Research Article Open Access
An Artificial Silk-elastin-like Protein Suppresses Cells Adhesion without Apoptosis
Satoshi Somamoto1,2 and Yasuhiko Tabata1*1Department of Biomaterials, Institute for Frontier Medical Sciences, Kyoto University, 53 Kawara-cho Shogoin, Sakyo-ku Kyoto 606-8507, Japan
2Sanyo Chemical Industries Ltd., 1-40, Goryo Ohara, Nishikyo-ku, Kyoto 615-8245, Japan
- Corresponding Author:
- Dr. Yasuhiko TABATA
Department of Biomaterials
Institute for Frontier Medical Sciences
Kyoto University, 53 Kawara-cho Shogoin
Sakyo-ku Kyoto 606-8507, Japan
Tel: +81-75-751-4121
Fax: +81-75-751-4646
E-mail: yasuhiko@frontier.kyoto-u.ac.jp
Received date: May 08, 2012; Accepted date: June 14, 2012; Published date: June 16, 2012
Citation: Somamoto S, Tabata Y (2012) An Artificial Silk-elastin-like Protein Suppresses Cells Adhesion without Apoptosis. J Biotechnol Biomater 2:139. doi:10.4172/2155-952X.1000139
Copyright: © 2012 Somamoto S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Visit for more related articles at Journal of Biotechnology & Biomaterials
Abstract
The objective of this study is to evaluate the effect of an artificial silk-elastin-like protein (SELP) on the adhesion and apoptotic behavior of cells. When MC3T3-E1 cells were cultured on dishes coated with different concentrations of SELP, the cells adhesion was suppressed with the increasing coating concentration. As controls, the coating with fibronectin enhanced the number of cells adhered in the concentration dependent manner. The poly (vinyl alcohol) (PVA) and agarose coating suppressed the cell adhesion. Elastin did not affect the cell adhesion behavior. The shape of cells cultured on the SELP, PVA, and agarose-coated dishes was spherical and spreaded cells were observed on the SELP- and fibronectin-coated ones. The level of caspase 3 and 9 was evaluated. The level was significantly high for the PVA- and agarose-coated dishes compared with non-coated dishes, whereas it was low for the fibronectincoated ones. The level was decreased with an increase in the concentration of SELP coated. The number of cells free of annexin V activity for the SELP group as low as that for the fibronectin group. The cell number was high for the PVA and agarose groups. The actin filaments were detected for the SELP-coated dishes, in remarked contrast to the PVA-coated ones. Pretreatment with the antibody of integrin αV or α5 significantly decreased the caspase 3 level for cells cultured on the SELP-coated dishes. It is concluded that the SELP has an unique property which allows cells to suppress their adhesion, but not to induce their apoptosis.
Keywords
Artificial silk-elastin-like protein; Cells adhesion; Apoptosis; Integrin
Introduction
Recently, the evaluation of cell-substrate interaction has been increasingly noted in terms of the manipulating technology for cell proliferation and differentiation. The substrates of cell culture have been investigated aiming at their property to enhance the cell adhesion which is necessary for the subsequent proliferation [1,2]. However, the suppression nature of cell adhesion is also attractive. When cells cannot adhere to the substrate, they tend to interact to each other to form their aggregates that are known as an embryoid body. This cell aggregate formation is often observed in the stage of cell differentiation. Without the formation of embryoid body, the differentiation of embryonic stem (ES) cells is not always induced [3,4]. On the other hand, it is reported that the aggregate of cells shows metabolic and functional activities higher than the cells existing in the single or separate manner [5-7]. In these connections, the culture substrate with a property to suppress the cell adhesion will be interesting in terms of cell biology. The interaction between cell and surface plays a major biological role in cellular behavior. Cell surface interaction influence many aspects of cell physiology, such as adhesion, spreading, activation, recruitment, migration, proliferation and differentiation. In these connections, the culture substrate with a property to suppress the cell adhesion will be interesting in terms of cell biology.
There have been reported on some materials which have the potential to suppress the cell adhesion to form cell aggregates (spheroids) [8-15]. It is well recognized that once detached from the substrate to adhere, cells are normally programmed to die due to their activation of apoptosis pathway [16]. This apoptosis of cells is often of problem in cell culture experiments, because it is difficult to continue the cell culture and their biological functions decrease. Therefore, if the substrate has a property not only to prevent the cells adhesion, but also to suppress their apoptosis, it will be a promising tool for cell researches.
Silk-elastin-like protein (SELP) is composed of a repeating aminoacid block naturally present a protein found in Bombyx mori (silkworm) silk (GAGAGS) and that of mammalian elastin (GVGVP). The SELP is structured from four silk blocks, seven elastin blocks, and one lysinesubstituted elastin block. The protein readily forms a stable hydrogel at concentrations of 4 wt% or higher as the temperature is elevated from 25 to 37°C. The biocompatibility of SELP has been investigated by the direct injection to guinea pigs subcutaneously and intradermally. No sign of significant, harmful immunological reaction or inflammation was observed [17]. The SELP medical and pharmaceutical applications to tissue engineering have been studied as the injectable polymer and the drug delivery system [18-20].
The research in new biomaterials for engineering of cell adhesion surface is still facing a great boost. Hence attempt, was made to examine the SELP interaction of cells with substrate and how they may influence cell behavior. To study the cell adhesion and apoptosis on SELP, nonadhesive PVA- or agarose-coated, and fibronectin-coated surfaces were chose negative and positive controls, respectively [21-25]. Cells were cultured on the culture dishes coated with different concentrations of SELP and other substances, such as the fibronectin, cell adhesion protein, the elastin, component of SELP, and the PVA. The adhesion and apoptotic behavior of cells were evaluated and compared between the SELP and other substances. In the present work, we examined the attachment, morphology, proliferation and apoptosis of cells cultured on the dishes coated with SELP.
Materials and Methods
Materials
A silk-elastin-like protein polymer (SELP), which is composed of four silk-like blocks, seven elastin-like blocks, and one modified elastin block containing a lysine(K) substitution MDPVVLQRRDWENPGVTQLNRLAAHPPFASDPMGAGSGAGAGS [ (GVGVP)4 GKGVP (GVGVP)3 (GAGAGS)4]12 (GVGVP)4 GKGVP (GVGVP)3 (GAGAGS) 2 GAGAMDPGRYQDLRSHHHHHH) was kindly supplied from Sanyo Chemical Co., Ltd., Kyoto, Japan. Poly (vinyl alcohol) (PVA, degree of polymerization = 1800, degree of saponification = 87 ~ 89 mole%) was obtained from Japan Vam & Poval Co., Ltd., Tokyo, Japan. Elastin (from bovine neck ligament), fibronectin (from bovine plasma), and agarose were purchased from Wako Co., Ltd., Kyoto, Japan.
MC3T3-E1 cells culture
cell line of preosteoblasts (MC3T3-E1) was used and cultured in Dulbecco’s Modified Eagle’s medium (DMEM, Gibco Life technologies Co., Carlsbad, CA) contained with 10 wt% fetal bovine serum (FBS) (Gibco Life technologies Co., Carlsbad, CA) and 1 wt% penicillin and streptomyciz (Sigma-Aldrich Co., St. Louis, MO) or only contained with 1 wt% penicillin and streptomycin for FBS-free DMEM under a humidified 5 % CO2-95 % air atmosphere at 37°C. Cells proliferated were detached from dishes by a trypsin- ethylenediaminetetraacetate (EDTA) solution (Sigma-Aldrich Co., St. Louis, MO)and used for the following cell culture experiments.
Cell culture on SELP-coated dishes and cell observation
SELP, elastin, fibronectin, PVA or agarose solution in 10 mM phosphate-buffered saline (PBS, pH=7.0) was prepared at concentration of 0.005, 0.05, 0.5, 5, 50, 100, 500, and 1,000 μg/ml, and 50 μl of solution was placed into a polystyrene dish (7.15 mm in diameter, Cat. No. 1860-096, Asahi Glass Co., Ltd, Tokyo, Japan). The dishes were left for 2 hr at 37°C, and then washed twice with PBS. Cells (1.5 × 103 cells/cm2) were seeded onto the SELP-, elastin-, fibronectin-, PVA- or agarose-coated dishes, and the cell culture in the DMEM medium was continued. Photographs of cells attached on the SELP-, elastin-, fibronectin-, PVA- or agarose -coated dishes were taken on phase-contrast microscope (IX70, Olympus Optical Co., Japan) 2 and 24 hr later to view the morphology of cells.
Cell adhesion assay
Cell adhesion was evaluated according to the method reported previously [1]. Cells proliferated were washed twice with FBS-free DMEM to remove cell adhesive factors from serum. The cell suspension in FBS-free DMEM (6.0 × 104 cells/cm2) was added to SELP-, elastin-, fibronectin-, PVA- or agarose coated plates, and culture for both 2 hr and 24 hr at 37°C. Non-adhesive cells were removed by washing with FBS-free DMEM, and a cell-counting reagent (Cell count Reagent SF, Nacalai Tesque Co., Ltd, Kyoto, Japan) was added to each well of 96- well multi-well culture plate. After 2 hr of incubation with the reagent, the absorbance of each well of 96-well multi-well culture plate (Asahi Glass Co., Ltd, Tokyo, Japan) was measured using a UV-microplate reader (VERSA max, Molecular Devices Inc, Sunnyvale, CA) at 490 nm. The number of cells adhered on the dishes coated with 50 μg/ml of fibronectin solution was defined as 100 % cell adhesion and used to normalize the adherent cell numbers.
Caspase assay
As a measure of cell apoptosis, the caspase activity of cells was evaluated based on the conventional method [26]. Cells (6.0 × 104cells/cm2) were cultured on SELP-, elastin-, fibronectin-, PVA- or agarose-coated dishes or different concentrations of SELP-coated ones for 24 hr. The cells were lysed with a cell lysis buffer of caspase assay kit (BioVision Inc., Mountain View, CA). The caspase activity of cell lysates was measured by spectrophoto metrical detection of p-nitro aniline (p-NA) generated through the enzymatic cleavage of caspasespecific substrates, Ac-DEVDpNA and Ac-LEHD-pNA for caspase 3 and 9, respectively. The cell lysates treated were measured using a UVmicroplate reader (VERSA max, Molecular Devices Llc., Sunnyvale, CA) at 405 nm. The caspasae activity of cells cultured on a PVA-coated plate (100 μg/ml) was defined as 100 % and used to normalize the caspase activity.
Annexin V–FITC/PI assay
As another method to evaluate the apoptosis of cells, an annexin V–FITC/PI assay was performed with the Annexin V–FITC Apoptosis Detection Kit I (Becton Dickinson Co., Franklin Lakes, NJ) according to the instructions of manufacturer referring the standard settings reported by Ulbrich et al. The Annexin V–FITC is used to quantitatively determine cells that are actively undergoing programmed cell death. In the early stage of apoptosis, it is well recognized that cells lose their membrane asymmetry and the phosphatidyl serine (PS) is translocated from the inner layer of plasma membrane to the outer layer and exposed to the external environment [27-29]. Cells (6.0 × 104 cells/cm2) were cultured on SELP-, elastin-, fibronectin-, PVA- or agarose-coated dishes for 24 hr, and then were stained with the annexin V–FITC and propidium iodide (PI). The cells stained (1 × 104) were applied to a FACScan flow cytometer (Becton Dickinson Co., Franklin Lakes, NJ) to view the percentage of cell apoptosis. The annexin V–FITC-negative and PI-negative cells population was regarded as normal healthy cells. The annexin V–FITC-positive and PI-negative, annexin V-FITCpositive and PI-positive, and annexin V-FITC-negative and PI-positive cells populations were classified into the early apoptosis, late apoptosis, and necrosis cells.
Actin staining
Filamentous actin fiber of cells was observed by the conventional immunostaining method [30]. Briefly, cells (3.0 × 1004 cells/cm2) cultured on different dishes for 24 hr were rinsed twice with PBS and fixed in 10 vol% formaldehyde solution at 4°C for 20 min. After washing with PBS, cells were treated with 2 wt% bovine serum albumin solution in PBS at room temperature for 20 min. Next, cells were incubated with fluorescein isothiocyanate (FITC)-conjugated phalloidin (10 μg/ ml, Sigma-Aldrich Co., St. Louis, MO) at room temperature for 1 hr. Following PBS washing two times, cells were mounted in a FluorSave Reagent (Calbiochem Inc., San Diago, CA) and viewed on a confocal fluorescence microscope (Fluoview FV1000, Olympus Optical Co., Japan).
Blocking assay by integrin antibodies and lactose
To evaluate the effect of integrin receptors and elastin-binding protein (EBP) on the apoptosis of cells, the blocking assay was performed. Integrin subunits were blocked by each integrin antibodies. EBP was blocked by lactose that is commonly used as EBP antagonist [31,32]. Cells proliferated were washed twice with FBS-free DMEM and cells washed were treated with 10 μg/ml anti-integrin-αV antibodies (BioLegend Inc., San Diego, CA), 10 μg/ml anti-integrin-α5 antibodies (Abcam Plc., Cambridge, UK) or 10 mM lactose of a EBP antagonist for 30 min at 37°C. Next, the cells were then centrifuged at 1,000 rpm for 5 min and the cell pellet was resuspended in FBS-free DMEM. Cells (6.0 × 104 cells/cm2) treated and non-treated were cultured on the SELPcoated dishes for 24 hr at 37°C. Then, the caspase 3 activity of cells was determined as described above.
Phosphorylation of Akt
Phosphorylation of Akt was evaluated based on the conventional method reported. Cells (3 × 104 cells/cm2) were cultured for different time periods on SELP-coated dishes. The cells were treated with a lysis buffer (50 mmole/l Tris–HCl, 150 mmole/l NaCl, 1 mmole/l ethylenediaminetetraacetate (EDTA), 0.1 wt% Nonidet P-40 ; pH 7.4) containing a protease inhibitor mixture (Cat. No. P8340, Sigma- Aldrich Co., St. Louis, MO) for cell lysation. The protein content of cell lysates was measured with a Micro BCA protein assay kit (Thermo Fisher Scientific Inc., Waltham, MA). The cell lysates prepared were applied to sodium dodecylsulfate polyacrylamide electrophoresis (SDSPAGE) and transferred to an Immobilon-P membrane (Millipore Inc., Milford, MA). The membrane was immunoblotted with an antibody against phospho-Akt (Ser473) or Akt (Lot No. 17, Cell Signaling Technology Inc., Beverly, MA) and β-actin (Sigma-Aldrich Co., St. Louis, MO) as an internal control as the diluted ratios of 1:1000, 1:1000, and 1:5000, respectively. After incubation with a peroxidaseconjugated anti-mouse or anti-rabbit secondary antibody (Lot. No. FK928983 or LB142282, Sigma-Aldrich Co., St. Louis, MO), the target proteins were immunologically visualized with Super Signal West Pico Chemiluminescent Substrate (Lot. No. LF144837, Pierce, Thermo Fisher Scientific, Rockford, IL). Primary culture was performed 5 times independently.
Statistical analysis
All the results were statistically analyzed by the Tukey-Kramer test p < 0.05 was considered to be statistically significant. Data were expressed as the mean ± the standard deviation.
Results
Cells adhesion onto dishes coated with SELP and other substances
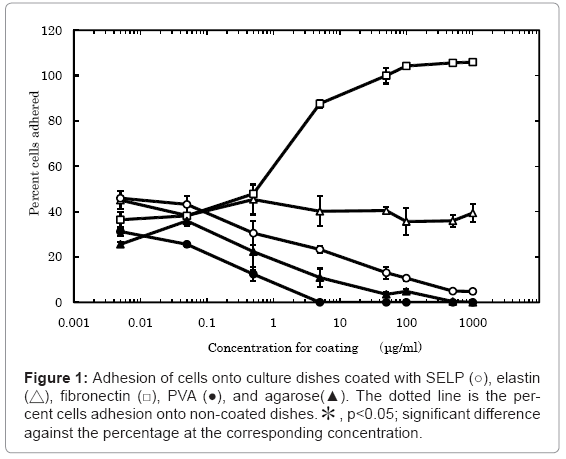
We first tested the ability of MC3T3-E1 cell lines to adhere percentage cells to the dishes coated with SELP, elastin, fibronectin, PVA and agarose (Figure 1). About 40 % of cells applied were adhered onto the non-coated polystyrene culture dishes. The number of cells adhered decreased with an increase in the concentration of SELP, PVA, and agarose coated. On the contrary, the cells adhered more with an increase in the fibronectin-concentration manner. The elastin coating did not affect the cell adhesion.
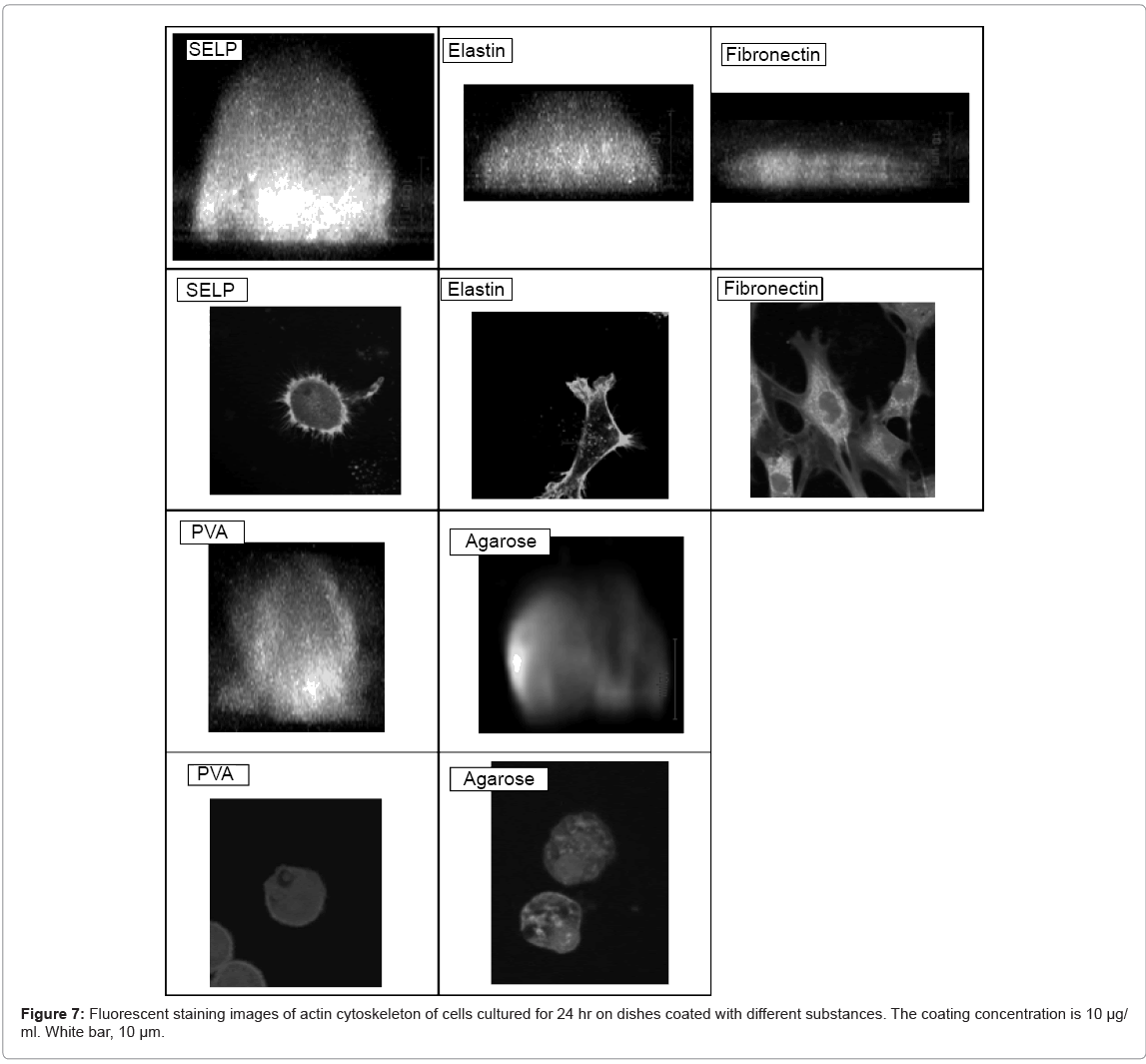
High magnification images demonstrated that the cell was studied 2 hr and 24 hr after cell seeding on surface of these materials (Figure 2). Low cell density (5 × 103 cells/ml) was selected to observe morphology of single cells. The shape of cells cultured on the SELP-, PVA-, and agarose-coated dishes was spherical while adhered and spreaded cells were observed for the elastin- and fibronectin-coated ones.
Caspase activity of cells cultured on dishes coated with SELP and other substances
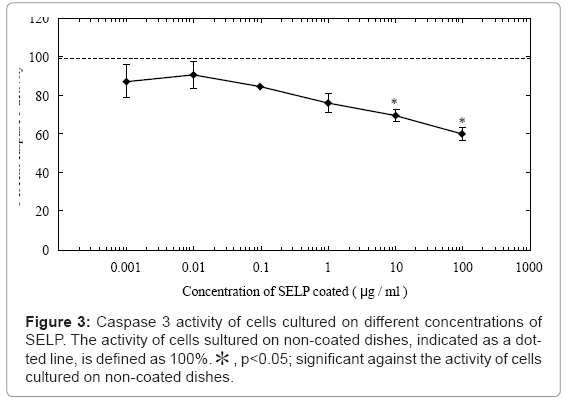
We next examined caspase 3 activity level of cells cultured on the SELP-coated substrate (Figure 3). The caspase 3 activity decreased with an increase in the coating concentration. When the SELP concentration became 10 μg/ml or higher, the activity was significantly low compared with that of cells cultured on non-coated dishes. Based on this finding, the coating concentration of SELP was fixed at 10 μg/ml.
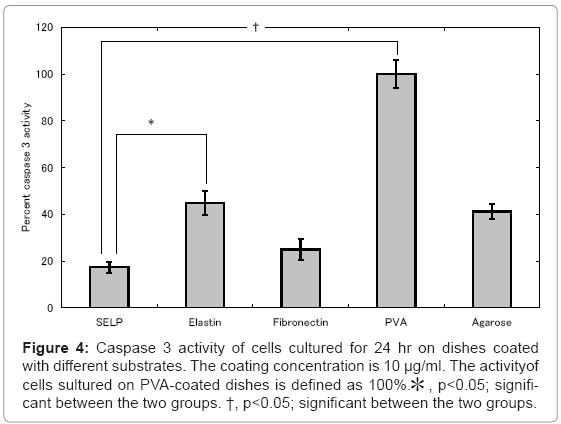
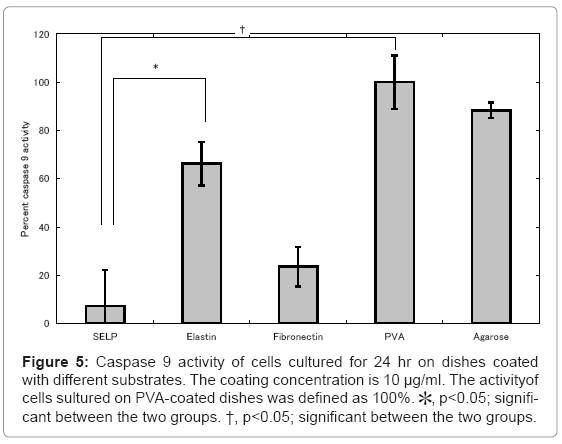
Figures 4 and 5 show the activity of caspase 3 and 9 for cells cultured on SELP-, elastin-, fibronectin-, PVA-, and agarose-coated dishes. Cells cultured on the PVA-coated dishes showed the highest level of caspase 3 and 9. Low activity of caspase 3 and 9 was observed for cells cultured on the fibronectin-coated dishes compared with these of PVA-coated ones. For the SELP-coated dishes, the level of caspases was as low as that of fibronectin-coated dishes.
Figure 4: Caspase 3 activity of cells cultured for 24 hr on dishes coated with different substrates. The coating concentration is 10 μg/ml. The activityof cells sultured on PVA-coated dishes is defined as 100%.* , p<0.05; significant between the two groups. †, p<0.05; significant between the two groups.
Figure 5: Caspase 9 activity of cells cultured for 24 hr on dishes coated with different substrates. The coating concentration is 10 μg/ml. The activityof cells sultured on PVA-coated dishes was defined as 100%. *, p<0.05; significant between the two groups. †, p<0.05; significant between the two groups.
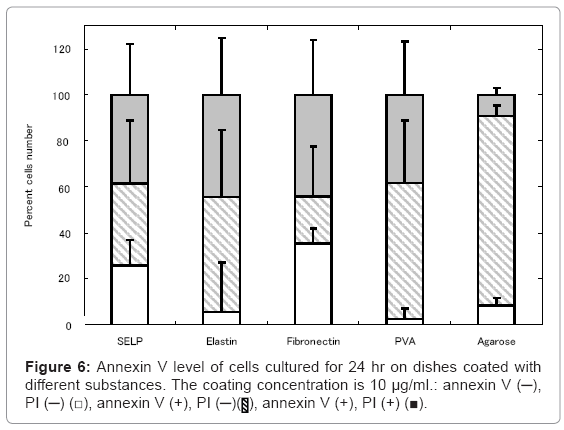
Annexin V assay
Our next logical step to examine the a widely used assay detect PS exposure on the cell surface on SELP, elastin, fibronectin, PVA, and agarose –coated dishes, which is one of the hall mark of early apoptosis by its binding to fluorochromogenate conjugated annexin-V [33]. The percentage of cells free of annexin V activity was as low as that of the fibronectin-coated dishes on which no cells were apoptosed to die. On the contrary, the higher percentage of annexin V –positive cells was observed for the PVA- and agarose-coated dishes.
Actin staining of cells adhered on dishes coated with SELP and other substances
Figure 7 shows the fluorescence actin staning and the morphology of cells adhered on SELP-, elastin-, fibronectin-, PVA-, and agarosecoated dishes. The shape of cells cultured on the SELP-coated dishes was of dome-like form. The actin filaments were randomly organized along the rim of cell surface. On the contrary, no actin filaments were observed for cells cultured on the PVA- and agarose-coated dishes. For cells cultured on the fibronectin-coated dishes, actin filaments were formed inside the cell.
Blocking effect of integrin receptors and EBP on the cell apoptosis
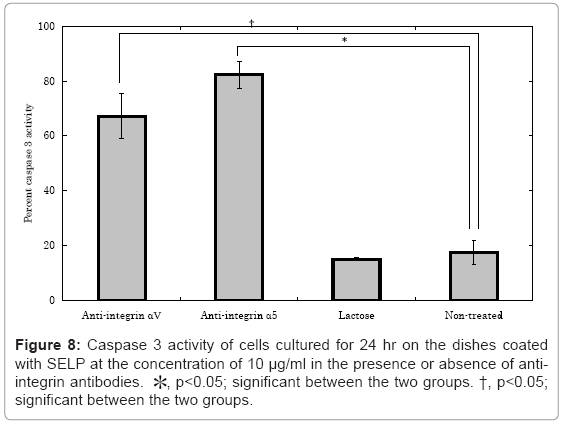
The effect of integrin αV, integrin α5, and lactose on the caspase 3 activity of cells cultured on the SELP-coated dishes Figure 8. The caspase 3 activity of cells cultured on the SELP-coated dishes “significantly” increased to the normal level when the anti-αV and anti-α5 integrin antibodies were pre-treated. However, the lactose treatment did not affect the level of caspase 3 activity.
Akt phosphorylation of cells cultured on SELP-coated dishes
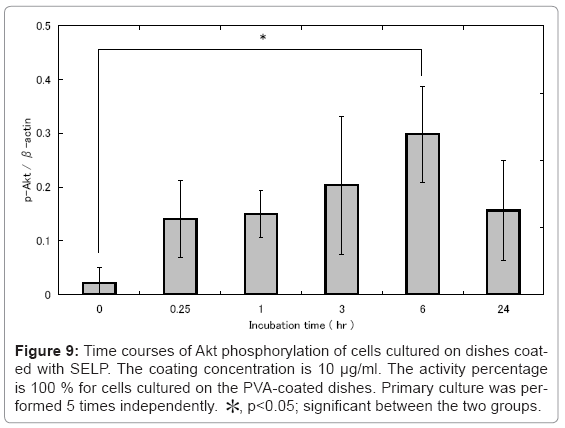
Figure 9 the time profile of Akt phosphorylation for cells cultured on SELP-coated dishes in different time intervals from 0 to 24 hr. Interestingly, the Akt phosphorylation was observed 6 hr after incubation and thereafter decreased as low as that of cells cultured on the non-treated culture dishes.
Figure 9: Time courses of Akt phosphorylation of cells cultured on dishes coated with SELP. The coating concentration is 10 μg/ml. The activity percentage is 100 % for cells cultured on the PVA-coated dishes. Primary culture was performed 5 times independently. *, p<0.05; significant between the two groups.
Discussion
The present study indicates that SELP has a nature to suppress the cells adhesion, but does not induce their apoptosis. Normally, in case cells cannot adhere onto a substrate of solid, they are apoptosed to die [16]. However, on the SELP-coated dishes, cells remained unadhered without the apoptosis. The apoptosis suppression was observed in the SELP concentration-dependent manner (Figure 3). The amount of SELP adsorbed onto dishes increased with an increase in the coating concentration, but leveled off at the concentration of 10 μg/ml (data not shown). In addition, the extent of cell adhesion suppression was also saturated at the SELP coating concentration of 10 μg/ml (data not shown). Taken together, it is expected that the SELP presence on the dishes manipulates the behavior of cell adhesion. However, the reason that the SELP has a property to suppress cell adhesion is not clear at present. On the other hand, PVA and agarose suppressed the cell adhesion and the consequent cells death was observed.
It is reported that some types of integrin signaling rescue apoptosis [34,35]. It is demonstrated that melanoma cells with a resistance to the detachment of extracellular matrix (ECM) over express integrin αVβ3 [36]. In addition, the αVβ3 over expression induced the Akt activation of melanoma cells even though they are in a detachment condition. It is known that cells lacking the ECM attachment undergo apoptosis [16]. It is reported that integrin αV and α5 were associated with cell apoptosis by detachment [34,35]. The blocking of integrin αVβ3 and α5β1 induced the apoptosis of fibroblasts and human colon cancer cells [34,35]. The Akt activity activation of MDCK cells inhibited the apoptotic death induced by their ECM detachment [34]. It is reported that elastin peptide rescue cellular apoptosis induced by the ceramide and indicated that EP treatment promotes Akt activation via EBP [32]. The apoptosis of cells on the SELP-coated dishes was promoted by pretreatment of cells with anti-integrin αV and α5 antibodies (Figure 8) and the cells showed akt activation (Figure 9). The phosphorylation of akt decreases at 24 hr after incubation compared with 6 hr. The signal of SELP to cells may reach to the maximum level at 6 hr and then decrease to the homeostatic level. On the other hand, the phosphorylation of akt did not change from 0 hr to 24 hr after incubation in cells cultured on PVA-coated dishes (data not shown). As an elastin receptor, the elastin receptor complex (ERC) of EBP, neuraminidase-1 (Neu-1), and cathepsin A/protective protein is reported [37]. EBP is an enzymatically inactive, spliced variant of β-galactosidase [38]. It binds an elastin peptide with a high affinity, while the elastin-EBP interaction can be regulated by galactosugars. For example, lactose is an antagonist of EBP commonly used. However, the pretreatment of cells did not rescue their apoptosis (Figure 8). The Akt kinase is one of anti-apoptotic substance via integrin signaling [16,39,40]. The Akt inactivates the caspase 9 of pro-apoptotic substance [41]. Taken together, it is possible that the SELP-induced apoptosis suppression is drived through the the integrin- akt pathway. SELP has an inherent property to form a hydrogel as the temperature increases from 25 to 37°C. This hydrogel formation may act to suppress the cell adhesion and apoptosis. Alternatively, the higher expression of annexin-V in both PVA, agarose coated dishes with the after examination apoptotic markers may be an indication that PS externalization is an early apoptotic events preceding the occurrence of other characteristic change. The most plausible explanation in our opinion for this difference, though, is dissociation of PS surface expression from apoptotic. Several line of classical evidence from the literature support this mechanism that is distinct from one leading to the typical events of apoptosis, including cytochrome c release, caspase activation, DNA fragmentation and that requires a sustained elevation of cytosolic Ca2+ levels.
Organization of actin was not observed for cells cultured on PVAcoated dishes. During the apoptosis process, it is reported that tensin is cleaved by effector caspases, such as caspase 3, 6, and 7 [42]. This cleavage of tensin is a critical step to disrupt the organization of cell actins in the apoptic condition, while their morphological change was induced [42]. On the SELP-coated dishes, round-shaped cells were observed, which is similar to the case of PVA-coated dishes. However, the organization of actin was observed in cells adhered on SELP-coated dishes although the pattern was different from that of fibronectincoated dishes. Integrins play a central role in organizing the actin cytoskeleton at the site of adhesion to the extracellular matrix [43]. Both of itegrins and actin cytoskeleton regulate signaling pathways [44]. It is reported that the actin cytoskeleton supports cell survival and the disruption of actin cytoskeleton induces apoptotic pathways. In mammary epithelial cells, the inhibition of actin polymerization is sufficient to activate apoptosis [45]. In addition, the drug-induced loss of the actin organization induced cell rounding and apoptotic cell death, which is correlated with suppression of Akt and activation of caspases [46]. It is conceivable that the organization of actin and the activation of akt in cells cultured on SELP-coated dishes contributed to the suppression of caspases.
There have been reported that elastin affects the proliferation [47,48], differentiation [49], migration [50,51], and anti-apoptosis of cells [32]. On the other hand, it is known that the SELP modifies the proliferation [52] and differentiation of cells [19]. However, there are no research reports to investigate whether the SELP has other biological activities which elastin has. The present study experimentally confirmed the anti-apoptotic activity of SELP. Further investigation is needed to clarify the molecular site of SELP to induce the activity of apoptosis suppression and the difference from elastin [53].
Conclusions
The SELP coating effectively gave the culture substrate a cell nonadherent property, but suppressed the cell apoptosis, in contrast to other substances of cell non-adherent property, such as agarose and PVA (Figures 1, 4, 5 and 6). The shape of cells cultured on the SELP and PVA-coated dishes was spherical, while the actin filaments around the surface of cells were detected only SELP-coated dishes (Figure 7). Pretreatment with the antibody of integrin αV or α5 significantly increased the caspase 3 level (Figure 8). It is concluded that the SELP with a property to suppress cells adhesion interacts with the integrin to rescue their apoptosis.
References
- Kurihara H, Nagamune T (2005) Cell adhesion ability of artificial extracellular matrix proteins containing a long repetitive Arg-Gly-Asp sequence. J Biosci Bioeng 100: 82-87.
- Ishii-Watabe A, Kanayasu-Toyoda T, Suzuki T, Kobayashi T,Yamaguchi T, et al. (2007) Influences of the recombinant artificial cell adhesive proteins on the behavior of human umbilical vein endothelial cells in serum-free culture. Biologicals 35: 247-57.
- Doetschman TC, Eistetter H, Katz M, Schmidt W, Kemler R (1985) The in vitro development of blastocyst-derived embryonic stem cell lines: formation of visceral yolk sac, blood islands and myocardium. J Embryol Exp Morphol 87: 27-45.
- Valamehr B, Jonas SJ, Polleux J, Qiao R, Guo S, et al. (2008) Hydrophobic surfaces for enhanced differentiation of embryonic stem cell-derived embryoid bodies. Proc Natl Acad Sci U S A 105: 14459-14464.
- Khalil M, Shariat-Panahi A, Tootle R, Ryder T, McCloskey P, et al. (2001) Human hepatocyte cell lines proliferating as cohesive spheroid colonies in alginate markedly upregulate both synthetic and detoxificatory liver function. J Hepatol 34: 68-77.
- Ma M, Xu J, Purcell WM (2003) Biochemical and functional changes of rat liver spheroids during spheroid formation and maintenance in culture: I. morphological maturation and kinetic changes of energy metabolism, albumin synthesis, and activities of some enzymes. J Cell Biochem 90: 1166-1175.
- Ma M, Xu J, Purcell WM (2003) Biochemical and functional changes of rat liver spheroids during spheroid formation and maintenance in culture: II. nitric oxide synthesis and related changes. J Cell Biochem 90: 1176-1185.
- Lee JH, Kopecek J, Andrade JD (1989) Protein-resistant surfaces prepared by PEO-containing block copolymer surfactants. J Biomed Mater Res 23: 351-68.
- Ikada Y (1994) Surface modification of polymers for medical applications. Biomaterials 15: 725-736.
- Uyama Y, Kato K, lkada Y (1998) Surface modification of polymers by grafting. Grafting/Characterization Techniques/Kinetic Modeling 137: 1-39.
- Campillo-Fernández AJ, Unger RE, Peters K, Halstenberg S, Santos M, et al. (2009) Analysis of the biological response of endothelial and fibroblast cells cultured on synthetic scaffolds with various hydrophilic/hydrophobic ratios: influence of fibronectin adsorption and conformation. Tissue Eng Part A 15: 1331-1341.
- Wei J,Igarashi T, Okumori N, Igarashi T, Maetani T, et al. (2009) Influence of surface wettability on competitive protein adsorption and initial attachment of osteoblasts. Biomed Mater 4: 045002.
- Nonckreman CJ, Fleith S, Rouxhet PG, Dupont-Gillain C (2010) Competitive adsorption of fibrinogen and albumin and blood platelet adhesion on surfaces modified with nanoparticles and/or PEO. Colloids Surf B Biointerfaces 77: 139-149.
- Yu Q, Zhang Y, Chen H, Zhou F, Wu Z, et al. (2010) Protein adsorption and cell adhesion/detachment behavior on dual-responsive silicon surfaces modified with poly(N-isopropylacrylamide)-block-polystyrene copolymer. Langmuir 26: 8582-8588.
- Hayashi K, Tabata Y (2011) Preparation of stem cell aggregates with gelatin microspheres to enhance biological functions. Acta Biomater 7: 2797-803.
- Grossmann J (2002) Molecular mechanisms of "detachment-induced apoptosis--Anoikis". Apoptosis 7: 247-260.
- Cappelloa J, Crissmana JW, Crissmana M, Ferraria FA, Textora G, et al. (1998) In-situ self-assembling protein polymer gel systems for administration, delivery, and release of drugs. J Control Release 53: 105-117.
- Megeed Z, Cappello J, Ghandehari H (2002) Controlled release of plasmid DNA from a genetically engineered silk-elastinlike hydrogel. Pharm Res 19: 954-959.
- Haider M, Cappello J, Ghandehari H, Leong KW (2008) In vitro chondrogenesis of mesenchymal stem cells in recombinant silk-elastinlike hydrogels. Pharm Res 25: 692-699.
- Hwanga D, Moolchandani V, Dandub R, Haider M,Cappello J, et al. (2009) Influence of polymer structure and biodegradation on DNA release from silk-elastinlike protein polymer hydrogels. Int J Pharm 368: 215-219.
- Costachel O, Fadei L, Badea E (1969) Tumor cell suspension culture on non adhesive substratum. J Cancer Res Clin Oncol 72: 24-31.
- Carlsson J, Gabel D, Larsson E, Pontén J, Westermark B (1979) Protein-coated agarose surfaces for attachment of cells. In Vitro Cell Dev Biol Plant 15: 844-850.
- Pierschbacher M, Hayman EG, Ruoslahti E (1983) Synthetic peptide with cell attachment activity of fibronectin. Proc Natl Acad Sci U S A 80: 1224-1227.
- Nuttelman CR, Henry SM, Anseth KS (2002) Synthesis and characterization of photocrosslinkable, degradable poly(vinyl alcohol)-based tissue engineering scaffolds. Biomaterials 23: 3617-3626.
- Gupta S, Webster TJ, Sinha A (2011) Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J Mater Sci Mater Med 22: 1763-1772.
- Fortneya JE, Zhaoa W, Wengerb Sl, Gibson LF (2001) Bone marrow stromal cells regulate caspase 3 activity in leukemic cells during chemotherapy. Leuk Res 25: 901-907.
- Thiagarajan P, Tait JF (1990) Binding of annexin V/placental anticoagulant protein I to platelets. Evidence for phosphatidylserine exposure in the procoagulant response of activated platelets. Journal of Biological Chemistry 265: 17420-17423.
- Fadok VA, Laszlo DJ, Noble PW, Weinstein L, Riches DW, et al. (1993) Particle digestibility is required for induction of the phosphatidylserine recognition mechanism used by murine macrophages to phagocytose apoptotic cells. Journal of Immunology 151: 4274-4285.
- Fadok VA, Savill JS, Haslett C, Bratton DL, Doherty DE, et al. (1992) Different populations of macrophages use either the vitronectin receptor or the phosphatidylserine receptor to recognize and remove apoptotic cells. Journal of Immunology 149: 4029-4035.
- Sachiko I, Masaaki I, Akihiro U, Yasuhiko T (2008) Attachment, proliferation and adipogenic differentiation of adipo-stromal cells on self-assembled monolayers of different chemical compositions. J Biomater Sci Polym Ed 19: 893-914.
- Duca L, Blanchevoye C, Cantarelli B, Ghoneim C, Dedieu S, et al. (2007) The elastin receptor complex transduces signals through the catalytic activity of its Neu-1 subunit. J Biol Chem 282: 12484-12891.
- Cantarellia B, Ducaa L, Blanchevoyea C, Poitevinb S, Martiny L, et al. (2009) Elastin peptides antagonize ceramide-induced apoptosis. FEBS Lett 583: 2385-2391.
- Zhang G, Gurtu V, Kain SR,Yan G (1997) Early detection of apoptosis using a fluorescent conjugate of annexin V. Biotechniques 23: 525-531.
- Murillo CA, Rychahou PG, Evers BM (2004) Inhibition of a5 integrin decreases PI3K activation and cell adhesion of human colon cancers. Surgery 136: 143-149.
- Zoppi N, Barlati S, Colombi M (2008) FAK-independent ab3 integrin-EGFR complexes rescue from anoikis matrix-defective fibroblasts. Biochim Biophys Acta 1783: 1177-1188.
- Tzukert K, Shimony N, Krasny L, Urieli-Shoval S, Gorodetsky R, et al. (2009) Human melanoma cells expressing the alphavbeta3 integrin are partially protected from necrotic cell death induced by dynamic matrix detachment. Cancer Lett 290: 174-181.
- Callahan JW (1999) Molecular basis of GM1 gangliosidosis and Morquio disease, type B. Structure-function studies of lysosomal beta-galactosidase and the non-lysosomal beta-galactosidase-like protein. Biochim Biophys Acta 1455: 85-103.
- Privitera S, Prody CA, Callahan JW, Hinek A (1998) The 67-kDa enzymatically inactive alternatively spliced variant of beta-galactosidase is identical to the elastin/laminin-binding protein. J Biol Chem 273: 6319-6326.
- Lee JW, Juliano RL (2000) a5b1 integrin protects intestinal epithelial cells from apoptosis through a phosphatidylinositol 3-kinase and protein kinase B-dependent pathway. Mol Biol Cell 11: 1973-1987.
- Pinkse GG, Bouwman WP, Jiawan-Lalai R, Terpstra OT, Bruijn JA, et al. (2006) Integrin signaling via RGD peptides and anti-beta1 antibodies confers resistance to apoptosis in islets of Langerhans. Diabetes 55: 312-317.
- Cardone MH, Roy N, Stennicke HR, Salvesen GS, Franke TF, et al. (1998) Regulation of cell death protease caspase-9 by phosphorylation. Science 282: 1318-1321.
- Kook S, Kima DH, Shima SR, Kima W, Chun JS, et al. (2003) Caspase-dependent cleavage of tensin induces disruption of actin cytoskeleton during apoptosis. Biochem Biophys Res Commun 303: 37-45.
- Hynes RO (1992) Integrins: versatility, modulation, and signaling in cell adhesion. Cell 69: 11-25.
- Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116: 167-179.
- Martin SS, Leder P (2001) Human MCF10A mammary epithelial cells undergo apoptosis following actin depolymerization that is independent of attachment and rescued by Bcl-2. Mol Cell Biol 21: 6529-6536.
- Flusberg DA, Numaguchi Y, lngber DE (2001) Cooperative control of Akt phosphorylation, bcl-2 expression, and apoptosis by cytoskeletal microfilaments and microtubules in capillary endothelial cells. Mol Biol Cell 12: 3087-3094.
- Landeau JM, Pellat B, Hornebeck W (1994) Increased secretion of latent elastase activity following contact between human skin fibroblasts and elastin derived peptides. Cell Biol Int 18: 111-117.
- Annabi N, Fathi A, Mithieux SM, Martens P, Weiss AS, et al. (2010) The effect of elastin on chondrocyte adhesion and proliferation on poly (varepsilon-caprolactone)/elastin composites. Biomaterials 32: 1517-1525.
- Betre H, Setton LA, Meyer DE, Chilkoti A (2002) Characterization of a genetically engineered elastin-like polypeptide for cartilaginous tissue repair. Biomacromolecules 3: 910-916.
- Senior RM, Griffin GL, Mecham RP (1980) Chemotactic activity of elastin-derived peptides. J Clin Invest 66: 859-862.
- Senior RM, Griffin GL, Mecham RP (1982) Chemotactic responses of fibroblasts to tropoelastin and elastin-derived peptides. J Clin Invest 70: 614-618.
- Scheller J, Henggeler D, Vivini A, Conrad U (2004) Purification of spider silk-elastin from transgenic plants and application for human chondrocyte proliferation. Transgenic Res 13: 51-57.
- García-Arévalo C, Alessandra Girotti MP, Javier Arias F, Rodríguez-Cabello JC (2012) A comparative study of cell behavior on different energetic and bioactive polymeric surfaces made from elastin-like recombinamers. Soft Matter 8: 3229-3249.
Relevant Topics
- Agricultural biotechnology
- Animal biotechnology
- Applied Biotechnology
- Biocatalysis
- Biofabrication
- Biomaterial implants
- Biomaterial-Based Drug Delivery Systems
- Bioprinting of Tissue Constructs
- Biotechnology applications
- Cardiovascular biomaterials
- CRISPR-Cas9 in Biotechnology
- Nano biotechnology
- Smart Biomaterials
- White/industrial biotechnology
Recommended Journals
Article Tools
Article Usage
- Total views: 16789
- [From(publication date):
July-2012 - Dec 20, 2025] - Breakdown by view type
- HTML page views : 11855
- PDF downloads : 4934