased. More important, the structured model predicted an increased specific growth rate and a decreased minimum substrate concentration for growth Smin. Using experimentally determined parameters, it could be demonstrated that at low maximal growth rates PHB synthesis allowed for growth, whereas in its absence Smin became infinite. Additional reduction of maintenance in dependence on the rate of PHB formation was considered as the stoichiometry of PHB synthesis from MTBE resulted in a gain of energy equivalents. Our model thus showed that coupling of growth on slowly metabolized substrates with PHB synthesis is energetically advantageous. Hence, we propose a not yet considered role of PHB in delivering energy to compensate high maintenance costs while growing on recalcitrant xenobiotics.
| Keywords |
| Recalcitrance; Methyl tert-butyl ether (MTBE); Biodegradation; Poly-3-hydroxybutyrate (PHB); Structured growth model |
| Abbreviations |
| CoA: Coenzyme A; ETBE: Ethyl tert-butyl ether; 2-HIBA: 2-hydroxyisobutyric acid; 3-HBA: 3-hydroxy butyric acid; MTBE: Methyl tert-butyl ether; PGA: 3-phosphoglycerate; PHB: Poly- 3-hydroxybutyrate; TBA: tert-butyl alcohol |
| Introduction |
| Organisms need above all energy which is required for sustaining their viability and for biosyntheses. The latter finally results in growth and propagation. Propagation means preservation of the species and is therefore a major feature of life. The rate of propagation itself is a determinant of competition. The question, however, which factors control the growth rate, is not simple to answer. A general explanation focuses on the energy production rate. This is comprehensible with heterotrophic substrates if we consider the different energy contents (degrees of reduction) in the various compounds which range from methane as the most reduced state to formate and carbon dioxide as the most oxidized ones [1,2]. As the expenses for synthesizing one unit of biomass are nearly constant taking into account an almost constant macromolecular composition of a growing cell [3], different quantities of the various heterotrophic substrates will be required to satisfy the expenditure for biosynthetic work. Consequently, kinetics of key enzymes in the metabolism of a defined substrate will apparently determine the overall growth rate. |
| Besides the biosynthetic needs, the cell will require energy in a steady manner to maintain itself (turnover and repair) and to compensate the influence of external factors (homeostasis). The efforts sum up to the specific maintenance rate, which becomes an increasing fraction of total energy flow when growth rate decreases. This effect is known for long time and included into an extended Monod growth model [4,5]. Accordingly, heterotrophic growth requires that a flow of carbon from a substrate into the central metabolism is accompanied by the production of energy at rates above the demand for biomass maintenance. Whereas microbial growth on easily degradable substrates normally meets these requirements, numerous organic compounds can be found in nature supporting only carbon fluxes at insufficient rates. At such a threshold situation, any process that reduces maintenance will increase the chance that productive degradation can occur. In case of groundwater pollutants, this is one of the factors which would enable bioremediation processes. |
| Organic pollutants are potential heterotrophic substrates. The problem of various compounds, however, is their xenobiotic character, i.e. they bear structures and bonds which are uncommon in natural systems. A good example for the recalcitrance of xenobiotic compounds are the fuel additives methyl tert-butyl ether (MTBE), ethyl tert-butyl ether (ETBE) and related compounds which are widely used since a few decades and nowadays constitute a serious threat of our water resources [6-8]. The inert ether bond and a tertiary carbon atom appear to complicate microbial degradation of these compounds in the environment and also under laboratory conditions. In line with this, only a couple of bacterial strains are presently known to grow as pure cultures on MTBE [9-14]. The typically low growth rates range from 0.001 h-1 to 0.05 h-1 and the presently most effective strain is Aquincola tertiaricarbonis L108 [15]. There are other bacteria that degrade MTBE, but require growth-supporting substrates like n-alkanes [16-19]. In these latter cases, the absence of autarkic growth on MTBE is likely due to incomplete degradation or degradation at rates too low to sustain growth. |
| We have formerly calculated the energy production rates required for growth on MTBE and ETBE [20]. The results showed that only small differences of the degradation kinetics or energy production rates distinguish an organism capable of autarkic growth from an organism relying on auxiliary substrates. Productive growth of A. tertiaricarbonis on fuel oxygenates and their intermediates, e.g. tert-butyl alcohol (TBA), has been explained by the employment of a set of efficient enzymes acquired by horizontal gene transfer [15,20,21]. In particular, the use of a novel cobalamin-dependent mutase converting the MTBE metabolite 2-hydroxyisobutyryl-coenzyme A (2-HIBA-CoA) into 3-hydroxybutyryl-CoA (3-HBA-CoA) seems to be important [21]. 3-HBA-CoA is a central metabolite since it can be easily mineralized and used for the synthesis of major cell constituents and the bacterial storage compound poly-3-hydroxybutyrate (PHB), which is normally produced only under carbon excess conditions [22,23]. This metabolic connection and the observed synthesis and accumulation of the storage polymer during the carbon-limited growth on MTBE [12,24] led us to speculate about possible effects of PHB on growth energetics. |
| Here, an existing growth model [20] is refined for considering PHB synthesis. Growth-coupled PHB accumulation surprises in view of the observed difficulty of many bacteria to utilize MTBE as a heterotrophic growth substrate. Our structured model, however, clearly shows that it is not a metabolic burden but energetically advantageous for the bacteria to synthesize PHB while growing on substrates supporting only low growth rates. Under these threshold conditions, synthesis and storage of PHB reduces maintenance rates and, thus, increases the fraction of total energy flow that can be used for synthesizing other cell constituents. |
| Materials and Methods |
| Calculations |
| The calculations were performed on the basis of the YATP concept [25] as applied recently in the context of MTBE utilization [20]. |
| Cultivation |
| The investigations were performed with A. tertiaricarbonis L108 on MTBE and TBA. Strain L108 was grown on mineral salts solution containing (in mg l-1): NH4Cl, 760; KH2PO4, 340; K2HPO4, 485; CaCl2 * 6 H2O, 27; MgSO4 * 7 H2O, 71.2, and 1 ml l-1 of trace element solution; the trace element solution was composed of (in g l-1 ): FeSO4 * 7 H2O, 4.98; CuSO4 * 5 H2O, 0.785; CoCl2 , 5; MnSO4 * 4 H2O, 0.81; ZnSO4 * 7 H2O, 0.44; Na2MoO4 * 2 H2O, 0.25. The medium was supplemented with a vitamin mixture to reach a final concentrations of (in μg l-1 ): biotin, 20; folic acid, 20; pyridoxine-HCl, 100; thiamine-HCl, 50; riboflavin, 50; nicotinic acid, 50; DL-Ca-pantothenate, 50; p-aminobenzoic acid, 50; lipoic acid, 50, and cobalamin, 50. |
| The cultivation was performed in a Biostat UD (B. BRAUN Biotech, Melsungen, Germany) with a working volume of 4 l. The fermenter and the equipment were explosion-proof to enable work with MTBE. The strain was grown under fed-batch conditions with a feed of the carbon substrates to hold the actual concentration between 0.3 to 1.0 g l-1. Aeration was performed via the headspace to reduce loss of substrate by volatilization. The fermenter was run with 1 bar over-pressure. Oxygen concentration in the fermenter broth was held beyond 20% of saturation via auto-regulation of the stirred speed. The pH was kept at 7.0 by automatic titration of 1 N NaOH. |
| Samples were taken for the measurement of biomass concentration, poly-3-hydroxy butyrate content of biomass, and substrate concentration. |
| Analytics |
| Biomass concentration was determined as the optical density at 700 nm (OD700) and as bacterial dry mass after drying to constancy samples of centrifuged and washed biomass at 105°C. MTBE and TBA were determined by gas chromatography (GC) with a headspace technique [21]. Poly-3-hydroxybutyrate were determined after extraction from biomass and hydrolysis of the polymer as the propionyl ester of 3-hydroxybutyrate by GC as shown elsewhere [26]. |
| Model |
| Model development |
| An extant energy balance model for growth on MTBE [20] was extended to account for PHB accumulation. The model calculates the metabolic costs of PHB formation based on the assumption that, once it has been formed, the storage of this product does not require energy. The model thus distinguishes metabolically active biomass fraction (indexed in equations as actBM) requiring maintenance energy and a PHB fraction not requiring maintenance as it is not subject to modification, turnover or repair. The total biomass Xtotal of a bacterial population is thus regarded as being composed of variable fractions of the two “biopolymers” active biomass and PHB (eq. 1). |
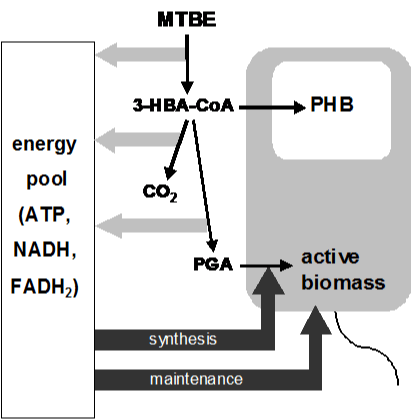
| The treatment of the biomass as a fractionated entity in equation 1 allows establishing interconnections between the fractions (e.g. energy flow), while accounting for deviating properties of the fractions (e.g. maintenance energy requirements). The carbon and energy flow of MTBE considered in the growth model is depicted in the metabolic scheme in Figure 1, including synthesis of PHB and active biomass from MTBE as well as its mineralization. In this scheme, PHB is assumed to be only accumulated but not degraded during growth. |
| Stoichiometry of active biomass synthesis |
| For calculations, the stoichiometry found in the MTBE-degrading strain A. tertiaricarbonis L108 was used as this strain is well studied [13,15,20,21] and also shows growth-coupled PHB synthesis (Figure 2). The stoichiometry for growth on MTBE and its complete degradation in A. tertiaricarbonis has been described in detail in the previous study [20]. Briefly, MTBE degradation proceeds via tert-butyl alcohol (TBA) and 2-HIBA. The latter is then CoA-activated and isomerized by a novel CoA-carbonyl mutase to 3-HBA-CoA. Assuming a synthetase reaction (forming AMP from ATP) for CoA activation of 2-HIBA [20], the balance for the conversion of MTBE to 3-HBA-CoA reads. |
| MTBE + 2 O2 + 2 ATP --> 3-HBA-CoA + CO2 + 2 NADH (2) |
| In our model, 3-HBA-CoA is transformed into the central precursor 3-phosphoglycerate (PGA) before being used for the synthesis of active biomass (eq. 3) [20]. |
| 3-HBA-CoA + 1 ATP + 3 H2O --> PGA + CO2 + 3 NADH + FADH2 (3) |
| The elementary composition of the active biomass fraction was assumed to be that of a standard microbial cell [3], i.e. C4H8O2N with a molar mass MactBM of 102 g mol-1. ATP requirements were based on the YATP concept [25], according to which the synthesis of 3 moles of biomass of the above composition requires 29.1 mol ATP. The balance equation for biomass synthesis from PGA is then |
| 4 PGA + 3 NH3 + 29.1 ATP + 5.5 NADH --> 3 C4H8O2N + 10H2O (4) |
| Combining equations 2 and 3, 4 moles of PGA are synthesized from 4 moles of MTBE according to |
| 4 MTBE + 12 ATP --> 4 PGA + 8 CO2 + 20 NADH + 4 FADH2 (5) |
| We assume that energy and reduction equivalents in equations 4 and 5 are freely convertible and that NADH and FADH2 are converted into η and (η-1 ) energy equivalents (ATP), respectively. The coefficient η is equivalent to the P/O quotient (number of energy-rich bonds in ATP formed per atom oxygen consumed). The total amount of energy equivalents required for the synthesis of 3 moles of biomass (eq. 4) from 4 moles of MTBE ΣATPsyn is then |
| Oxidation of MTBE to carbon dioxide via the mutase pathway in A. tertiaricarbonis yields 9 NADH and 2 FADH2 [20]. The molar gain of ATP per mol of MTBE γATPMTBE is thus equivalent to |
| The molar fraction of MTBE that needs to be dissimilated in order to satisfy the energy demand of biomass synthesis Sd is thus |
| and the theoretical yield of active biomass from MTBE with a molar mass MMTBE of 88 g mol-1 is |
| Stoichiometry of PHB synthesis |
| Now, we have to develop the stoichiometry of PHB formation from MTBE. For elongation of the polymer [PHB]n, the PHB synthase uses 3-HBA-CoA, which is synthesized from MTBE according to equation 2. Consequently, PHB synthesis from MTBE follows equation 10 where two NADH are produced while only two molecules of ATP are consumed. |
| MTBE + 2 ATP + [PHB]n --> [PHB]n+1 + CO2+ 2 NADH (10) |
| Taking into account the equivalence of ATP and reduction equivalents, the molar turnover of ATP during synthesis of PHB from MTBE γATPPHB is |
| According to eq. 10, a monomeric unit in PHB (C4H8O2, MPHB = 86 g mol-1 ) is formed from one molecule of MTBE and the corresponding yield YPHB is |
| Actual growth yield |
| Up to this point we have only regarded the energy balance of catabolic and anabolic reactions without regard of the energy that an organism constantly needs for non-productive purposes (homeostatis, turnover and repair). This energy is expended per unit time and can be lumped in the maintenance coefficient. The actual growth yield coefficient of active biomass YactBM includes thus a growth rate-dependent term (accounting for continuous maintenance that counteracts growth) reducing the stoichiometrically determined maximum growth yield according to Pirt´s fundamental equation [4,5]. YactBM can then be calculated as |
| where μ is the specific growth rate and YmaxactBM is the theoretical growth yield that can be calculated based on the above described stoichiometry of active biomass synthesis according to equation 9. The substratebased maintenance coefficient is mS. The fractional composition of biomass in a population can now be used to calculate the overall yield coefficients Ytotal including PHB synthesis (eq. 14). |
| Kinetics |
| Maintenance requires energy in excess to that needed for biosynthesis and at a specific energy production rate below mS growth becomes negative. An extended Monod model including a biomass decay term b (eq. 15) accounts for this. |
| Equation 15 implies the existence of a substrate concentration Smin at which the effective specific growth rate is zero [20,27,28] (eq. 16). |
| At μmax ≤ b, consequently, growth is not possible. In the previous model [20], the term b was derived from |
| Equation 17 is derived from equation 13 [4,29] and is valid for bacteria consisting only of active biomass. Due to the present model, however, the biomass of the population consists of fractions of PHB and active biomass and only for the latter fraction maintenance energy is required. Hence, we have to subtract the fraction of b that would otherwise consider biomass decay due to PHB maintenance. In doing so, the modified term bf (eq. 18) has to be used instead of b for calculating μ and Smin according to equati vaons 15 and 16, respectively. In other words, when synthesizing PHB in parallel to active biomass, growth becomes possible at μmaxlues below b. |
| Moreover, in the case of PHB accumulation from MTBE, we have to include into the overall balance the energy equivalents that were gained during synthesis of this polymer at η > 1. These will further reduce the maintenance requirements. For doing this, the substrate-based coefficient mS has to be transformed into an energy equivalent-based maintenance coefficient me according to |
| According to the present model and in conformity with equation 18, the modified term meactBM has to be used for further calculations (eq. 20). |
| From meactBM the amount of energy equivalents formed during PHB synthesis has to be subtracted. The latter can be calculated from the molar turnover of ATP during PHB synthesis γATPPHB (eq. 11) multiplied by the molar fraction of PHB present in the bacterial population (fPHB/MPHB) and the rate by which PHB is formed. Due to assuming growthassociated PHB synthesis, we used a Monod equation-type term multiplied by a factor n for expressing the rate of PHB synthesis. This leads to a reduced maintenance coefficient meactBM with |
| The factor n determines the degree of coupling between growth and PHB synthesis. The degree of the reduction of maintenance energy associated with active biomass by supplying extra energy from PHB synthesis can be expressed by the coefficient with |
| Results |
| Growth coupled synthesis of PHB |
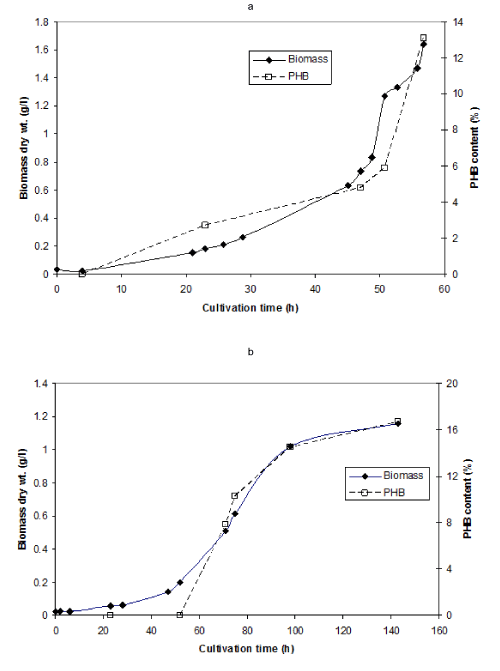
| Cultivation of A. tertiaricarbonis L108 on MTBE or TBA was performed in a fermenter with a fed-batch regime aiming to avoid exhaustion of the carbon substrates during cultivation. This was necessary in order to avoid any degradation of PHB under substrate-deficient conditions. Continuous biomass increase becomes evident from Figures 2A and B indicating that the requirement was apparently fulfilled. Biomass accumulated with a rate of 0.048 h-1 (R2 = 0.99) in the case of MTBE over the entire growth phase; this value is in accord with former data [15]. In the case of TBA, the rate amounted to 0.077 h-1 (R2 = 0.97) which is slightly lower than formerly observed. Accompanied with biomass formation, synthesis of PHB was observed. This amounted to around 15% with both substrates. The constant growth rates found over almost the entire growth phase indicate unlimited conditions; hence, PHB accumulation follows growth-coupled conditions. |
| Effect of the PHB fraction on growth stoichiometry |
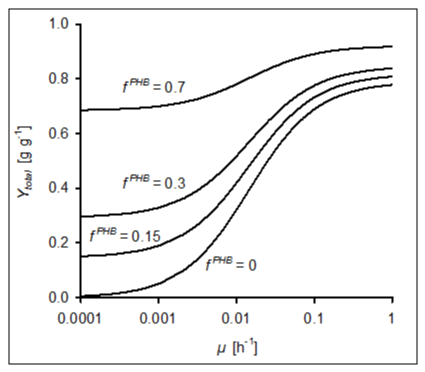
| With the structured growth model developed in this study, effects of growth-coupled PHB synthesis on growth yields over a wide range of growth rates were calculated. For doing this, the following assumptions for YmaxactBM and mS were made. For η = 2, which follows from the experimentally observed biomass synthesis in A. tertiaricarbonis [15], Sd attains a value of 0.405 mol (eq. 8). According to equation 9, this results in a maximum (theoretical) growth yield Ymax actBM of 0.789 g active biomass (g MTBE)-1 . With respect to mS, we used the previously determined value of 0.21 mmol MTBE g-1 h-1 which is valid for A. tertiaricarbonis growing on MTBE using the mutase pathway [20]. Then, the overall growth yield of the growing culture can be calculated with equation 14. Unlike the constant yield of PHB (eq. 12), the actual growth coefficient of active biomass YactBM depends on the specific growth rate μ (eq. 13). This effect on the yield of total biomass at four different contents of PHB is shown in Figure 3. PHB contents of 15%, i.e. the observed accumulation in A. tertiaricarbonis growing on MTBE (Figure 2), and 70% as a likely upper limit for growth-associated PHB accumulation [30-32] were included. It can be seen that the influence of the maintenance coefficient on the yield decreased with lower fractions of active biomass and at increased growth rates. For high growth rates, maximum yields of 0.789, 0.817, 0.845, and 0.921 g g-1 were calculated for PHB contents of 0, 15, 30, and 70%, respectively. At very low growth rates, on the other hand, Ytotal tends to mainly consist of the μ-independent YPHB fraction. |
| Effect of the PHB fraction on growth kinetics |
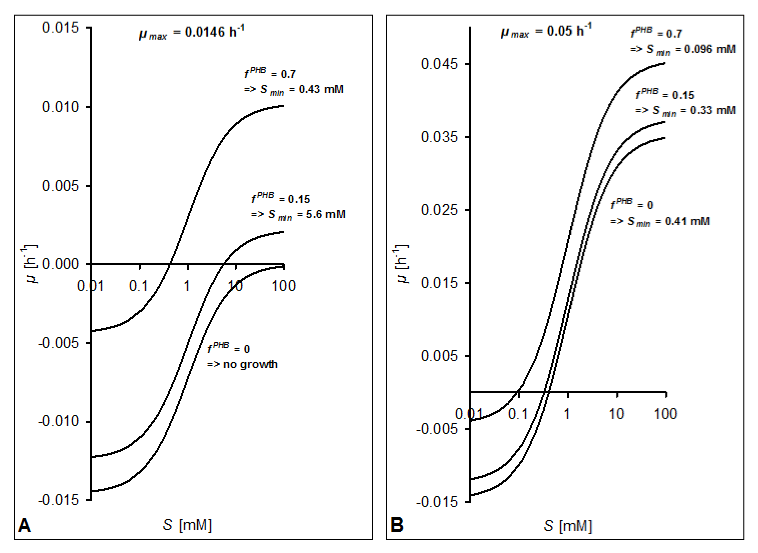
| According to equation 15 and using the modified term bf (eq. 18) of the present model for describing biomass decay due to maintenance, growth-coupled PHB formation will increase μ with increasing fractions of PHB compared with the specific growth rate of a population not showing PHB synthesis. Figure 4A illustrates this effect assuming a KS = 1 mM and a value of b = 0.0146 h-1 for A. tertiaricarbonis, resulting from the above used YmaxactBM = 0.789 g g-1 = 0.0694 g mmol-1 and mS = 0.21 mmol g-1 h-1 [20] that is regarded as independent of the growth rate (see also [33]). Without PHB synthesis, growth only takes place at μmax > 0.0146 h-1. Due to the reduction of maintenance expenditures by PHB formation, growth would already be possible at μmax = 0.0146 h-1 above a Smin = 5.6 mM considering a PHB content of 15% (Figure 4A). At the same μmax, a more than tenfold reduction of Smai will be achieved when 70% PHB are accumulated. PHB formation thus helps to significantly reduce Smin. Figure 4B shows graphs considering the same KS and b values as in Figure 4A but using a μmax = 0.05 h-1, which is close to the maximal growth rate observed with A. tertiaricarbonis growing on MTBE [15]. Due to the higher μmax, the influence of PHB accumulation on μ and Smin is less pronounced. Still, a 3-fold reduction of Smin can be observed when shifting from 15% PHB to 70%. |
| Effect of energy gain from PHB synthesis |
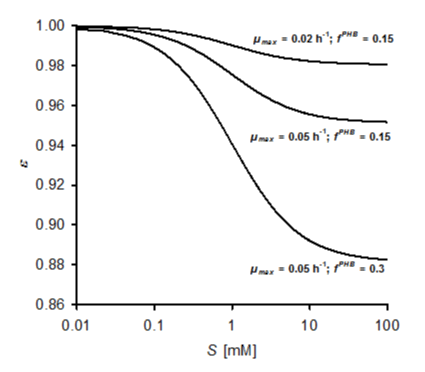
| The amount of ATP saved due to the presence of a PHB fraction not requiring maintenance can be calculated from equation 20 by comparing the energy equivalent-based maintenance coefficient me (eq. 19) with the modified term meactBM (eq. 20). However, not just the presence of a PHB fraction but also its synthesis from MTBE results in an ATP gain. According to equations 10 and 11, this yield amounts to 2 moles per mole of PHB synthesized at η = 2. This additional energy gain is considered in equation 21 giving a modified term meFactBM for the energy equivalent-based maintenance coefficient. The extent of maintenance reduction is expressed as the quotient ε of meFactBM and meactBM (eq. 22). This relative maintenance coefficient decreases with increasing substrate concentration in an asymptotic manner approaching limits that depend on μmax. Figure 5 shows examples for e assuming a coupling factor n = 1, mS = 0.21 mmol g-1 h-1, KS = 1 mM, η = 2 and a PHB fraction of 15 and 30%. In the case of 15% PHB, the maximal reduction of maintenance due to energy formation from PHB synthesis (at infinite substrate concentration) is less than 2% at μmax = 0.02 h-1 but reaches about 5% at a μmax = 0.05 h-1. At the higher μmax, a PHB fraction of 30% results in a maximal reduction of 12%. |
| In A. tertiaricarbonis, growth and PHB synthesis are strongly coupled (Figure 2), indicating that the factor n (eq. 21) approaches values around 1 which enables stable growth. Consequently, at least at realistic PHB fractions of up to 30% the reducing effect of energy gain from PHB synthesis on maintenance is quite low (Figure 5). On the other hand, significantly higher values of n express some uncoupling of PHB formation from growth leading to imbalances between the fraction of active biomass and storage polymer and finally terminate growth. For demonstrating the effect of higher coupling values, however, calculations accounting for such situations were included in the current model. Table 1 gives values for ε and Smin at a substrate concentration S = 1 mM and coupling values n = 1, 2 and 5. For comparison with the previous calculations, again KS = 1 mM, mS = 0.21 mmol g-1 h-1 and η = 2 were used. These figures make evident that there is a significant reduction of maintenance demands at higher PHB fractions and coupling values solely due to the energy gain accompanying the accumulation of PHB from MTBE. In case of a low μmax = 0.01 h-1 , growth is not possible at PHB fractions of 15 and 30% and n = 1. Even higher coupling values would still result in unrealistic high Smin values at the lower PHB fractions tested for μmax = 0.01 h-1 . At a very high PHB content of 70%, however, maintenance and consequently Sminare significantly reduced. With Smai = 0.42 mM at fPHB = 0.7 and n = 5 (Table 1), a minimum substrate concentration is reached at μmax = 0.01 h-1 comparable to the one obtained at μmax = 0.05 h-1 without PHB accumulation (Smin = 0.41 mM, Figure 4B). Considering PHB synthesis at the latter μmax, effects are more pronounced. At a PHB fraction of 70% and n = 5, there would be even a condition at which excess energy would be generated by the process of PHB synthesis with regard to maintenance demands, i.e. resulting in negative values for meFactBM and bf. More realistically, maintenance was already significantly reduced at n = 2, which still represents strong coupling of PHB and active biomass synthesis. |
| Discussion |
| The results with cultivation of A. tertiaricarbonis L108 have shown that growth on MTBE and its degradation product TBA is accompanied by the synthesis of PHB. The storage product PHB is thus formed despite the known difficulties of microbes to productively utilize MTBE as sole carbon and energy source. The present model, however, gives an explanation of this unexpected effect according to which PHB accumulation turns even into an energetic advantage. In an actual field study on the bioremediation of MTBE-polluted groundwater treated by constructed wetlands, degradation of MTBE was established and brought about stable and complete [34] whereby degradative microorganisms were accumulated. As strain L108 was derived from the same site, it would be interesting to look for details on the consortium which has accumulated in this biofilter. |
| MTBE and other newly released chemicals represent a challenge for the autopurification capacity of microbial communities. The evolution and adaptation of catabolic routes to novel substrates requires that the extant enzymatic equipment brings about at least some degradation and growth and that appropriate enzymatic capacities become arranged in a way that an organism or a consortium can gain benefit from the degradation. In the case of MTBE, the relatively effective growth of A. tertiaricarbonis L108 has been explained by the fact that this organism has acquired an appropriate range of special enzymes, including a P450 monooxygenase attacking MTBE [35], a phthalate dioxygenases-like enzyme hydroxylating TBA [36] and a cobalamin-dependent mutase that isomerizes 2-HIBA [21]. In view of this and the fact that MTBE is an energy-rich and highly bioavailable substrate, the modest growth of most degraders required an explanation, also in view of the desirable improvement of degradation rates. In earlier work [20], we could show that growth on MTBE was kinetically limited, i.e. MTBE was processed at rates that hardly delivered enough energy to balance the bacterial maintenance requirements. The difficulty to isolate and maintain MTBE-degrading strains can easily be explained by the previous model. In view of this, it was surprising that despite this kinetic limitation, MTBE-dependent growth is coupled with significant PHB synthesis in several degrader strains including A. tertiaricarbonis L108. With the extended model presented here, which accounts for the energetics of PHB formation we could show that the conversion of MTBE to PHB and its accumulation delivers additional energy that can be used to relieve A. tertiaricarbonis from its maintenance expenditures. |
| The most obvious effect of differentiating between fractions of active biomass and PHB is the increase of the growth yield due to the independence of YPHB of the specific growth rate. In addition, even very high growth rates and a P/O quotient of up to η = 3 would still result in lower yields in the absence of PHB accumulation as the maximum growth yield of active biomass Ymax actBM would still be lower than YPHB. However, the resulting benefits for a microbial population coupling PHB synthesis with growth are not that clear. At least, more carbon from MTBE is assimilated and the PHB formed is available as the classical bacterial source of stored energy and carbon. Besides, PHB accumulation led to higher resistance to stress and improved survivability in the absence of available carbon and energy sources [37,38] which is important to microbes that live in such poor environments like groundwater [39] as this holds for A. tertiaricarbonis L108 [13]. The effects on growth kinetics, on the other hand, are much more dramatic. According to our model, growth is stimulated by a combination of (i) the reduction of the fraction of active biomass resulting in reduced total biomass-related maintenance, and (ii) the contribution of the PHB synthesis pathway to the energy budget. The visible effects are an increase of the specific growth rate and a reduction of the minimum MTBE concentration required for growth. Consequently, growth can take place even when μmax attains lower values. |
| Growth-associated accumulation of PHB seems to be a widespread response to occurrence of alternating feast and famine condition [32,33,40-42]. The kind of regulation may vary but usually reacts to imbalances in the metabolism, i.e. an excess of substrate being taken up to that actually required for growth. Alternatively, PHB formation may serve as a sink of reduction equivalents for maintaining redox balance [43]. In this connection, growth-associated hyperproduction of PHB in Azotobacter vinelandii UWD, which results in up to 75% PHB accumulation [31], has been explained by the lack of an active NADH dehydrogenase complex in this strain [44,45], resulting in the demand for an alternative NAD recycling reaction. Besides these metabolic bottlenecks and impairment of central enzyme activities, other reasons may result in growth-coupled PHB synthesis. In A. tertiaricarbonis and other MTBE degraders, the growth-coupled accumulation of PHB may amount to moderate values of 15 to 30% during growth on MTBE and TBA as well as simple substrates, such as glucose and pyruvate. This is in a typical range for many combinations of bacteria and substrates [40]. However, the advantage of this PHB synthesis is not really obvious when growing on substrates supporting high growth rates. In line with this, in many bacteria PHB synthesis is not growthcoupled but only occurs under nitrogen exhaustion or other nutrient limitations [23,44]. This strict control of PHB synthesis may be relaxed in A. tertiaricarbonis and other MTBE degraders and, according to the structured model, turns out to be beneficial while growing on fuel oxygenates. Here we thus propose a further role of PHB formation, the delivery of energy to compensate for high maintenance costs while growing on substrates only supporting low growth rates. |
| We are not aware of such phenomena with other substrates than MTBE, but it is not unlikely, that it might also apply to other xenobiotic compounds. In the case of MTBE and other fuel oxygenates, degrader strains were very often found among the Rubrivivax-Roseateles- Leptothrix-Ideonella-Aquabacterium and Comamonadaceae branches of the Betaproteobacteria [9,12,13,14,46] whose representatives are known to typically synthesize PHB [47,48]. However, direct correlations between growth on xenobiotics and PHB synthesis have not been documented so far. Generally, the effects on growth stoichiometry and kinetics resulting from the structured model should be observable with any other bioavailable substrate which also sustains only slow growth. In case of an unfavorable stoichiometry of the degradation pathway, i.e. a negative turnover of energy equivalents during PHB synthesis from the substrate, reduction of maintenance due to PHB formation may be less pronounced. As long as the degradation efficiency of the xenobiotic compound is far away from rates attainable with simple substrates, PHB accumulation should be advantageous. This widens the range of environmental conditions under which growth-coupled degradation of so-called recalcitrant pollutants and as a consequence further evolutionary adaptation to their utilization is possible. Hence, future screening for suitable degrader strains should see PHB synthesis not as a disadvantageous metabolic burden but as an indicator of superior degradation capacity. |
References
|
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 | Figure 5 |
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 14632
- [From(publication date):
specialissue-2012 - Nov 08, 2025] - Breakdown by view type
- HTML page views : 9998
- PDF downloads : 4634
