Special Issue Article Open Access
Use of Dissolved Organic Carbon to Biostimulate Rapid Rhizodegradation of Perchlorate in Soil
Dawit D.Yifru1 and Valentine A. Nzengung2*
1Geosyntec Consultants, 1255 Roberts Blvd. Suite 200, Kennesaw, GA 30144, USA
2Department of Geology, University of Georgia, Athens, Georgia 30602, USA
- *Corresponding Author:
- Valentine A. Nzengung
Department of Geology
University of Georgia, Athens, Georgia 30602, USA
Tel: 706-542-2699
Fax: 706-542-2425
E-mail: vnzengun@uga.edu
Received March 26, 2012; Accepted March 16, 2012; Published March 18, 2012
Citation: Yifru DD, Nzengung VA (2012) Use of Dissolved Organic Carbon to Biostimulate Rapid Rhizodegradation of Perchlorate in Soil. J Bioremed Biodeg S7:003. doi: 10.4172/2155-6199.S7-003
Copyright: © 2012 Yifru DD, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Bioremediation & Biodegradation
Abstract
The main limitation to biodegradation of perchlorate in most soils and groundwater tends to be the availability of an adequate supply of organic carbon or electron donors. This study investigated the use of electron sources provided as Dissolved Organic Carbon (DOC) from chicken litter extract and acetate to enhance and sustain rhizodegradation of perchlorate in contaminated soils. This approach should reduce the residence time of perchlorate in vegetated soils and minimize the well-documented plant uptake of perchlorate at contaminated field sites and by food crops. Willow trees (Salix nigra) planted in 20 L soil bioreactors were dosed with perchlorate-contaminated water multiple times. The rate of biodegradation of perchlorate in willow-planted soil bioreactors provided with electron sources as 300 mg L-1 DOC was very rapid and described by zero-order kinetics with a maximum rate constant of 24 mg L-1 day-1. For planted control experiments in which DOC was limiting, the removal of perchlorate primarily by biodegradation was described by pseudo-first-order kinetics with a maximum rate constant of 0.35 day-1. The fraction of perchlorate phytoaccumulated in the control plants was an order of magnitude higher than in plants grown in the DOC-dosed bioreactors. The results of this study confirms that the slow buildup of DOC in the rhizosphere by root exudation and organic matter decomposition is insufficient to sustain a high rate of biodegradation and/or rhizodegradation of perchlorate and perhaps other degradable contaminants in vegetated contaminated soils. It is recommended that an optimum design of phytoremediation of perchlorate should include enhancement of rhizodegradation by providing an optimum and sustained supply of carbon source.
Keywords
Perchlorate; Rhizodegradation; Dissolved organic carbon; Phytoaccumulation
Introduction
Contamination of freshwater resources, fresh produce and some processed foods by perchlorate (ClO4-) is a growing problem in the United States. The anion perchlorate is a naturally occurring as well as an anthropogenic chemical which occurs as salts of ammonium, sodium and potassium. The natural sources of perchlorate include Chilean caliche which is mined for use as a natural source of NaNO3 in fertilizer and evaporite deposits in arid climates. Fertilizer manufactured from Chilean caliche may contain approximately 0.5-2 mg g-1 perchlorate [1]. The main anthropogenic source of perchlorate is ammonium perchlorate (NH4ClO4), which is used as an oxygenadding component in propellants for rockets, missiles and fireworks. Ammonium perchlorate is highly soluble in water and dissociates to ammonium (NH4+) and perchlorate (ClO4-) ions. Perchlorate is very stable under typical surface and ground water conditions.
Perchlorate is not only found in the drinking water supplies of over 15 million people in the USA [2], but is also found in foods such as lettuce [3], dairy milk [4] and breast milk [5]. The health concerns over perchlorate result from its interference with the function of the human thyroid gland. Perchlorate competitively inhibits thyroid iodide uptake, which results in disruption of the normal thyroid hormone production. In January 2006, the U.S. Environmental Protection Agency (USEPA) set a preliminary cleanup goal for perchlorate of 24.5 μg L-1 in drinking water [6].
Anoxic bioremediation and phytoremediation processes have shown great promise as cost-efficient and sustainable approaches for the removal of perchlorate from contaminated soils and water [7-9]. Under favorable root zone conditions, an adequate supply of carbon source is required to mineralize perchlorate [8-12]. In the natural environment, the sources of electrons and organic carbon may include soil organic matter, exudates of plant roots such as ethanol, acetate, organic acids, sugars, and dead root biomass. Perchlorate reducing microbes are ubiquitous [13] in soils, sediment and water. Under reducing conditions, microbes use perchlorate as a terminal electron acceptor in the absence of nitrate, thereby degrading perchlorate to innocuous chloride ion. When suitable environmental conditions exist, perchlorate degradation follows the pathway: ClO4 - → ClO3 - → ClO2 - → Cl- [14,15]. The dismutation of ClO2 - into Cl- and O2, an important step in the reductive pathways of perchlorate, is mediated by an enzyme chlorite dismutase [15]. Degenerate primers were recently developed by Bender et al. [16] for the amplification of the chlorite dismutase gene cld [17].
At the bench scale, phytoremediation has been shown to be a promising technology for the clean up of perchloratecontaminated surface water and groundwater [9,10,18,19]. Previous hydroponics studies have identified two predominant mechanisms of phytoremediation of perchlorate as relatively slow uptake and phytodegradation, and rapid rhizodegradation when suitable environmental conditions are created in the rhizosphere [9,18-20]. Uptake and phytodegradation poses ecological risk because uptake and slow phytodegradation results in phytoaccumulation of a fraction of the perchlorate taken-up into plants. A growing number of studies have documented the uptake and accumulation of perchlorate in plant leaf tissues, including tobacco [21], lettuce [3,22], grass [23], terrestrial and aquatic plants [24]. The detection of perchlorate in dairy and breast milk [4,5] provides direct evidence of perchlorate accumulation in the food chain. Unlike uptake and phytodegradation, rhizodegradation is a desirable process because perchlorate-respiring microbes in the root zone rapidly degrade ClO4 - to Cl-. The most important benefits of rhizodegradation are the rapid removal of perchlorate from the root zone and minimization of perchlorate accumulation in plants.
Although previous research has shown that plant exudates enhance rhizodegradation of perchlorate, the natural supply of plant exudates alone may not be sufficient to sustain rapid rhizodegradation of perchlorate at some field sites [25]. This explains the phytoaccumulation of high concentrations of perchlorate in the leaf tissue of field plants and food crops. To overcome this potential limitation, this study evaluated the use of external sources of carbon derived from agricultural waste and synthetic sources to biostimulate rapid biodegradation and rhizodegradation of perchlorate in planted soil systems. Specifically, two sources of organic carbon were applied to perchlorate-contaminated soils in planted soil bioreactors. The lag-time, rates of perchlorate removal and the perchlorate fraction in leaf tissue of willows (Salix nigra) grown in acetate (synthetic carbon source), and chicken litter extract (natural carbon source) amended soil were compared to results of the no carbon amended controls. The lag-time in this study was considered as the duration of the initial slow phase of perchlorate removal, predominantly by plant uptake from the soil pore water, which preceded the rapid rhizodegradation phase.
Materials and Methods
Chemicals
Sodium acetate trihydrate (CH3COONa.3H2O) and sodium hydroxide (NaOH, 50% w/w) solution were obtained from J.T.Baker® (Phillipsburg, NJ) and used as a synthetic organic carbon source and to prepare the ion chromatography (IC) eluent, respectively. Multiple concentrations of perchlorate standard solutions were purchased from AccuStandard™ (New Haven, CT) and SPEX CertiPrep (Metuchen, NJ). Peters Professional® (St. Louis, MO) (4.6% ammonical nitrogen, 19.4% urea nitrogen, 12% phosphate and 12% potash) was diluted to make the desired strength of nutrient solution. Almatis Ac. Inc. provided DD-6 alumina sorbent with 48 × 100 U.S. screen mesh and having a surface area of 360 m2 g-1.
Preparation of dissolved organic carbon sources
Solutions of acetate and chicken litter extracts were used as carbon sources. Chicken litter extract solution was prepared by mixing 100 g of solid chicken litter obtained from a University of Georgia poultry farm, with 1 L of deionized water. After shaking the mixture overnight and centrifugation for 30 minutes at 1000 × g, the supernatant was filtered sequentially through a Whatman® 42 and then a 0.45 μm filter paper (Pall Corp., Ann Arbor, MI). The raw extract was diluted as needed to provide the desired 300 mg L-1 of DOC to the root zone solution. The 300 mg L-1 DOC source from acetate (CH3COO-) was prepared by dissolving 1.7 g of CH3COONa.3H2O in 1L of perchlorate contaminated groundwater added to the soil bioreactors. The DOC initial concentration of 300 mg L-1 was selected because multiple previous tests performed by our research group has confirmed that an initial DOC concentration of an order of magnitude greater than the initial perchlorate concentration biostimulates and sustains rapid rhizodegradation of perchlorte. To ensure uniform distribution of the DOC, all pore water was drained from each bioreactors and the stock solution of DOC mixed in before reapplying at the soil surface. The concentrations of DOC in solution were measured on a Shimadzu 5050 Total Organic Carbon analyzer.
Trees
Willow tree (salix nigra) cuttings purchased from Ernst Conservation Seed (PA) were pre-rooted in 0.39 % aqueous solutions of Peter’s Professional® plant growth media under natural light in a greenhouse maintained at a temperature of approximately 28°C. For each experiment, pre-rooted cuttings having similar stem diameter, root system and total biomass were used. Willow trees were selected for this study because of a high water uptake and high rate of perchlorate removal, robustness and high rate of survival under the humid greenhouse conditions.
Soil bioreactors
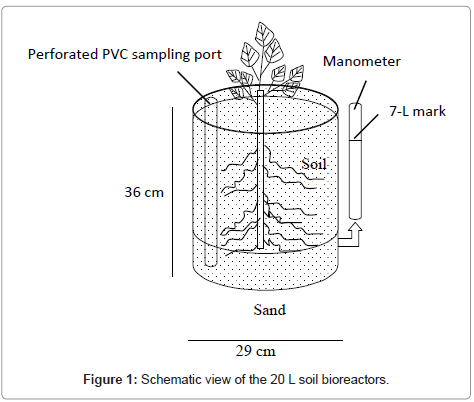
The pre-rooted trees were transferred to 20 L buckets containing a layer of sand and topped with soil (Figure 1). The bottom 7 cm of the bucket was filled with clean sand topped by 25 cm of sandy loam soil collected from Athens, Georgia. The sandy loam soil had a pH of 5.79 and contained 4.96 % organic carbon, 16.7 % clay, 21.3 % silt and 62 % sand. The soil was obtained from depths of 50 to 100 cm from a site that has not been used for agriculture nor received any fertilizer or pesticide treatment in recent years. The 25 cm layer of soil in each bucket was topped with a 3 cm layer of clean sand. The plant growth nutrients were provided as 0.39 % diluted Peters Professional® solution. Triplicate planted soil bioreactors were prepared for acetate, chicken litter and control experiments. A water gauge (manometer) prepared from vinyl tubing was used to monitor water level inside each soil bioreactor. A sampling port made up of perforated PVC was installed in each bioreactor on the opposite side of the water gauge.
Once the trees were acclimated for about two weeks, each planted bioreactor was dosed with either diluted chicken litter extract or acetate and perchlorate-contaminated groundwater from the Longhorn Army Ammunition Plant, Karnack, TX. The concentration of perchlorate in the groundwater was 32 mg L-1. In order to obtain a similar concentration of perchlorate in all bioreactors, a NaClO4 solution prepared in the lab was added to some of the bioreactors. The constant volume of water in the bioreactors was maintained by replacing the evapotranspired water with nutrient solution dissolved in deionized water. The perchlorate-contaminated water and the DOC were homogenized in each bioreactor by recirculation; the solution was pumped from the bottom of the sampling port and expelled at the soil surface. The pore-water (water within the saturated soil) in the bioreactors was then sampled and analyzed on a daily basis, with occasional exceptions.
Before taking samples from each soil bioreactor, deionized water was added on the soil surface to replace the evapotranspired water and to bring the water level up to approximately 7L water mark. A 10 ml pipette (Costar Corp., Cambridge, MA) was used to purge 40 to 50 ml of water from multiple depths within the sampling port, which was disposed on the soil surface. This procedure was performed prior to taking a sample in order to thoroughly mix the water in the bioreactor and take a representative sample. A 1.5 ml sample was taken from each bioreactor and diluted as needed to the working perchlorate concentration range of 0.002 to 1.5 mg L-1. These samples were analyzed for perchlorate on a Dionex® DX 500 IC. Each planted soil bioreactor was sampled on a daily basis until the concentration of perchlorate decreased to below IC detection limit of 2 μg L-1.
Once the concentrations of perchlorate in the willow planted soil bioreactors had reached non-detectable levels, a fraction of the willow tree leaves were harvested before the buckets were re-spiked with perchlorate. This process was repeated four times over the course of the study. For the second and subsequent dosing events, the water within the bioreactors was pumped out and dosed with perchlorate before slowly reapplied onto the soil surface. The reactors were not dosed with DOC during the second and third dosing events. During the fourth dosing event, DOC was added to selected buckets to achieve an initial concentration of approximately 300 mg L-1.
Plant tissue extraction
Plant leaves harvested for perchlorate analysis during and immediately after the complete removal of each dose of perchlorate added to the bioreactors were weighed and washed with deionized water before drying overnight at 75°C. The dried samples were weighed and pulverized with a mortar and pestle. The extraction of leaves and clean-up procedure of Ellington and Evans [26] was followed. The extracted perchlorate concentrations were measured in mg L-1 and converted to the mass of perchlorate recovered per mass of dried or fresh weight (dw or fw) of the leaf material (mg kg-1).
Analysis
Water samples taken from the rhizosphere were diluted as needed to the working perchlorate concentration range of 0.002 to 1.5 mg L-1. Each prepared sample was placed in two 5 ml Dionex® autosampling vials and was analyzed immediately after sampling. The Dionex® DX- 500 IC was outfitted with an IONPAC® AG16 guard column (4 × 50 mm) and an IONPAC® AS16 analytical column (4 × 250 mm). The IC was equipped with a Dionex® AI-450 Chromatography Automation System, an Advanced Computer Interface Module (ACI) and an ASRS-ULTRA II Self-Regenerating Suppressor (4 mm) at a 300 mA setting. The samples were loaded into sixty 5-ml vials auto sampler. A 100 mM and 50 mM sodium hydroxide (NaOH) eluent at a flow rate of 1 ml min-1 were used to measure perchlorate in mg L-1 and μg L-1 levels, respectively. The eluent was prepared using NaOH (50% w/w) solution and deionized, degassed (in the VWR Scientific Aqusonic, Model 150D) water. Calibration and check standards were made by diluting 1000 μg mL-1 perchlorate anion standard (SPEX CertiPrep, Inc.®) and 0.5 μg mL-1 and 1 μg mL-1 standards (AccuStandard, Inc.®). A new calibration curve was created each time the ion chromatograph was turned on, or after the eluent had been changed (every 1 to 2 days). For quality control, all samples were run in duplicate, and an external standard and a blank were run after every two samples. The standard was used to ensure that the percent error remained below 5 %, and to monitor any instrumental drift, while the blank checked for any carry-over from the previous sample. To confirm the perchlorate peak in plant tissue extracts, some duplicate samples were spiked with a solution containing 100 μg L-1 perchlorate.
Results and Discussion
Over a period of 90 days, the planted bioreactors each treated four doses of 17-53 mg L-1 perchlorate to below the method detection limit (MDL) of 2 μg L-1. At the termination of each experiment, there was no significant difference in the plant biomass of trees grown with and without an external supply of electron source. Similarly, the volume of water evaporated from the soil surface and transpired by the willow trees in each experiment was similar at a rate of about 300 mL day-1.
Dose 1
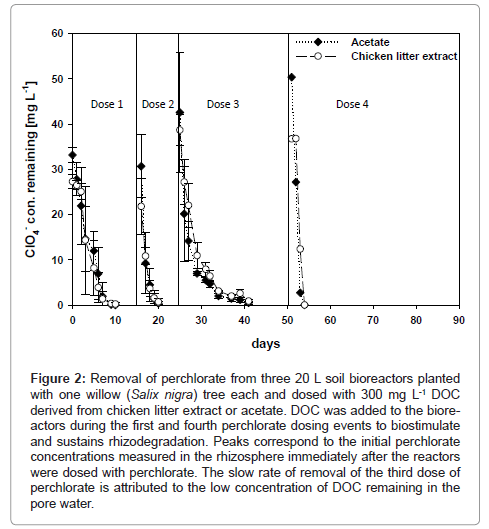
Initial perchlorate concentrations of approximately 33 mg L-1 were reduced to the MDL within 9 days in planted bioreactors treated with DOC as chicken litter extract and acetate (Figure 2). There was no significant difference in the effectiveness of the two DOC sources to stimulate perchlorate reduction by bacteria. In bioreactors supplied with acetate, no lag-time preceded the faster rhizodegradation phase, while a lag-phase attributed to slow plant uptake of perchlorate lasted for 3 days in chicken litter extract amended bioreactors. The 3 days lag-phase was likely the time needed for perchlorate degrading microorganisms to adapt to metabolizing the relatively more complex DOC provided as chicken litter extract. Since acetate is a natural plant exudate, the root zone bacterial should be well adapted to metabolize acetate. Following the biostimulation of rhizodegradation, perchlorate removal was very fast in both acetate and chicken litter bioreactors and was described by a zero-order kinetic equation with a degradation rate constant of 4.53 ± 0.65 mg L-1 day-1. The concentration of perchlorate in willow leaves harvested immediately after the complete removal of the first dose of perchlorate was 11.7 ± 2.7 mg kg-1 fresh weight (fw).
Figure 2: Removal of perchlorate from three 20 L soil bioreactors planted with one willow (Salix nigra) tree each and dosed with 300 mg L-1 DOC derived from chicken litter extract or acetate. DOC was added to the bioreactors during the first and fourth perchlorate dosing events to biostimulate and sustains rhizodegradation. Peaks correspond to the initial perchlorate concentrations measured in the rhizosphere immediately after the reactors were dosed with perchlorate. The slow rate of removal of the third dose of perchlorate is attributed to the low concentration of DOC remaining in the pore water.
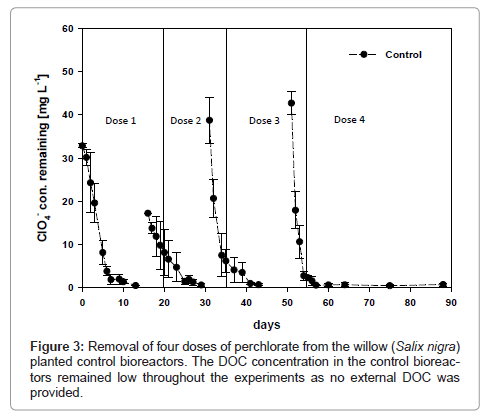
The planted soil bioreactors provided with no external electron sources (controls) contained an initial DOC concentration of 54.6 ± 5.3 mg L-1 derived from residual soil organic carbon and plant exudates (Figure 3). Removal of the first dose of perchlorate required more than 13 days compared to 9 days for chicken litter or acetate amended bioreactors with a first-order rate constant of 0.36 ± 0.02 day-1. As a result, the concentration of perchlorate in willow leaves harvested immediately after treatment of the first perchlorate dose was 103.7 ± 16.8 mg kg-1 fw, an order of magnitude higher than in the leaves of DOC amended trees. The slower rate of perchlorate removal amounted to longer residence times and higher perchlorate fraction uptake and phytoaccumulation in the willow trees.
Dose 2
The second dose of 30 mg L-1 perchlorate applied to DOC-amended bioreactors was completely degraded within 5 days. The kinetic data was still described by the zero-order equation and the degradation rate constant for the second dose of perchlorate doubled from 4.53 ± 0.65 mg L-1 day-1 for the first dose to 8.66 ± 3.23 mg L-1 day-1. The doubling of the perchlorate removal rate observed after the second dosing event suggested that microbial activity had increased in the rhizosphere. The DOC concentration decreased from an average of 309.1 ± 21.7 to 280.7 ± 71.6 mg L-1, while the perchlorate concentration in the willow leaves increased to 39.0 ± 18.5 mg kg-1 fw. Complete removal of the second dose of 17.2 mg L-1 perchlorate from the control bioreactors required 15 days, compared to only 5 days for DOC amended bioreactors. Because perchlorate was available for plant uptake for a longer time, the amount of perchlorate in the control leaves increased to 185.6 ± 88.5 mg kg-1. At the end of the treatment of the second dose in control bioreactors, the concentration of DOC remaining in solution was 32.0 ± 12.0 mg L-1.
Dose 3
In the DOC-amended bioreactors, the removal of the third dose of perchlorate, initial concentration of 42 mg L-1, was observed to be slower. More than 18 days was required to reach the MDL and the kinetic data was poorly described by the zero-order kinetic model and better described by the first-order model (Table 1). As the microbial population increased and the available DOC was progressively consumed or oxidized by bacteria to yield electrons used in perchlorate mineralization, the rates of rhizodegradation of subsequent doses of perchlorate decreased and were progressively poorly described by the zero-order kinetic model. This led to the persistence of perchlorate in the root zone of the trees as confirmed by the higher perchlorate concentration measured in the leaves, which increased from 39.0 ± 18.5 mg kg-1 fw to 95.1 ± 50 mg kg-1 fw. The slower rate of perchlorate degradation and increased perchlorate concentration in the willow leaves corresponded to the low DOC concentration remaining in solution, which had reduced from 280.7 ± 71.6 mg L-1 to 32.8 ± 7.2 mg L-1 when the third dose was completely removed to below the MDL.
| Experiment | Average Initial ClO4-[mg L-1] | DOC [mg L-1] | ClO4- in leaves [mg kg-1 fw] | ClO4- in leaves [mg kg-1 dw] | K [mg L-1day-1] Zero order | R2 | K [day-1] 1st order | R2 |
|---|---|---|---|---|---|---|---|---|
| Acetate 1st dose | 33.2 ± 1.7 | 293.2 ± 12 | 11.9 ± 1.8 | 47.5 ± 7.1 | 4.21 ± 0.61 | 0.93 | ||
| Acetate 2nd dose | 30.7 ± 7.0 | 246.3 ± 82 | 33.8 ± 25.1 | 120.9 ± 92.8 | 10.81 ± 1.9 | 0.85 | ||
| Acetate 3rd dose | 42.6 ± 13.3 | 29.1 ± 1.3 | 69.4 ± 58.3 | 246.2 ± 206.1 | 1.80 ± 0.58 | 0.58 | 0.24 ± 0.04 | 0.95 |
| Acetate 4th dose | 50.4 | 300.0 | 104.7 | 488.6 | 23.8 | 0.99 | ||
| CLE 1st dose | 27.3 ± 1.7 | 325 ± 16.6 | 11.7 ± 3.8 | 44.3 ± 12.0 | 4.85 ± 0.62 | 0.98 | ||
| CLE 2nd dose | 21.8 ± 6.2 | 315.1±50.3 | 44.1 ± 12 | 167.6 ± 48.2 | 6.50 ± 2.94 | 0.91 | ||
| CLE 3rd dose | 38.7 ± 3.4 | 36.4 ± 9.3 | 120.8 ± 29.6 | 479.6 ± 84.1 | 1.73 ± 0.24 | 0.71 | 0.23 ± 0.05 | 0.95 |
| CLE 4th dose | 37.0 | 300.0 | 136.4 | 409.1 | 18.4 | 0.97 | ||
| Control 1st dose | 32.8 ± 0.5 | 54.6 ± 5.3 | 103.7 ± 16.8 | 416.3 ± 60.0 | 3.23 ± 0.91 | 0.85 | 0.36 ± 0.02 | 0.94 |
| Control 2nd dose | 17.2 ± 0.1 | 32 ± 12 | 185.6 ± 88.5 | 817.7 ± 353.6 | 1.31 ± 0.10 | 0.85 | 0.35 ± 0.05 | 0.90 |
| Control 3rd dose | 38.7 ± 5.4 | 18.3 ± 5.6 | 210.6 ± 97.8 | 908.7 ± 397.1 | 2.68 ± 0.43 | 0.68 | 0.33 ± 0.03 | 0.93 |
| Control 4th dose | 42.8 ± 2.7 | 386.6 ± 27 | 1540 ± 132.2 | 3.2 ± 0.72 | 0.50 | (0.17 ± 0.1)† | 0.88 |
† - second order degradation rate constant [L mg-1 day-1] for the 4th dose in the control bioreactors.
Table 1: The average initial concentration of perchlorate and dissolved organic carbon in solution, and concentration of perchlorate in willow leaves. CLE: chicken litter extract. The leaves were collected immediately following the complete treatment of perchlorate dose. fw = fresh weight, dw = dry weight.
Likewise, the rate of perchlorate removal from the control bioreactors was described by first-order kinetics with a degradation rate constant of 0.33 ± 0.03 day-1 and perchlorate phytoaccumulated in the leaves of the control trees increased to 210.6 ± 97.8 fw. The similarity of the biodegradation kinetics at low concentrations of DOC following the third dosing event in DOC-amended reactors and control experiments further confirmed the limiting effect of electron and DOC sources during phytoremediation of perchlorate-contaminated soils.
Dose 4
At the fourth dosing, 300 mg L-1 DOC as chicken litter extract or acetate was added together with 45 mg L-1 perchlorate to the willow planted bioreactors. Unlike the third dose, the rhizodegradation reaction was described by the zero-order kinetic model and the rhizodegradation rate increased to the highest observed rate of 21.1 ± 3.8 mg L-1 day-1. Immediately after the complete removal of the fourth dose of perchlorate from solution, the concentration of perchlorate phytoaccumulated increased to 120.5 ± 22.4 mg kg-1 fw. In the control bioreactors no DOC was added with the fourth dose of perchlorate. As a result, the rate of perchlorate removal was much slower and described by a second-order kinetic model with rate constant of 0.17 ± 0.09 L mg-1 day-1. The phytoaccumulated perchlorate increased to 387 ± 27 mg kg-1 fw, a factor of three higher than in plants grown in the DOCamended bioreactors and dosed similarly.
The results of the control experiments provide strong evidence that electron sources normally available as DOC is limiting at most field sites where plants growing over perchlorate-contaminated soil and groundwater are found to phytoaccumulate significant amounts of perchlorate. By providing suitable external sources of DOC, it is clear that suitable environmental conditions that promote rapid rhizodegradation of perchlorate are created and the fraction taken up by plants is significantly minimized. The rate of perchlorate removal from the soil bioreactors and the amount of perchlorate detected in the leaf tissue indicates that a higher fraction uptake of perchlorate correlates directly with the residence time of perchlorate in the rooted media. The residence time of perchlorate in the rhizosphere is longest when the DOC concentration in soils is insufficient to sustain a high rate of rhizodegradation.
Sorption of perchlorate onto soil
At the end of the experiments, when the concentration of perchlorate in pore water had decreased to the MDL, multiple soil samples were collected from below the top 5 cm in each of the 20-L planted soil bioreactors and analyzed for perchlorate. Perchlorate was detected in all soil samples with the lowest concentration of 0.40 ± 0.29 mg kg-1 measured in bioreactors amended with acetate and the highest concentration of 3.19 ± 0.88 mg kg-1 in the control bioreactors. The chicken litter-amended soils contained 1.11 ± 0.35 mg kg-1 perchlorate. The sorption of perchlorate to most soils has been shown to be weak. Perchlorate binds to soil by deposition to the soil surface and in some soils sorption (anion replacement) may occur [27]. The perchlorate concentrations reported above were leached from the soil with deionized water and therefore represent the deposited fraction whose mobility is influenced mainly by hydrologic factors. To obtain the anion exchanged fraction would have required leaching with 0.1 M NaOH.
Implications for biostimulation of perchlorate degradation
Perchlorate-degrading microbes are ubiquitous in the environment [13]. The limitation to rhizodegradation of perchlorate in most perchlorate-contaminated soils is the lack of an adequate supply of DOC consumed by perchlorate degraders and utilized as electron sources to mineralize perchlorate. The control experiments demonstrate that DOC is limiting in most vadose zone source areas even though the site may be vegetated [28]. The slow rate of rhizodegradation under DOC limiting conditions tends to increase the residence time of perchlorate in the rhizosphere, which leads to higher fraction uptake, slow phytodegradation and phytoaccumulation of perchlorate in plant leaf tissue. Therefore, it is necessary to biostimulate and/or enhance rhizodegradation by providing cheap and commonly available carbon and electron sources, such as acetate and organic rich waste products.
This study has provided evidence that the slow build up of DOC in the rhizosphere by root exudation, root turnover and organic matter decomposition is insufficient to sustain a high rate of rhizodegradation of perchlorate and perhaps other degradable contaminants. An optimum design of phytoremediation of perchlorate should include enhancement of rhizoremediation by providing a sustained supply of electron donors to the root zone. The ecological benefits of enhancement of rhizodegradation include rapid attainment of site cleanup goals, minimization of the undesired uptake and phytoaccumulation of perchlorate, and avoidance of the potential recycling of perchlorate during phytoremediation.
Acknowledgements
Financial support for this research was provided by the USEPA through grant number RD 83109001.
References
- Urbansky ET, Brown SK, Magnuson ML, Kelty CA (2001) Perchlorate levels in samples of sodium nitrate fertilizer derived from Chilean caliche. Environ Pollut 112: 299-302.
- USEPA (U.S. Environmental Protection agency) (1999) Region 9 perchlorate update.
- Hogue C (2003) Environment of lettuce and rocket fuel. Chem Eng News 81: 11.
- Kirk AB, Smith EE, Tian K, Anderson TA, Dasgupta PK (2003) Perchlorate in milk. Environ Sci Technol 37: 4979-4981.
- Kirk AB, Martinelango PK, Tian K, Dutta A, Smith EE, et al. (2005) Perchlorate and iodide in dairy and breast milk. Environ Sci Technol 39: 2011-2017.
- USEPA (2006) EPA issues guidance for protective cleanups of perchlorate.
- Hatzinger PB, Whittier MC, Arkins MD, Bryan CW, Guarini WJ (2002) In-Situ and Ex-Situ bioremediation options for treating perchlorate in groundwater. Remediation Journal 12: 69-86.
- Nzengung VA, Wang C, Harvey G (1999) Plant-mediated transformation of perchlorate to chloride. Environ Sci Technol 33: 1470-1478.
- Yifru DD, Nzengung VA (2008) Organic carbon biostimulates rapid rhizodegradation of perchlorate. Environ Toxicol Chem 27: 2419-2426.
- Wang C, Lippincott L, Meng X (2008) Kinetics of biological perchlorate reduction and pH effect. J Hazard Mater 153: 663-669.
- Struckhoff GC (2009) Plant-assisted bioremediation of perchlorate and the effect of plants on redox conditions and biodiversity in low and high organic carbon soil. University of Iowa libraries.
- Shrout JD, Parkin GF (2006) Influence of electron donor, oxygen, and redox potential on bacterial perchlorate degradation. Water Res 40: 1191-1199.
- Coates JD, Michaelidou U, Bruce RA, O'Connor SM, Crespi JN, et al. (1999) Ubiquity and diversity of dissimilatory (per)chlorate-reducing bacteria. Appl Environ Microbiol 65: 5234-5241.
- Rikken GB, Kroon AGM, van Ginkel CG (1996) Transformation of (per)chlorate into chloride by a newly isolated bacterium: reduction and dismutation. Appl Microbiol Biotechnol 45: 420-426.
- van Ginkel CG, Rikken GB, Kroon AG, Kengen SW (1996) Purification and characterization of chlorite dismutase: a novel oxygen-generating enzyme. Arch Microbiol 166: 321-326.
- Bender KS, O'Connor SM, Chakraborty R, Coates JD, Achenbach LA (2002) Sequencing and transcriptional analysis of the chlorite dismutase gene of Dechloromonas agitata and its use as a metabolic probe. Appl Environ Microbiol 68: 4820-4826.
- Bender KS, Rice MR, Fugate WH, Coates JD, Achenbach LA (2004) Metabolic primers for detection of (per) chlorate-reducing bacteria in the environment and phylogenetic analysis of cld gene sequences. Appl Environ Microbiol 70: 5651-5658.
- Yifru DD, Nzengung VA (2006) Uptake of N-Nitrosodimethylamine (NDMA) from water by phreatophytes in the absence and presence of perchlorate as a co-contaminant. Environ Sci Technol 40: 7374-7380.
- van Aken B, Schnoor JL (2002) Evidence of perchlorate (ClO4-) reduction in plant tissues (poplar tree) using Radio-Labeled 36ClO4-. Environ Sci Technol, 36: 2783-2788.
- Nzengung VA, Penning H, O'Niell W (2004) Mechanistic changes during phytoremediation of perchlorate under different root zone conditions. Int J Phytoremediation 6: 63-83.
- Ellington JJ, Wolfe NL, Garrison AW, Evans JJ, Avants JK, et al. (2001) Determination of perchlorate in tobacco plants and tobacco products. Environ Sci Technol 35: 3213-3218.
- EWG (Environmental Working Group) (2005) Suspect salads: toxic rocket fuel found in first tests of grocery store lettuce.
- Smith PN, Yu L, McMurry ST, Anderson TA (2004) Perchlorate in water, soil, vegetation and rodents collected from the Las Vegas Wash, Nevada, USA. Environ Pollut 132: 121-127.
- Nzengung VA, McCutcheon SC (2003) Phytoremediation of Perchlorate. In Phytoremediation: Transformation and Control of Contaminants. Wiley, New York.
- Yifru D, Nzengung VA (2007) Uptake of Perchlorate by Vegetation Growing at Field Sites in Arid and Subhumid Climates. Remediation Journal 17: 53-68.
- Ellington JJ, Evans JJ (2000) Determination of perchlorate at parts-per-billion levels in plants by ion chromatography. J Chromatogr A 898: 193-199.
- Urbansky ET, Brown SK (2003) Perchlorate retention and mobility in soils. J Environ Monit 5: 455-462.
- Shrout JD, Struckhoff GC, Parkin GF, Schnoor JL (2006) Stimulation and molecular characterization of bacterial perchlorate degradation by plant-produced electron donors. Environ Sci Technol 40: 310-317.
Relevant Topics
- Anaerobic Biodegradation
- Biodegradable Balloons
- Biodegradable Confetti
- Biodegradable Diapers
- Biodegradable Plastics
- Biodegradable Sunscreen
- Biodegradation
- Bioremediation Bacteria
- Bioremediation Oil Spills
- Bioremediation Plants
- Bioremediation Products
- Ex Situ Bioremediation
- Heavy Metal Bioremediation
- In Situ Bioremediation
- Mycoremediation
- Non Biodegradable
- Phytoremediation
- Sewage Water Treatment
- Soil Bioremediation
- Types of Upwelling
- Waste Degredation
- Xenobiotics
Recommended Journals
Article Tools
Article Usage
- Total views: 15122
- [From(publication date):
specialissue-2013 - Dec 18, 2025] - Breakdown by view type
- HTML page views : 10371
- PDF downloads : 4751