Research Article Open Access
Chemical Synthesis, Characterization and Bioactivity Evaluation of Hydroxyapatite Prepared from Garden snail (Helix aspersa)
| Anjuvan Singh1* and K. M. Purohit2 | |
| 1Department of Biotechnology, Lovely School of Sciences, Lovely Professional University, Phagwara, India | |
| 2Department of Chemistry, National Institute of Technology, Rourkela –769008, Orissa, India | |
| Corresponding Author : | A. Singh Lecturer, Department of Biotechnology Lovely School of Sciences Lovely Professional University Phagwara, INDIA Tel: +91-7837651937 E-mail: anjuvan@gmail.com |
| Received March 31, 2011; Accepted April 28, 2011; Published May 06, 2011 | |
| Citation: Singh A, Purohit KM (2011) Chemical Synthesis, Characterization and Bioactivity Evaluation of Hydroxyapatite Prepared from Garden snail (Helix aspersa). J Biotechnol Biomaterial 1:104. doi: 10.4172/2155-952X.1000104 | |
| Copyright: © 2011 Singh A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited. | |
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
Early years, bio-nano-composites materials are interesting in advanced engineering applications fields. In order to, biological properties of the 10Ce-TZP/Al2O3 nano-composite was evaluated by the addition of HA nanopowder for medical applications. In this research, a bio-nano-composite consisting of CeO2- stabilized zirconia (Ce-TZP), Al2O3 and Hydroxyapatite (HA) powders proposed to use for medical application .The first step, nano-powders of Ce-TZP and Al2O3, were blended by fast milling machine. Then, nano-powder of HA was added to the alumina - zirconia (ZA) mixture from 10 to 40 vol%, and homogenized again. In final step, mixed powder was cold pressed and sintered at1600°C for 120 min. Crystal phase of the sintered samples were characterized by X-ray diffraction(XRD),the density and bending strength were measured and cellular response test was performed with osteoblastic cell-lines by MG63 cell-lines and the morphology of the proliferated cells was observed with FESEM too. From the strength and cellular response evaluations of samples, specimens with 30 vol% HA showed the best result, also XRD patterns confirmed this results.
| Keywords |
| Snail shell; Hydroxyapatite; Calcination; β-tricalcium phosphate; Bioactivity |
| Introduction |
| Hydroxyapatite [Ca10(PO4)6 (OH)2] based bioceramics are successfully used as implants as it is chemically similar with the inorganic constituent of biological hard tissue[1]. It is present in bone, teeth and tendons to give these organs stability, hardness and function. On account of its chemical similarity with the biological calcified tissue it is remarkably biocompatible [2]. Due to the formation of strong bond with the hard tissue, it is widely used in orthopedics or in dental implants. |
| HAP is also a potential implant material due to its excellent osteoconductive properties[3-4]. HAP has been shown to stimulate osteoconduction and is a material that can be integrated into bone without provoking an immune reaction. The biological response to HAP implants is influenced by its properties. The application of HAP as useful biocompatible materials largely depends on the purity and morphology of the powder. HAP can be prepared by different routes like chemical precipitation, sol-gel route, combustion synthesis, plasma etc [5-10]. The purity in the final HAP powder and stoichemetry (molar ratio of Ca/P = 1.67) can be well controlled in chemical precipitation route. The different chemical processes use precursors like Ca(NO3)2, Ca(OH)2 etc. as the source of Calcium[Ca] and (NH4)2 HPO4 , H3PO4etc. as the source of Phosphorus [P] during synthesis of HAP. The extremely pure HAP powder is very costly and needs high quality precursors. The most of the sources of Ca2+ contains different types and level of impurities mainly silica. Snail shell consists of CaCO3 with minor amount of MgCO3 and other matters can be potential precursors for the production of HAP. |
| The composition of human bone is an inorganic/organic hybrid consisting of 70% (wt) apatitic calcium phosphates and 30% (wt) organic (largely collagen) [11]. The apatitic calcium phosphate of bone mineral consists of carbonate, small amount of sodium, magnesium and other trace elements. The submicroscopic crystals of calcium phosphates |
| The objective of the present paper is to synthesize pure and biocompatible HAP using snail shell as precursor following chemical precipitation method [13]. The powders are characterized using XRD, DTA/ TGA, FTIR, SEM and BET surface area. Hydroxyapatite prepared by precipitation route also has the feature of small size, low crystallinity and high surfacial activation which can meet different demands [14]. Keeping the above points in mind, the present study was aimed to produce and to enhance the bioactivity of stoichemetric HAP prepared from garden snail shell (Helix aspersa) and to evaluate its bioactivity in simulated body fluid. |
| Materials and Methods |
| Garden snail shells (SS) were collected and their shell covering was removed carefully. Shells were washed with tap water followed by distilled water to remove the mud, sand and other impurities. The cleaned shells were dried in the direct sunlight for 2 days. Dry and cleaned SS were calcined at 1000°C for 2 hours so that all organic matters and proteins escape out. The calcined SS was treated with concentrated nitric acid to convert it to Ca(NO3)2. 130 ml. of 1.63(N) ammonical Ca(NO3)2 solution was added drop wise to a mixture of ammonical (NH4)2 HPO4 solution with constant stirring with the help of magnetic stirrer. The pH of the solution was maintained at 10. Hydroxyapatite was formed as per the following reaction: |
| 10Ca(NO3)2 + 6(NH4)2 HPO4 + 2H2O ΓΆΒ?Β? Ca10(PO4)6 (OH)2 + 12NH4NO3 + 8HNO3 |
| The resulting suspension was boiled for 10 minutes and cooled in an ice bath overnight to obtain a white gelatinous precipitate. The precipitate was filtered and filtered cake (residue) was dried in the oven at 80°C. The dried sample of hydroxyapatite was ground to powder. |
| The thermal analysis of the Snail shell was performed by (NETZSCH-Geratebau GmbH Thermal Analyser) at a heating rate of 10°C/minutes from ambient to 1200°C to study the weight loss and thermal behavior. The powder samples of SS, calcined SS and synthesized HAP samples were examined with high resolution X-ray Diffractometer (PW-1830, Philips, Netherlands) using Cu-Kα radiation. The X-ray diffraction (XRD) patterns were recorded in the steps of 0.01° interval with 1s counting time at each step. Fourier transform infra red (FTIR) spectrum of synthesized HAP powder was obtained over the wave numbers 400 - 4500cm-1. The powder was dispersed into pellets of KBr (mixed in 1:4 ratio) and the spectra was recorded with a Perkin- Elmer (S2000) IR spectrometer. The particle size analysis of HAP was done by Malvern Particle Size Analyzer (Model - Micro-P, UK). The surface morphology of SS and the prepared HAP powder were studied by Scanning Electron Microscope (SEM) while the surface area of the prepared HAP samples was determined with BET surface area analyzer (QUANTACHROME Model:Autosorb1). |
| In vitro bioactivity evaluation |
| The in vitro bioactivity evaluation of synthesized HAP powder from garden snail shell was performed in a stimulated body fluid (SBF) media of pH 7.4 at a ratio of 1 mg/ml in a water bath at 37°C. The changes in the pH of SBF medium were measured at pre-determined time intervals using a pH meter. Scanning electron microscopy (SEM) was used to identify the apatite formation on surface of the samples and to evaluate the surface morphology of the samples after immersion in SBF medium for 2, 8 and 15 days respectively. |
| Preparation of (Synthetic Body Fluid) SBF |
| SBF is known to be a metastable buffer solutions [15,16] and even a small, undesired variance in both of the preparation steps and the storage temperatures, may drastically affect the phase purity and hightemperature stability of the produced HA powders, as well as the kinetics of the precipitation processes. |
| Merck-grade NaCl (99.5%), NaHCO3 (99.5%), KCl (99.0%), Na2HPO4.2H2O (99.5%), MgCl2.6H2O(99.0%), Na2SO4, (CH2OH)3CNH2 (99.5%), CaCl2.H2O(99.0%) and HCl (37 vol%, Carlo- Erba, Rome, Italy) were used in the preparation of the SBF of this study |
| SBF solutions [17-21] were prepared by dissolving appropriate quantities of the above chemicals in deionized water. Reagents were added, one by one after each reagent was completely dissolved in 700 ml of water, in the order given in Table 1. A total of 40 ml of 1 M HCl solution was consumed for pH adjustments during the preparation of 1l of SBF solutions. A 15 ml aliquot of this acid solution was added just before the addition of the sixth reagent, viz., (CaCl2). 2H2O. Otherwise, the solution would display slight turbidity. The remaining part of the HCl solution was used during subsequent titration. Following the addition of the eight reagent (tris(hydroxymethyl) aminomethane), the solution temperature was raised from ambient to 37°C. This solution was then titrated with 1 M HCl to a pH of 7.4 at 37°C. During the titration process, the solution was also continuously diluted with consecutive additions of de-ionized water to make the final volume equal to 1l. It was observed in this study that the prepared SBF solutions can be stored at 5°C for a month without degradation. |
| Biodegradation test |
| Biodegradation test of calcined HAP prepared from garden snail shell (Helix aspersa) was done by taking Tris-HCl buffer solution. 0.05MTris- HCl solution was prepared using distilled water. The pH of solution was maintained 7.4 at 37°c by adding 1MHCl. Calcined HAP in the form of pallets were soaked in Tris-HCl buffer solution for one week then the samples were dried at 100°c and final weight loss of sample was determined by the formulae as given below: |
 |
| Where, W1 = initial weight of sample |
| W2 = final weight of sample after soaking in Tris-HCl solution. |
| Results and Discussion |
| DTA/TGA of snail shell (SS) |
| DTA/TGA analysis of Snail Shell showed the weight loss at temperature between 90°C - 120°C that is due to the physically adsorbed water. Over a wide range of temperature from 250°C - 400°C the weight loss is due to the decomposition of MgCO3 combined with the combustion of hydrocarbons. The weight loss along with endothermic peak at 750°C - 850°C indicates the decomposition of CaCO3 following the reaction. |
 |
| So it is confirmed from the thermal analysis that Snail Shell mainly contains CaCO3 along with small amount of MgCO3 and other organic matters. |
| XRD Analysis |
| A typical XRD profile of SS and calcined SS HAP has been shown in Figure 2. The raw SS showed the presence of CaCO3 phase, where as CaO was detected in the calcined Snail shell. The appearance of calcined SS was soft, porous and white in colour. However, due to delay in recording some amount of CaO was converted to Ca(OH)2by adsorbing moisture from the atmosphere which is depicted in Figure 2b. |
| The XRD phase analysis of HAP powder has been shown in Figure 3. Three-high-intensity peaks located at 2θ = 31.7°, 32.2° and 32.9° with Cu-Kα radiation are difficult to be exactly recognized from their diffraction patterns. An XRD pattern reveals the formation of HAP and is well resembled with the standard JCPDS file. The unindexed peak at 30.750 (Figure 3b) may be due to β-tricalcium phosphate; which indicates the initiation of conversion of HAP to β-tricalcium phosphate on heating HAP above 800°C. The calcined HAP exhibits well crystallized sharp peaks of characteristics HAP. The HAP powders, thus synthesized from Snail Shell precursor, are very pure and chemical analysis of powders confirms the same observation. |
| FTIR Analysis |
| Infrared characterization was carried out for the sample to study the spectral characteristics indicative of the chemical bonding in the synthesized HAP powder. The spectrum (Figure 4) can be divided into four regions with peaks having wave numbers around 3500, 1420, 1100 and 600cm-1. The peak observed around 3431.8cm-1 is due to the presence of -OH bond [22]. This peak is mainly due to O-H stretching vibration in HAP [23]. The peak at 1036.2 cm-1 is associated with the stretching modes of the P-O bonds of HAP [23,24]. The double peak at 603.1 cm-1 and 567.4cm-1 are due to bending modes of P-O bonds in phosphate groups [24]. Thus, the presence of PO4 3- - group in HAP is almost confirm from IR studies. The pH of the medium during synthesis of HAP was maintained using ammonium solution and it was removed from the suspension with repeated washing with distilled water. In spite of all efforts to remove ammonia from the solution, there is a possibility of small amount of it in the HAP powder. The IR analysis shows a small broad peak at 1422.6cm-1; which is characteristics peak of NH4 +- group [25-27]. |
| Particle size analysis |
| Particle size analysis of HAP powder was carried out following Laser technique and pattern of particle size distribution is plotted in Figure 5. Average particle size was found to be 2.63 µm. Small amount of fine particles (0.2-0.3 µm) are also present in the synthesized powder. |
| Surface area measurement |
| The surface areas of the hydroxyapatite powder and calcined HAP are determined are 83 and 15m2/gm respectively. Powders are agglomerated during calcinations; but HAP powders have to be calcined to remove volatile impurities like ammonia. |
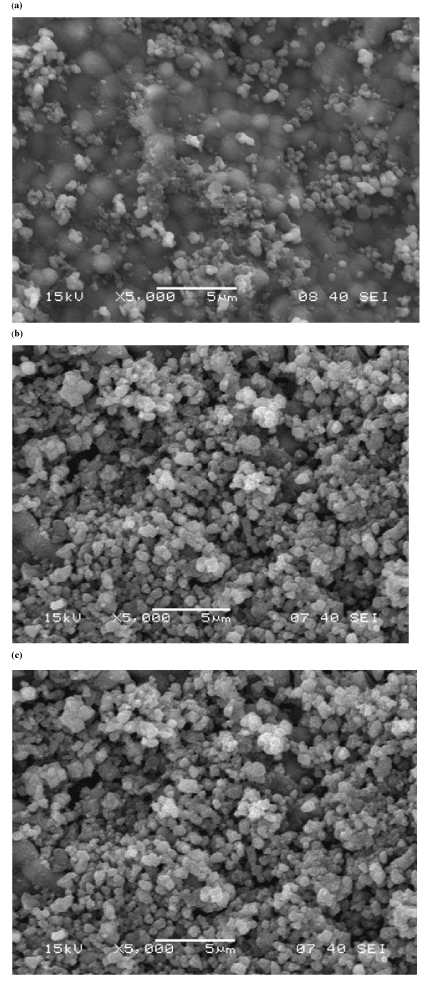
| Scanning Electron Microscope (SEM) |
| The morphologies of as synthesized and calcined HAP powders are shown in Figure 6. Uncalcined HAP powders are almost regular and round in shape; where as calcined HAP powders are agglomerated. The microstructure as reveals from SEM is in well- agreement with the particle size analysis and BET surface area analyzer results. |
| Bioactivity evaluation |
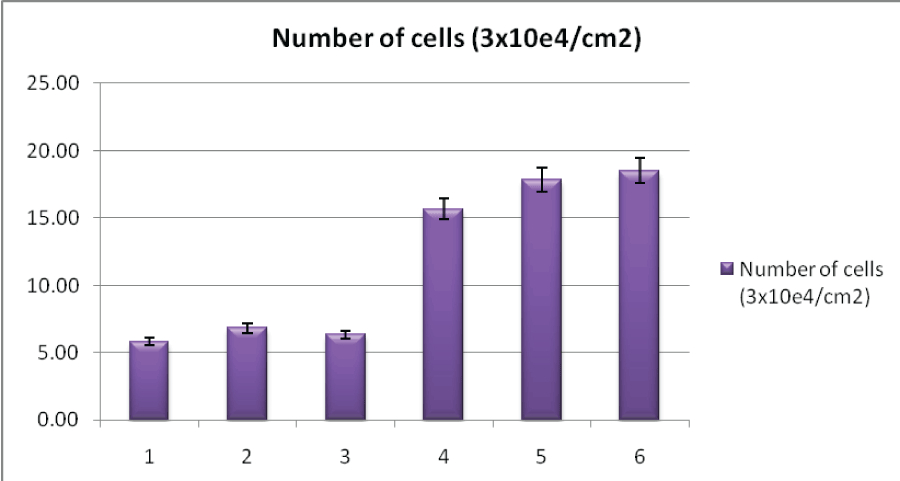
| The SEM micrographs of the surfaces of the immersed HAP powder after soaking in SBF for various periods of time are shown in Figure 7. Tiny agglomerated bone-like apatite particles could be formed on the surface of the HAP powders. |
| Figure 7 shows that tiny agglomerated bone-like apatite particles could be formed on the surface of the HA powders soaked for 2 days, 8 days and 15 days respectively. The number and the size of these agglomerated particles increased with increasing soaking times. Identification and evaluation of apatite formation on the surface of a material in SBF is useful for predicting the in vivo bone bioactivity of the material, not only qualitatively but also quantitatively [28-31].The results indicated that the synthesized HAP powder from Garden Snail shell (Helix aspersa) showed the high bioactivity in SBF solution. |
| In-vitro biodegradation |
| Biodegradation of calcined HAP samples in the form of pallets were carried out in Tris-HCl solution. HAP samples were soaked in Trisbuffer solution at pH 7.4 and temperature 37°C for 7 days [32]. When porous HA was soaked in Tris-buffer solution, the loss of calcium ion took place which resulted in the increase in pH of the buffer from 7.4 to 8.2 which confirms the biodegradation of HAP. The calcined HAP prepared from garden snail shell by chemical precipitation method showed weight loss of 4.5%. Thus it appears that the ageing time in Tris-HCl solution may also affect the weight loss behavior. |
| Conclusions |
| A stoichiometric, pure and thermally stable hydroxyapatite powder was synthesized from Snail shell (Helix aspersa) by chemical precipitation method. XRD analysis indicated the phase purity and crystallinity of hydroxyapatite powder. TG/DTA result showed that Snail shell is mainly composed of calcium carbonate (CaCO3). Fine particle size of hydroxyapatite was produced. The present work is based on the utilization of biological waste (Snail shell) to produce hydroxyapatite for Bio-medical applications. The prepared HAP powder showed high bioactivity similar to that in biological apatite and higher bioactivity in comparison with conventional HAP. Thus, prepared HAP from garden snail shell (Helix aspersa) might be more useful for treatment of oral bone defects in comparison with conventional HAP, and might be more effective as a bone replacement material to promote bone formation. |
| An attempt will be made in future to synthesize porous HAP and study its bio-compatibility. Mass production of biocompatible HAP for biological application may be possible at simple and low cost through this route. |
| Acknowledgements |
| The authors like to acknowledge H.O.D, Department of Chemistry, National Institute of Technology, Rourkela, for providing laboratory facilities for carrying out research work. |
References
- Layrotte P, Ito A, Tateishi T (1998) Sol-gel Synthesis of Amorphous Calcium Phosphate and Sintering into Microporous Hydrozyapatite Bioceramics. J. Am. Ceram. Soc. 81: 1421.
- Li P, Ohtsuki C, Kokubo T, Nakanishi K, Soga N, et al. (1992) Apatite Formation Induced by Silica Gel in a Simulated Body Fluid. J. Am. Ceram. Soc. 75: 2094- 2097.
- de Groot K (1980) Bioceramics Consisting of Calcium Phosphate Salts, Biomaterials. 1: 47-50
- Jarcho M (1981) Calcium Phosphate Ceramics as Hard Tissue Prosthetics. Clin Orthop Rel Res. 157: 259-278.
- Brown PW, Fulmer M (1991) Kinetics of Hydroxyapatite Formation at Low Temperature. J. Am. Ceram. Soc.74: 934-940.
- Young RA, Holcomb DW (1982) Variability of Hydroxyapatite Preparations. Calcified Tissue Int. 34: 17-32
- Arita IH, Wilkinson DS, Mondragon MA, Castano VM (1995) Chemistry and Sintering Behaviour of Thin Hydroxyapatite Ceramics with Controlled Porosity. Biomaterials 16: 403-408.
- Brendel T, Engel A, Russel C (1992) Hydroxyapatite Coatings by a Polymeric Route. J. Mater Sci: Mater. Med 3: 175-179.
- Partenfelder UME, Engel A ,Russel CA (1991) Pyrolytic Route for the Formation of Hydroxyapatite/Fluoroapatite Solid Solutions. J Mater. Sci: Mater. Med 4: 292-295.
- Roy DM , Linnehan SK (1974) Hydroxyapatite Formed From Coral Skeletal Carbonate by Hydrothermal Exchange. Nature 247: 220-222.
- Cowin SC, Vanburskirk WC, Ashaman RB (1987) Handbook of Bioengineering (eds) R Skalak and Schien C Newyork (McGRawHill).
- Sinha A, Ingle A, Munim KR, Vaidya SN, Sharma BP, et al (2001) Development of Calcium Phosphate based Bioceramics. Bull. Mater. Sci 24: 653-657.
- Jarcho M (1978) Hydroxyapatite Ceramic. U.S Patent, 4097935, July 4.
- Liu C, Huang Y, Shen W, Cui J. (2001) Kinetics of Hydroxyapatite Precipitation at pH 10 to 11. Biomaterials 22: 301-306.
- Ohtsuki C, Kokubo T, Yamamuro T (1992) Mechanism of HA formation of CaO{SiO2}P2O5 glasses in simulated body Fluid. J Non- Cryst Solids 143: 84-92
- Neuman W, Neuman M (1958) The chemical dynamics of bone mineral. Chicago: University of Chicago Press 34.
- Li P, Kangasniemi I, de Groot K, Kokubo T (1994) Bone-like hydroxyapatite induction by a gel-derived titania on a titanium substrate. J Am Ceram So 77:1307-1312.
- Li P, Nakanishi K, Kokubo T, de Groot K (1993) Induction and morphology of HA precipitated from metastable simulated body Fluids on sol gel prepared silica. Biomaterials 14: 963-968.
- Li P, Kangasniemi I, de Groot K, Kokubo T, Yli-Urpo AU (1994) Apatite crystallization from metastable calcium phosphate solution on sol gel prepared silica. J Non-Cryst Solids168: 281-286.
- Cho SO, Nakanishi K, Kokubo T, Soga N, Ohtsuki C, et al. (1995) Dependence of apatite formation on silica gel on its structure: e!ect of heat treatment. J Am Ceram Soc 78: 1769-1774.
- Kokubo T, Miyaji F, Kim HM, Nakamura T (1996) Spontaneous formation of bonelike apatite layer on chemically treated titanium metals. J Am Ceram Soc 79: 1127-1129.
- Russell SW, Luptak KA, Suchicital CTA, Alford TL, Pizziconi VB (1996) Chemical and Structural Evolution of Sol-Gel-Derived Hydroxyapatite Thin Films under Rapid Thermal Processing. J. Am. Ceram. Soc 79: 837-842.
- Sargin Y, Kizilyalli M, Telli C, Guler H (1997) A New Method for the Solid-State Synthesis of Tetracalcium Phosphate: A Dental Cement: X-ray Powder Diffraction and IR Studies. J. Eur. Ceram. Soc 17: 963-970.
- Ramanan SR, Ramannan V (2004) A Study of Hydroxyapatite Fibers Prepared via Sol-Gel Route. Material Letter 58: 3320-3323
- Cuneyt T (2000) Synthesis of Biomimetic Ca-Hydroxyapatite Powders at 37°C in Synthetic Body Fluids. Biomaterials 21:1429-1438.
- Caroline E, Victoria E, Gnanam FD (2002) Synthesis and Characterisation of Biphasic Calcium Phosphate. Trends Biomater.Artif.Organs 16: 12-14.
- Gomez-Morales J, Torrent-Burgues J, Boix T, Fraile J, Rodriguez-Clement R (2001) Precipitation of Stoichiometric Hydroxyapatite by a Continuous Method. Cryst.Res.Technol 36: 15-26.
- Williamson GK, Hall WH (1953) Acta Metall Sinica 1: 22.
- Hench LL, Wilson J (1993) An Introduction to Bioceramics. 2nd ed. London: World Scientific Publishing Co Ltd.
- Murugan R, Ramakrishna S (2005) Aqueous mediated synthesis of bioresorbable nanocrystalline hydroxyapatite. J Cryst Growth. 274: 209-213.
- Kokubo T, Takadama H (2006) How useful is SBF in predicting in vivo bone bioactivity?. Biomaterials 27: 2907-2915.
- Kwon SH, Jun YK, Hong SH, Lee IS, Kim HE (2002) Calcium phosphate bioceramics with various porosities and dissolution rate. J. Am. Ceram. Soc 85: 3129-3131.
Tables and Figures at a glance
| Table 1 |
Figures at a glance
 |
 |
 |
 |
| Figure 1 | Figure 2 | Figure 3 | Figure 4 |
 |
 |
 |
| Figure 5 | Figure 6 | Figure 7 |
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 15625
- [From(publication date):
May-2011 - Dec 18, 2025] - Breakdown by view type
- HTML page views : 10831
- PDF downloads : 4794
